Intestinal Microbial Dysbiosis and Colonic Epithelial Cell Hyperproliferation by Dietary α-Mangostin is Independent of Mouse Strain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Ethics Statement
2.3. Diet
2.4. Experimental Groups and Tissue Collection
2.5. Histology and Immunohistochemistry (IHC)
2.6. Bacterial Analyses
2.7. Statistical Analysis
2.8. Availability of Supporting Data
3. Results
3.1. Dietary α-MG Increases Fluid Content in Stool
| Strain | Group | Body Weight (g)/Experimental Day | Food Intake (g/Day) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6 | 12 | 19 | 26 | |||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| C57BL/6J | control | 18.4 | 0.6 | 19.2 | 1.1 | 19.5 | 1.1 | 21.0 | 1.5 | 20.2 | 0.6 | 2.1 | 0.2 |
| α-MG | 18.7 | 1.9 | 19.0 | 1.6 | 19.4 | 1.3 | 19.8 | 1.3 | 20.2 | 1.1 | 2.2 | 0.3 | |
| Balb/c | control | 19.9 | 1.1 | 20.6 | 1.0 | 20.5 | 1.1 | 20.4 | 1.0 | 21.8 | 1.4 | 2.3 | 0.4 |
| α-MG | 20.5 | 1.0 | 20.6 | 0.6 | 21.4 | 0.8 | 21.6 | 0.8 | 21.7 | 0.3 | 2.3 | 0.2 | |
| C3H | control | 25.2 | 1.2 | 25.0 | 1.4 | 25.3 | 0.8 | 26.2 | 1.1 | 26.8 | 1.7 | 2.7 | 0.2 |
| α-MG | 25.6 | 1.1 | 25.4 | 1.6 | 25.7 | 2.0 | 26.5 | 2.3 | 27.2 | 1.9 | 2.8 | 0.3 | |
| Athymic | control | 22.1 | 1.4 | 22.4 | 1.5 | 23.7 | 1.3 | 24.2 | 1.6 | 23.5 | 1.0 | 3.0 | 0.1 |
| FoxN1nu | α-MG | 23.0 | 0.9 | 23.6 | 1.0 | 24.2 | 0.8 | 24.8 | 1.2 | 25.0 | 1.4 | 2.9 | 0.1 |
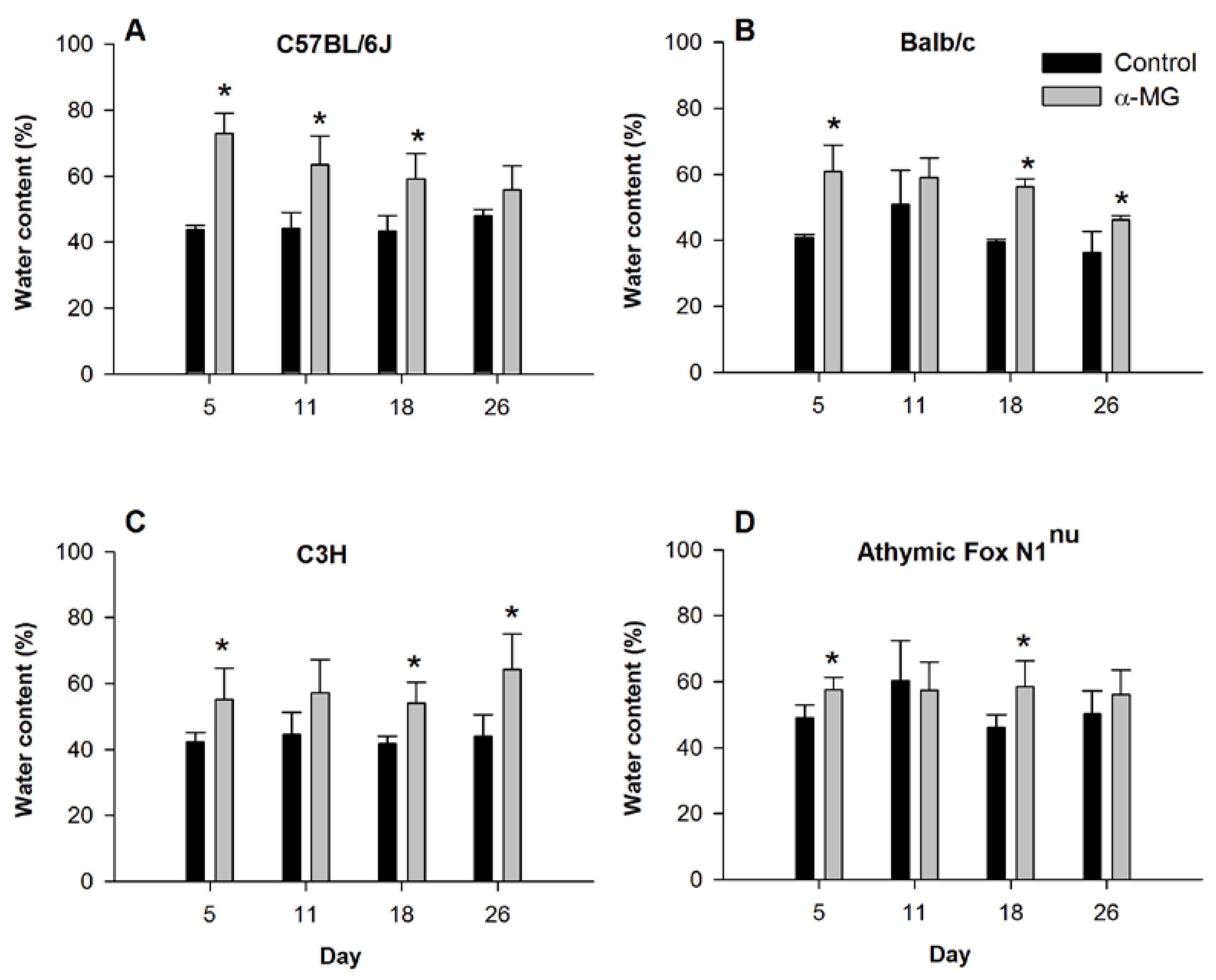
3.2. Colonic Epithelial Cell Proliferation and Immune Cell Infiltration are Stimulated by α-MG

3.3. Dietary α-MG Induces Dysbiosis in Mice with Different Genetic Backgrounds
3.3.1. C57BL/6J Strain
3.3.2. Balb/c Strain


3.3.3. C3H Strain
3.3.4. Athymic FoxN1nu Strain


4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sekirov, I.; Russell, S.L.; Antunes, L.C.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Castellarin, M.; Warren, R.L.; Freeman, J.D.; Dreolini, L.; Krzywinski, M.; Strauss, J.; Barnes, R.; Watson, R.; Allen-Vercoe, E.; Moore, R.A.; et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012, 22, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.D.; Gevers, D.; Pedamallu, C.S.; Michaud, M.; Duke, F.; Earl, A.M.; Ojesina, A.I.; Jung, J.; Bass, A.J.; Tabernero, J.; et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012, 22, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sorensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One 2010, 5, e9085. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.; St Amand, A.; Feldman, R.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. PNAS 2007, 104, 13780–13785. [Google Scholar] [CrossRef] [PubMed]
- Ott, S.; Musfeldt, M.; Wenderoth, D.; Hampe, J.; Brant, O.; Folsch, U.R.; Timmis, K.N.; Schreiber, S. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut 2004, 53, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Lupp, C.; Robertson, M.; Wickham, M.; Sekirov, I.; Champion, O.; Gaynor, E.C.; Finlay, B.B. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2007, 2, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. PNAS 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Knight, R.; Gordon, J.I. The effect of diet on the human gut microbiome: A metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 2009, 1. