Association of Polyphenols from Oranges and Apples with Specific Intestinal Microorganisms in Systemic Lupus Erythematosus Patients
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Nutritional Assessment
2.2. Anthropometric Measures
2.3. Microbiological Analyses
2.4. Statistical Analysis
3. Results
| SLE (N = 20) | Control (N = 20) | Reference | |
|---|---|---|---|
| Age (year) | 49.25 ± 10.71 | 46.95 ± 8.60 | [4] |
| BMI (kg·m−2) | 26.11 ± 5.31 | 25.17 ± 4.16 | [4] |
| Energy (kcal·day−1) a | 2189.65 ± 722.42 | 1858.53 ± 332.85 | [4] |
| Animal protein (g·day−1) a,b | 104.70 ± 27.60 | 100.85 ± 20.86 | This work |
| Saturated fatty acids (g·day−1) a,b | 25.19 ± 14.09 | 24.66 ± 6.04 | [4] |
| Dietary fiber (g·day−1) a,b | 18.06 ± 10.04 | 20.32 ± 8.52 | [4] |
| Total flavonoids (mg·day−1) a,b | 400.13 ± 259.94 | 436.34 ± 189.70 | This work |
| Anthocyanins (mg·day−1) a,b | 20.81 ± 30.30 | 29.73 ± 30.65 | |
| Dihydrochalcones (mg·day−1) a,b | 2.70 ± 4.42 | 3.26 ± 3.58 | |
| Dihydroflavonols (mg·day−1) a,b | 0.34 ± 0.80 | 1.96 ± 2.82 * | |
| Flavanols (mg·day−1) a,b | 298.54 ± 222.16 | 283.47 ± 160.21 | |
| Flavanones (mg·day−1) a,b | 38.22 ± 29.44 | 45.31 ± 32.81 | |
| Flavones (mg·day−1) a,b | 2.48 ± 2.39 | 3.96 ± 3.92 | |
| Flavonols (mg·day−1) a,b | 38.00 ± 30.26 | 52.05 ± 25.55 | |
| Isoflavones (mg·day−1) a,b | 5.52 ± 16.81 | 15.92 ± 66.13 |
| Group | Animal protein (g·day−1) | SFA (g·day−1) | Dietary fiber (g·day−1) | ||||
|---|---|---|---|---|---|---|---|
| R2 | β | R2 | β | R2 | β | ||
| Blautia (%) | L | 0.129 | 0.206 | 0.273 | −0.874 | 0.109 | 0.129 |
| C | 0.028 | −0.117 | 0.016 | 0.031 | 0.046 | −0.205 | |
| Clostridium (%) | L | 0.271 | 0.431 | 0.170 | 0.363 | 0.140 | −0.116 |
| C | 0.116 | 0.343 | 0.007 | 0.026 | 0.007 | −0.022 | |
| Lactobacillus (%) | L | 0.041 | −0.237 | 0.024 | −0.312 | 0.031 | 0.225 |
| C | 0.203 | −0.347 | 0.091 | −0.030 | 0.174 | 0.336 | |
| Lactococcus (%) | L | 0.345 | 0.447 | 0.213 | −0.193 | 0.218 | 0.150 |
| C | 0.065 | −0.210 | 0.029 | −0.086 | 0.029 | −0.085 | |
| Faecalibacterium (%) | L | 0.063 | −0.111 | 0148 | 0.641 | 0.086 | −0.232 |
| C | 0.143 | 0.272 | 0.075 | 0.028 | 0.074 | 0.000 | |
| Bacteroides (%) | L | 0.005 | −0.026 | 0.017 | 0.232 | 0.118 | 0.439 |
| C | 0.125 | 0.110 | 0.291 | 0.483 | 0.115 | −0.045 | |
| Bifidobacterium (%) | L | 0.121 | −0.126 | 0.147 | 0.402 | 0.299 | 0.567 |
| C | 0.323 | −0.154 | 0.302 | −0.046 | 0.371 | −0.309 | |



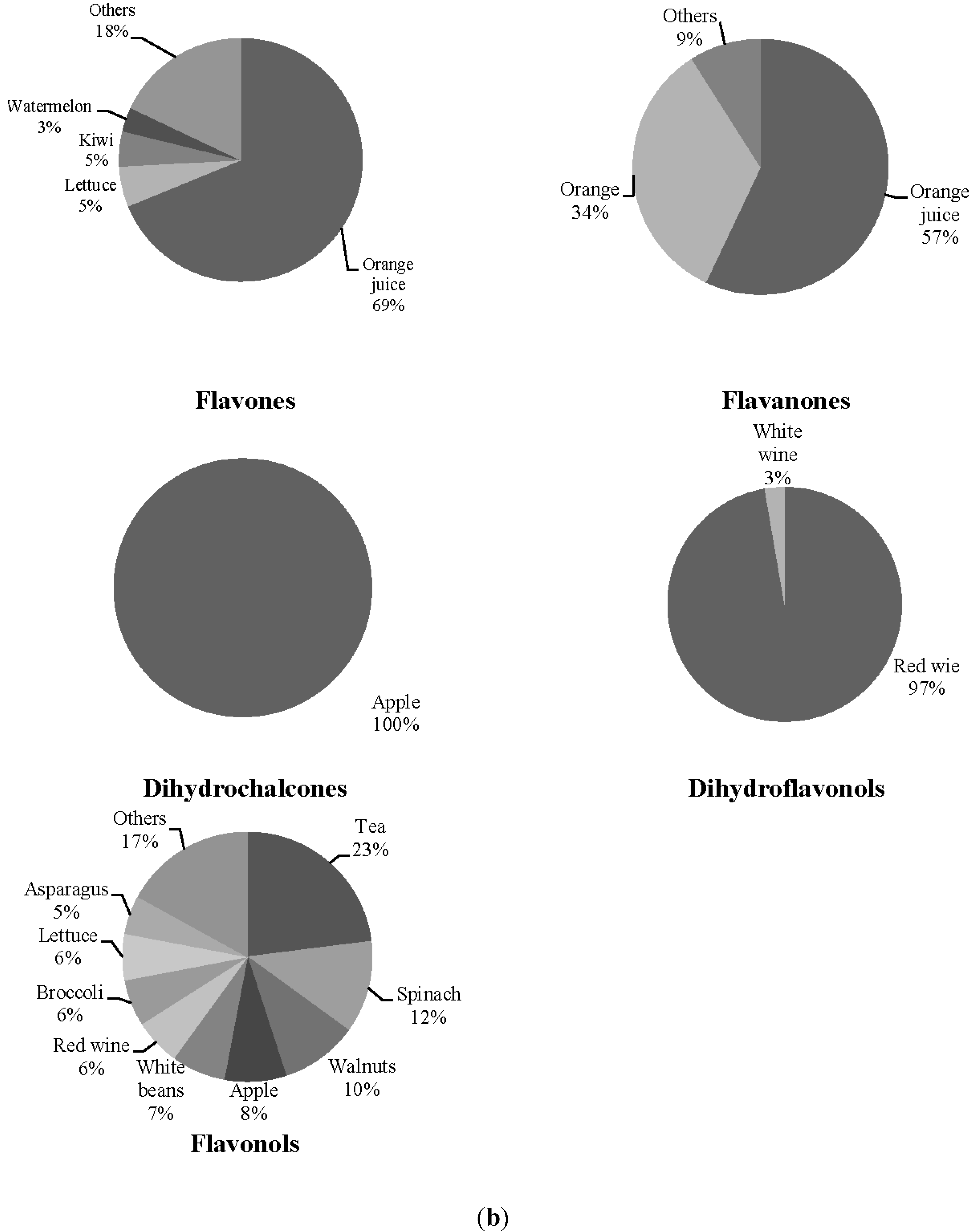
| SLE (N = 20) | Control (N = 20) | |
|---|---|---|
| Orange (g·day−1) | 58.43 ± 72.24 | 34.51 ± 42.75 |
| Orange juice (g·day−1) | 17.94 ± 33.69 | 51.51 ± 73.53 |
| Lettuce (g·day−1) | 50.63 ± 30.19 | 50.22 ± 42.66 |
| Watermelon (g·day−1) | 10.79 ± 19.11 | 7.01 ± 13.83 |
| Kiwi (g·day−1) | 21.19 ± 29.50 | 25.11 ± 62.61 |
| Tomato (g·day−1) | 84.06 ± 51.58 | 72.77 ± 48.13 |
| Apple (g·day−1) | 77.81 ± 81.87 | 58.72 ± 63.59 |
| Lentils (g/day) | 8.02 ± 5.12 | 9.31 ± 7.69 |
| Celery (g·day−1) | 0.18 ± 0.80 | 0.45 ± 1.51 |
