Alcohol: A Nutrient with Multiple Salutary Effects
Abstract
:1. Alcohol in Health and Disease
2. Alcohol and Plasma Lipoproteins
2.1. Alcohol and Endogenous Triglyceride Metabolism
2.2. Effects of Alcohol on Postprandial Lipemia
2.3. Alcohol and High Density Lipoprotein Metabolism
3. G-Protein Coupled Receptor (GPCR) 43
3.1. Acetate as CVD Therapy
3.2. Antidiabetic Effects of Acetate
3.3. Alcohol and Pancreatitis
3.4. Effects of Acute Alcohol on De Novo Lipogenesis (DNL) and Lipid Balances in Humans
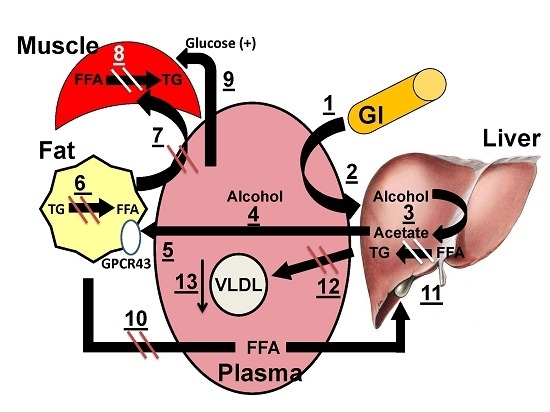
3.5. Mechanistic Model for the Salutary Effects of Acetate
| Analyte | Effect |
|---|---|
| Plasma FFA | Decrease |
| Plasma alcohol | Increase |
| Plasma acetate | Increase |
| Plasma TG | Decrease |
| Plasma Glucose | Decrease |
| HDL-C | Increase |
| Small, dense LDL | Decrease |
4. Conclusions

Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thun, M.J.; Peto, R.; Lopez, A.D.; Monaco, J.H.; Henley, S.J.; Heath, C.W., Jr.; Doll, R. Alcohol consumption and mortality among middle-aged and elderly U.S. Adults. N. Engl. J. Med. 1997, 337, 1705–1714. [Google Scholar]
- Ginsberg, H.; Olefsky, J.; Farquhar, J.W.; Reaven, G.M. Moderate ethanol ingestion and plasma triglyceride levels. A study in normal and hypertriglyceridemic persons. Ann. Intern. Med. 1974, 80, 143–149. [Google Scholar]
- Abramson, E.A.; Arky, R.A. Acute antilipolytic effects of ethyl alcohol and acetate in man. J. Lab. Clin. Med. 1968, 72, 105–117. [Google Scholar]
- Huang, Z.; Sjoholm, A. Ethanol acutely stimulates islet blood flow, amplifies insulin secretion, and induces hypoglycemia via nitric oxide and vagally mediated mechanisms. Endocrinology 2008, 149, 232–236. [Google Scholar]
- Nilsson, N.O.; Belfrage, P. Effects of acetate, acetaldehyde, and ethanol on lipolysis in isolated rat adipocytes. J. Lipid Res. 1978, 19, 737–741. [Google Scholar]
- Patsch, J.R.; Miesenbock, G.; Hopferwieser, T.; Muhlberger, V.; Knapp, E.; Dunn, J.K.; Gotto, A.M., Jr.; Patsch, W. Relation of triglyceride metabolism and coronary artery disease. Studies in the postprandial state. Arterioscler. Thromb. 1992, 12, 1336–1345. [Google Scholar]
- Pownall, H.J. Dietary ethanol is associated with reduced lipolysis of intestinally derived lipoproteins. J. Lipid Res. 1994, 35, 2105–2113. [Google Scholar]
- Barboriak, J.J.; Meade, R.C. Enhancement of alimentary lipemia by preprandial alcohol. Am. J. Med. Sci. 1968, 255, 245–251. [Google Scholar]
- Hartung, G.H.; Lawrence, S.J.; Reeves, R.S.; Foreyt, J.P. Effect of alcohol and exercise on postprandial lipemia and triglyceride clearance in men. Atherosclerosis 1993, 100, 33–40. [Google Scholar]
- Ellison, R.C.; Zhang, Y.; Qureshi, M.M.; Knox, S.; Arnett, D.K.; Province, M.A.; Investigators of the NHLBI family heart study. Lifestyle determinants of high-density lipoprotein cholesterol: The national heart, lung, and blood institute family heart study. Am. Heart J. 2004, 147, 529–535. [Google Scholar]