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Farias, C.; Slezak, K.; Fuller, Z.; Duncan, A.; Holtrop, G.; Louis, P. Effect of inulin on the human gut microbiota: Stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br. J. Nutr. 2009, 101, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Blaut, M.; Clavel, T. Metabolic diversity of the intestinal microbiota: Implications for health and disease. J. Nutr. 2007, 137, 751–755. [Google Scholar]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly) phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073–2085. [Google Scholar]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Taguri, T.; Tanaka, T.; Kouno, I. Antibacterial spectrum of plant polyphenols and extracts depending upon hydroxyphenyl structure. Biol. Pharm. Bull. 2006, 29, 2226–2235. [Google Scholar] [CrossRef] [PubMed]
- Yapwattanaphun, C.; Subhadrabandhu, S.; Sugiura, A.; Yonemori, K.; Utsunomiya, N. Utilization of some Garcinia species in Thailand. Acta Hort. (ISHS) 2002, 575, 563–570. [Google Scholar]
- Pedraza-Chaverri, J.; Cárdenas-Rodríguez, N.; Orozco-Ibarra, M.; Pérez-Rojas, J.M. Medicinal properties of mangosteen (Garcinia mangostana). Food Chem. Toxicol. 2008, 46, 3227–3239. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Orozco, F.; Failla, M.L. Biological activities and bioavailability of mangosteen xanthones: A critical review of the current evidence. Nutrients 2013, 5, 3163–3183. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Orozco, F.; Thomas-Ahner, J.M.; Berman-Booty, L.D.; Chitchumroonchokchai, C.; Mace, T.; Suksamrarn, S.; Bailey, M.T.; Clinton, S.K.; Lesinski, G.B.; et al. Dietary α-mangostin, a xanthone from mangosteen fruit, exacerbates experimental colitis and promotes dysbiosis in mice. Mol. Nutr. Food Res. 2014, 58, 1226–1238. [Google Scholar] [CrossRef] [PubMed]
- Chitchumroonchokchai, C.; Riedl, K.M.; Suksumrarn, S.; Clinton, S.K.; Kinghorn, A.D.; Failla, M.L. Xanthones in mangosteen juice are absorbed and partially conjugated by healthy adults. J. Nutr. 2012, 142, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Chitchumroonchokchai, C.; Thomas-Ahner, J.M.; Li, J.; Riedl, K.M.; Nontakham, J.; Suksumrarn, S.; Clinton, S.K.; Kinghorn, A.D.; Failla, M.L. Anti-tumorigenicity of dietary α-mangostin in an HT-29 colon cell xenograft model and the tissue distribution of xanthones and their phase II metabolites. Mol. Nutr. Food Res. 2013, 57, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Eckburg, P.B.; Bik, E.; Bernstein, C.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Walton, K.; Blue, R.; MacNaughton, K.; Magnes, S.T.; Lund, P.K. Mucosal healing and fibrosis after acute or chronic inflammation in wild type FVB-N mice and C57BL6 procollagen α1(I)-promoter-GFP reporter mice. PLoS One 2012, 7, e42568. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.B. HPLC analysis of selected xanthones in mangosteen fruit. J. Sep. Sci. 2007, 30, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Chaivisuthangkura, A.; Malaikaew, Y.; Chaovanalikit, A.; Jaratrungtawee, A.; Panseeta, P.; Ratananukul, P.; Suksamrarn, S. Prenylated xanthone composition of Garcinia mangostana (mangosteen) fruit hull. Chromatographia 2009, 69, 315–318. [Google Scholar] [CrossRef]