| Red wine (mL·day−1) | 6.79 ± 14.37 | 34.20 ± 52.34 * |
| White wine (mL·day−1) | 1.79 ± 7.99 | 8.95 ± 34.30 |
| Tea (mL·day−1) | 51.52 ± 156.22 | 93.82 ± 172.59 |
| Spinach (g·day−1) | 3.01 ± 6.15 | 4.44 ± 5.76 |
| Walnuts (g·day−1) | 6.014 ± 11.24 | 7.75 ± 10.77 |
| White beans (g·day−1) | 6.61 ± 4.20 | 6.82 ± 7.13 |
| Broccoli (g·day−1) | 6.16 ± 10.63 | 7.64 ± 9.47 |
| Asparagus (g·day−1) | 9.66 ± 9.96 | 8.80 ± 14.87 |
| Green beans (g·day−1) | 8.08 ± 15.56 | 11.59 ± 12.36 |
| Predictors | R2 | β | p | |
|---|---|---|---|---|
| SLE (N = 20) | ||||
| Lactobacillus a | Orange | 0.383 | 0.619 | 0.004 |
| Bifidobacterium b | Apple | 0.437 | 0.661 | 0.001 |
| Controls (N = 20) | ||||
| Faecalibacterium c | Red wine | 0.264 | 0.514 | 0.024 |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lopez, P.; Gonzalez-Rodriguez, I.; Gueimonde, M.; Margolles, A.; Suarez, A. Immune response to Bifidobacterium bifidum strains support Treg/Th17 plasticity. PLoS One 2011, 6, e24776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, P.; Gonzalez-Rodriguez, I.; Sanchez, B.; Ruas-Madiedo, P.; Suarez, A.; Margolles, A.; Gueimonde, M. Interaction of Bifidobacterium bifidum LMG13195 with HT29 cells influences regulatory-T-cell-associated chemokine receptor expression. Appl. Environ. Microbiol. 2012, 78, 2850–2857. [Google Scholar] [CrossRef]
- Maslowski, K.M.; Mackay, C.R. Diet, gut microbiota and immune responses. Nat. Immunol. 2011, 12, 5–9. [Google Scholar] [CrossRef]
- Hevia, A.; Milani, C.; López, P.; Cuervo, A.; Arboleya, S.; Duranti, S.; Turroni, F.; González, S.; Suárez, A.; Gueimonde, M.; et al. Intestinal dysbiosis associated with Systemic Lupus Erythematosus. mBio 2014, 5, e01548–e01614. [Google Scholar]
- Berer, K.; Mues, M.; Koutrolos, M.; Rasbi, Z.A.; Boziki, M.; Johner, C.; Wekerle, H.; Krishnamoorthy, G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 2011, 479, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Markle, J.G.; Frank, D.N.; Mortin-Toth, S.; Robertson, C.E.; Feazel, L.M.; Rolle-Kampczyk, U.; von Bergen, M.; McCoy, K.D.; Macpherson, A.J.; Danska, J.S. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 2013, 339, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Proal, A.D.; Albert, P.J.; Marshall, T.G. The human microbiome and autoimmunity. Curr. Opin. Rheumatol. 2013, 25, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Manichanh, C.; Rigottier-Gois, L.; Bonnaud, E.; Gloux, K.; Pelletier, E.; Frangeul, L.; Nalin, R.; Jarrin, C.; Chardon, P.; Marteau, P.; et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut 2006, 55, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Fava, F.; Gitau, R.; Griffin, B.A.; Gibson, G.R.; Tuohy, K.M.; Lovegrove, J.A. The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome at-risk population. Int. J. Obes. 2013, 37, 216–223. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Martinez, I.; Lattimer, J.M.; Hubach, K.L.; Case, J.A.; Yang, J.; Weber, C.G.; Louk, J.A.; Rose, D.J.; Kyureghian, G.; Peterson, D.A.; et al. Gut microbiome composition is linked to whole grain1induced immunological improvements. ISME J. 2013, 7, 269–280. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [PubMed]