- Rimm, E.B.; Williams, P.; Fosher, K.; Criqui, M.; Stampfer, M.J. Moderate alcohol intake and lower risk of coronary heart disease: Meta-Analysis of effects on lipids and haemostatic factors. BMJ 1999, 319, 1523–1528. [Google Scholar]
- Gaziano, J.M.; Buring, J.E.; Breslow, J.L.; Goldhaber, S.Z.; Rosner, B.; VanDenburgh, M.; Willett, W.; Hennekens, C.H. Moderate alcohol intake, increased levels of high-density lipoprotein and its subfractions, and decreased risk of myocardial infarction. N. Engl. J. Med. 1993, 329, 1829–1834. [Google Scholar]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.Y.; Lannoy, V.; Decobecq, M.E.; Brezillon, S.; Dupriez, V.; Vassart, G.; van Damme, J.; et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar]
- Nilsson, N.E.; Kotarsky, K.; Owman, C.; Olde, B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem. Biophys. Res. Commun. 2003, 303, 1047–1052. [Google Scholar]
- Hong, Y.H.; Nishimura, Y.; Hishikawa, D.; Tsuzuki, H.; Miyahara, H.; Gotoh, C.; Choi, K.C.; Feng, D.D.; Chen, C.; Lee, H.G.; et al. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology 2005, 146, 5092–5099. [Google Scholar]
- Ge, H.; Li, X.; Weiszmann, J.; Wang, P.; Baribault, H.; Chen, J.L.; Tian, H.; Li, Y. Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology 2008, 149, 4519–4526. [Google Scholar]
- Kimura, W.; Mossner, J. Role of hypertriglyceridemia in the pathogenesis of experimental acute pancreatitis in rats. Int. J. Pancreatol 1996, 20, 177–184. [Google Scholar]
- Lundquist, F.; Tygstrup, N.; Winkler, K.; Mellemgaard, K.; Munck-Petersen, S. Ethanol metabolism and production of free acetate in the human liver. J. Clin. Investig. 1962, 41, 955–961. [Google Scholar]
- Siler, S.Q.; Neese, R.A.; Hellerstein, M.K. De novo lipogenesis, lipid kinetics, and whole-body lipid balances in humans after acute alcohol consumption. Am. J. Clin. Nutr. 1999, 70, 928–936. [Google Scholar]
- Fushimi, T.; Suruga, K.; Oshima, Y.; Fukiharu, M.; Tsukamoto, Y.; Goda, T. Dietary acetic acid reduces serum cholesterol and triacylglycerols in rats fed a cholesterol-rich diet. Br. J. Nutr. 2006, 95, 916–924. [Google Scholar]
- Johnston, C.S.; Gaas, C.A. Vinegar: Medicinal uses and antiglycemic effect. MedGenMed 2006, 8, 61. [Google Scholar]
- Johnston, C.S.; Kim, C.M.; Buller, A.J. Vinegar improves insulin sensitivity to a high-carbohydrate meal in subjects with insulin resistance or type 2 diabetes. Diabetes Care 2004, 27, 281–282. [Google Scholar]
- Leeman, M.; Ostman, E.; Bjorck, I. Vinegar dressing and cold storage of potatoes lowers postprandial glycaemic and insulinaemic responses in healthy subjects. Eur J. Clin. Nutr. 2005, 59, 1266–1271. [Google Scholar]
- Liljeberg, H.; Bjorck, I. Delayed gastric emptying rate may explain improved glycaemia in healthy subjects to a starchy meal with added vinegar. Eur. J. Clin. Nutr. 1998, 52, 368–371. [Google Scholar]