- Johnson, J.; Petiwala, S.; Syed, D.; Rasmussen, J.; Adhami, V.; Siddiqui, I.A.; Kohl, A.M.; Mukhtar, H. α-Mangostin, a xanthone from mangosteen fruit, promotes cell cycle arrest in prostate cancer and decreases xenograft tumor growth. Carcinogenesis 2012, 33, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Gonzalez Peña, A.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.; Tiedje, J.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- McDonald, D.; Price, M.; Goodrich, J.; Nawrocki, E.P.; DeSantis, T.Z.; Probst, A.; Andersen, G.L.; Knight, R.; Hugeholtz, P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012, 6, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.; Bittinger, K.; Bushman, F.; DeSantis, T.Z.; Andersen, G.L.; Knight, R. PyNAST: A flexible tool for aligning sequences to a template alignment. Bioinformatics 2010, 26, 266–267. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C.E. The mathematical theory of communication. MD Comput. 1997, 14, 306–317. [Google Scholar] [PubMed]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Guillaume Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, H.H.; Wagner, H. Vegan: Community Ecology Package. Available online: http://www.cran.r-project.org/package=vegan (accessed on 12 February 2014).
- The R Core Team. R: A language and Environment for Statistical Computing. Available online: http://www.web.mit.edu/r_v3.0.1/fullrefman.pdf (accessed on 16 January 2014).
- Campbell, J.; Foster, C.; Vishnivetskaya, T.; Campbell, A.G.; Yang, Z.K.; Wymore, A.; Palumbo, A.V.; Chesler, E.J.; Podar, M. Host genetic and environmental effects on mouse intestinal microbiota. ISME J. 2012, 6, 2033–2044. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.G.; Yang, L.L.; Wang, C.C. Anti-inflammatory activity of mangostins from Garcinia mangostana. Food Chem. Toxicol. 2008, 46, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.Y.; Kwon, O.K.; Oh, S.R.; Lee, H.K.; Chin, Y.W. Mangosteen xanthones mitigate ovalbumin-induced airway inflammation in a mouse model of asthma. Food Chem. Toxicol. 2012, 50, 4042–4050. [Google Scholar] [CrossRef] [PubMed]
- Tewtrakul, S.; Wattanapiromsakul, C.; Mahabusarakam, W. Effects of compounds from Garcinia mangostana on inflammatory mediators in RAW264.7 macrophage cells. J. Ethnopharmacol. 2009, 121, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.; Ma, Q.; Guo, K.; Liu, J.; Wang, F.; Wu, E. Xanthones from mangosteen extracts as natural chemopreventive agents: Potential anticancer drugs. Curr. Mol. Med. 2011, 11, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Sindermsuk, J.; Deekijsermphong, S. The antibacterial activities of crude extract from the fruit hull of Garcinia mangostana on enteric pathogens and intestinal commensal flora. Bull. Depart. Med. Serv. 1989, 14, 421–426. [Google Scholar]
- Kitajima, S.; Morimoto, M.; Sagara, E.; Schimizu, C.; Ikeda, Y. Dextran sodium sulfate-induced colitis in germ-free IQI/Jic mice. Exp. Anim. 2001, 50, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Fite, A.; Macfarlane, S.; Furrie, E.; Bahrami, B.; Cummings, J.H.; Steinke, D.T.; Macfarlane, G.T. Longitudinal analyses of gut mucosal microbiotas in ulcerative colitis in relation to patient age and disease severity and duration. J. Clin. Microbiol. 2013, 51, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Pathmakanthan, S.; Thornley, J.P.; Hawkey, C.J. Mucosally associated bacterial flora of the human colon: Quantitative and species specific differences between normal and inflamed colonic biopsies. Microb. Ecol. Health Dis. 1999, 11, 169–174. [Google Scholar] [CrossRef]