- Lee, H.C.; Jenner, A.M.; Low, C.S.; Lee, Y.K. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res. Microbiol. 2006, 157, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Schoenroth, L.J.; Hart, D.A.; Pollard, K.M.; Fritzler, M.J. The effect of the phytoestrogen coumestrol on the NZB/W F1 murine model of systemic lupus. J. Autoimmun. 2004, 23, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Queipo-Ortuno, M.I.; Boto-Ordonez, M.; Murri, M.; Gomez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R.; Cardona, D.F.; Andres-Lacueva, C.; Tinahones, F.J. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am. J. Clin. Nutr. 2012, 95, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Massot-Cladera, M.; Perez-Berezo, T.; Franch, A.; Castell, M.; Perez-Cano, F.J. Cocoa modulatory effect on rat faecal microbiota and colonic crosstalk. Arch. Biochem. Biophys. 2012, 527, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Guglielmetti, S.; Fracassetti, D.; Taverniti, V.; Del, B.C.; Vendrame, S.; Klimis-Zacas, D.; Arioli, S.; Riso, P.; Porrini, M. Differential modulation of human intestinal bifidobacterium populations after consumption of a wild blueberry (Vaccinium angustifolium) drink. J. Agric. Food Chem. 2013, 61, 8134–8140. [Google Scholar] [CrossRef] [PubMed]
- Vendrame, S.; Guglielmetti, S.; Riso, P.; Arioli, S.; Klimis-Zacas, D.; Porrini, M. Six-week consumption of a wild blueberry powder drink increases bifidobacteria in the human gut. J. Agric. Food Chem. 2011, 59, 12815–12820. [Google Scholar] [CrossRef] [PubMed]
- Dolara, P.; Luceri, C.; De, F.C.; Femia, A.P.; Giovannelli, L.; Caderni, G.; Cecchini, C.; Silvi, S.; Orpianesi, C.; Cresci, A. Red wine polyphenols influence carcinogenesis, intestinal microflora, oxidative damage and gene expression profiles of colonic mucosa in F344 rats. Mutat. Res. 2005, 591, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Tzounis, X.; Rodriguez-Mateos, A.; Vulevic, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am. J. Clin. Nutr. 2011, 93, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Lopez, P.; Mozo, L.; Gutierrez, C.; Suarez, A. Epidemiology of systemic lupus erythematosus in a northern Spanish population, gender and age influence on immunological features. Lupus 2003, 12, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.M.; Cohen, A.S.; Fries, J.F.; Masi, A.T.; McShane, D.J.; Rothfield, N.F.; Schaller, J.G.; Talal, N.; Winchester, R.J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982, 25, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, A.; Valdes, L.; Salazar, N.; de los Reyes-Gavilan, C.G.; Ruas-Madiedo, P.; Gueimonde, M.; Gonzalez, S. Pilot study of diet and microbiota, interactive associations of fibers and polyphenols with human intestinal bacteria. J. Agric. Food Chem. 2014, 62, 5330–5336. [Google Scholar] [CrossRef] [PubMed]