- Ostman, E.M.; Elmstahl, H.G.L.; Bjorck, I.M. Inconsistency between glycemic and insulinemic responses to regular and fermented milk products. Am. J. Clin Nutr 2001, 74, 96–100. [Google Scholar]
- Sugiyama, M.; Tang, A.C.; Wakaki, Y.; Koyama, W. Glycemic index of single and mixed meal foods among common Japanese foods with white rice as a reference food. Eur. J. Clin. Nutr. 2003, 57, 743–752. [Google Scholar]
- Vu, C.N.; Ruiz-Esponda, R.; Yang, E.; Chang, E.; Gillard, B.; Pownall, H.J.; Hoogeveen, R.C.; Coraza, I.; Balasubramanyam, A. Altered relationship between plasma triglycerides to HDL cholesterol in patients with HIV/HAART-associated dyslipidemia: Further evidence for a unique form of metabolic syndrome in HIV patients. Metabolism 2013, 62, 1014–1020. [Google Scholar]
- Brown, A.G.; Smale, T.C.; King, T.J.; Hasenkamp, R.; Thompson, R.H. Crystal and molecular structure of compactin, a new antifungal metabolite from penicillium brevicompactum. J. Chem. Soc. Perkin. 1976, 1, 1165–1170. [Google Scholar]
- Boffetta, P.; Hashibe, M. Alcohol and cancer. Lancet Oncol. 2006, 7, 149–156. [Google Scholar]
- Rehm, J.; Baliunas, D.; Borges, G.L.; Graham, K.; Irving, H.; Kehoe, T.; Parry, C.D.; Patra, J.; Popova, S.; Poznyak, V.; et al. The relation between different dimensions of alcohol consumption and burden of disease: An overview. Addiction 2010, 105, 817–843. [Google Scholar]
- Glueck, C.J.; Lang, J.; Hamer, T.; Tracy, T. Severe hypertriglyceridemia and pancreatitis when estrogen replacement therapy is given to hypertriglyceridemic women. J. Lab. Clin. Med. 1994, 123, 59–64. [Google Scholar]
- Whitfield, J.B.; Hensley, W.J.; Bryden, D.; Gallagher, H. Some laboratory correlates of drinking habits. Ann. Clin. Biochem. 1978, 15, 297–303. [Google Scholar]
- Pownall, H.J.; Ballantyne, C.M.; Kimball, K.T.; Simpson, S.L.; Yeshurun, D.; Gotto, A.M., Jr. Effect of moderate alcohol consumption on hypertriglyceridemia: A study in the fasting state. Arch. Intern. Med. 1999, 159, 981–987. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pownall, H.J.; Rosales, C.; Gillard, B.K.; Gotto, A.M., Jr. Alcohol: A Nutrient with Multiple Salutary Effects. Nutrients 2015, 7, 1992-2000. https://doi.org/10.3390/nu7031992
Pownall HJ, Rosales C, Gillard BK, Gotto AM Jr. Alcohol: A Nutrient with Multiple Salutary Effects. Nutrients. 2015; 7(3):1992-2000. https://doi.org/10.3390/nu7031992
Chicago/Turabian StylePownall, Henry J., Corina Rosales, Baiba K. Gillard, and Antonio M. Gotto, Jr. 2015. "Alcohol: A Nutrient with Multiple Salutary Effects" Nutrients 7, no. 3: 1992-2000. https://doi.org/10.3390/nu7031992
APA StylePownall, H. J., Rosales, C., Gillard, B. K., & Gotto, A. M., Jr. (2015). Alcohol: A Nutrient with Multiple Salutary Effects. Nutrients, 7(3), 1992-2000. https://doi.org/10.3390/nu7031992