- Calder, P.; Albers, R.; Antoine, J.; Blum, S.; Bourdet-Sicard, R.; Ferns, G.A.; Folkerts, G.; Friedmann, P.S.; Frost, G.S.; Guarner, F.; et al. Inflammatory disease processes and interactions with nutrition. Br. J. Nutr. 2009, 101, 1–45. [Google Scholar]
- Romier, B.; Schneider, Y.J.; Larondelle, Y.; During, A. Dietary polyphenols can modulate the intestinal inflammatory response. Nutr. Rev. 2009, 67, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, S.; Macfarlane, G.T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003, 62, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Maslowski, K.; Vieira, A.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Duda-Chodak, A. The inhibitory effect of polyphenols on human gut microbiota. J. Physiol. Pharmacol. 2012, 63, 497–503. [Google Scholar] [PubMed]
- Walter, J. Ecological role of Lactobacilli in the gastrointestinal tract: Implications for fundamental and biomedical research. Appl. Environ. Microbiol. 2008, 74, 4985–4996. [Google Scholar] [CrossRef] [PubMed]
- Kemperman, R.A.; Gross, G.; Mondot, S.; Possemiers, S.; Marzorati, M.; Van de Wiele, T.; Dore, J.; Vaughan, E.E. Impact of polyphenols from black tea and red wine/grape juice on a gut model microbiome. Food Res. Int. 2013, 53, 659–669. [Google Scholar] [CrossRef]
- Dubourg, G.; Lagier, J.C.; Armougom, F.; Robert, C.; Audoly, G.; Papazian, L.; Raoult, D. High-level colonization of the human gut by Verrucomicrobia following broad-spectrum antibiotic treatment. Int. J. Antimicrob. Agents 2013, 41, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Collado, M.C.; Ben-Amor, K.; Salminen, S.; de Vos, W.M. The mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl. Environ. Microbiol. 2008, 74, 1646–1648. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Hullar, M.A.; Schwarz, Y.; Lampe, J.W. Human gut bacterial communities are altered by addition of cruciferous vegetables to a controlled fruit- and vegetable-free diet. J. Nutr. 2009, 139, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutierrez-Orozco, F.; Thomas-Ahner, J.M.; Galley, J.D.; Bailey, M.T.; Clinton, S.K.; Lesinski, G.B.; Failla, M.L. Intestinal Microbial Dysbiosis and Colonic Epithelial Cell Hyperproliferation by Dietary α-Mangostin is Independent of Mouse Strain. Nutrients 2015, 7, 764-784. https://doi.org/10.3390/nu7020764
Gutierrez-Orozco F, Thomas-Ahner JM, Galley JD, Bailey MT, Clinton SK, Lesinski GB, Failla ML. Intestinal Microbial Dysbiosis and Colonic Epithelial Cell Hyperproliferation by Dietary α-Mangostin is Independent of Mouse Strain. Nutrients. 2015; 7(2):764-784. https://doi.org/10.3390/nu7020764
Chicago/Turabian StyleGutierrez-Orozco, Fabiola, Jennifer M. Thomas-Ahner, Jeffrey D. Galley, Michael T. Bailey, Steven K. Clinton, Gregory B. Lesinski, and Mark L. Failla. 2015. "Intestinal Microbial Dysbiosis and Colonic Epithelial Cell Hyperproliferation by Dietary α-Mangostin is Independent of Mouse Strain" Nutrients 7, no. 2: 764-784. https://doi.org/10.3390/nu7020764
APA StyleGutierrez-Orozco, F., Thomas-Ahner, J. M., Galley, J. D., Bailey, M. T., Clinton, S. K., Lesinski, G. B., & Failla, M. L. (2015). Intestinal Microbial Dysbiosis and Colonic Epithelial Cell Hyperproliferation by Dietary α-Mangostin is Independent of Mouse Strain. Nutrients, 7(2), 764-784. https://doi.org/10.3390/nu7020764