- Centro de Enseñanza Superior de Nutrición Humana y Dietética (CESNID). Tablas de Composición de Alimentos por Medidas Caseras de Consumo Habitual en España, 1st ed.; McGraw-Hill Interamericana de España S.L: Barcelona, Spain, 2008; pp. 1–288. [Google Scholar]
- Marlett, J.A.; Cheung, T.F. Database and quick methods of assessing typical dietary fiber intakes using data for 228 commonly consumed foods. J. Am. Diet. Assoc. 1997, 10, 1139–1151. [Google Scholar] [CrossRef]
- United States Department of Agriculture (USDA). Agricultural Research Service. USDA National Nutrient Database for Standard Reference. Available online: http://www.ars.usda.gov/Services/docs.htm?docid=8964 (accessed on 14 February 2012).
- Neveu, V.; Perez-Jimenez, J.; Vos, F.; Crespy, V.; du, C.L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer, an online comprehensive database on polyphenol contents in foods. Database 2010, bap024. [Google Scholar]
- Auborn, K.J.; Qi, M.; Yan, X.J.; Teichberg, S.; Chen, D.; Madaio, M.P.; Chiorazzi, N. Lifespan is prolonged in autoimmune-prone (NZB/NZW) F1 mice fed a diet supplemented with indole-3-carbinol. J. Nutr. 2003, 133, 3610–3613. [Google Scholar] [PubMed]
- Lai, N.S.; Lin, R.H.; Lai, R.S.; Kun, U.C.; Leu, S.C. Prevention of autoantibody formation and prolonged survival in New Zealand Black/New Zealand White F1 mice with an ancient Chinese herb, Ganoderma tsugae. Lupus 2001, 10, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Sawai, C.; Anderson, K.; Walser-Kuntz, D. Effect of bisphenol A on murine immune function; modulation of interferon-gamma, IgG2a, and disease symptoms in NZB X NZW F1 mice. Environ. Health Perspect. 2003, 111, 1883–1187. [Google Scholar] [CrossRef] [PubMed]
- De La Serre, C.B.; Ellis, C.L.; Lee, J.; Hartman, A.L.; Rutledge, J.C.; Raybould, H.E. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, 440–448. [Google Scholar] [CrossRef]
- Ghanim, H.; Sia, C.L.; Upadhyay, M.; Korzeniewski, K.; Viswanathan, P.; Abuaysheh, S.; Mohanty, P.; Dandona, P. Orange juice neutralizes the proinflammatory effect of a high-fat, high-carbohydrate meal and prevents endotoxin increase and Toll-like receptor expression. Am. J. Clin. Nutr. 2010, 91, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Selma, M.V.; Espin, J.C.; Tomas-Barberan, F.A. Interaction between phenolics and gut microbiota, role in human health. J. Agric. Food Chem. 2009, 57, 6485–64501. [Google Scholar] [CrossRef] [PubMed]
- Celiz, G.; Audisio, M.C.; Daz, M. Antimicrobial properties of prunin, a citric flavanone glucoside, and its prunin 6″-O-lauroyl ester. J. Appl. Microbiol. 2010, 109, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Schantz, M.; Erk, T.; Richling, E. Metabolism of green tea catechins by the human small intestine. Biotechnol. J. 2010, 5, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Sembries, S.; Dongowski, G.; Jacobasch, G.; Mehrlander, K.; Will, F.; Dietrich, H. Effects of dietary fibre-rich juice colloids from apple pomace extraction juices on intestinal fermentation products and microbiota in rats. Br. J. Nutr. 2003, 90, 607–715. [Google Scholar] [CrossRef] [PubMed]
- Sembries, S.; Dongowski, G.; Mehrlander, K.; Will, F.; Dietrich, H. Physiological effects of extraction juices from apple, grape, and red beet pomaces in rats. J. Agric. Food Chem. 2006, 54, 10269–10280. [Google Scholar] [CrossRef] [PubMed]
- Konieczna, P.; Akdis, C.A.; Quigley, E.M.; Shanahan, F.; O’Mahony, L. Portrait of an immunoregulatory Bifidobacterium. Gut Microbes 2012, 3, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Daly, K.; Darby, A.C.; Hall, N.; Nau, A.; Bravo, D.; Shirazi-Beechey, S.P. Dietary supplementation with lactose or artificial sweetener enhances swine gut Lactobacillus population abundance. Br. J. Nutr. 2014, 111, 30–35. [Google Scholar] [CrossRef]
- Ruiz, L.; Hevia, A.; Bernardo, D.; Margolles, A.; Sanchez, B. Extracellular molecular effectors mediating probiotic attributes. FEMS Microbiol. Lett. 2014, 359, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Cerda, B.; Tomas-Barberan, F.A.; Espin, J.C. Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans, identification of biomarkers and individual variability. J. Agric. Food Chem. 2005, 53, 227–235. [Google Scholar] [CrossRef]
- Gross, G.; Jacobs, D.M.; Peters, S.; Possemiers, S.; van Duynhoven, J.; Vaughan, E.E.; van de Wiele, T. In vitro bioconversion of polyphenols from black tea and red wine/grape juice by human intestinal microbiota displays strong interindividual variability. J. Agric. Food Chem. 2010, 58, 10236–10246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duda-Chodak, A. The inhibitory effect of polyphenols on human gut microbiota. J. Physiol. Pharmacol. 2012, 63, 497–503. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuervo, A.; Hevia, A.; López, P.; Suárez, A.; Sánchez, B.; Margolles, A.; González, S. Association of Polyphenols from Oranges and Apples with Specific Intestinal Microorganisms in Systemic Lupus Erythematosus Patients. Nutrients 2015, 7, 1301-1317. https://doi.org/10.3390/nu7021301
Cuervo A, Hevia A, López P, Suárez A, Sánchez B, Margolles A, González S. Association of Polyphenols from Oranges and Apples with Specific Intestinal Microorganisms in Systemic Lupus Erythematosus Patients. Nutrients. 2015; 7(2):1301-1317. https://doi.org/10.3390/nu7021301
Chicago/Turabian StyleCuervo, Adriana, Arancha Hevia, Patricia López, Ana Suárez, Borja Sánchez, Abelardo Margolles, and Sonia González. 2015. "Association of Polyphenols from Oranges and Apples with Specific Intestinal Microorganisms in Systemic Lupus Erythematosus Patients" Nutrients 7, no. 2: 1301-1317. https://doi.org/10.3390/nu7021301
APA StyleCuervo, A., Hevia, A., López, P., Suárez, A., Sánchez, B., Margolles, A., & González, S. (2015). Association of Polyphenols from Oranges and Apples with Specific Intestinal Microorganisms in Systemic Lupus Erythematosus Patients. Nutrients, 7(2), 1301-1317. https://doi.org/10.3390/nu7021301





