Hydrolysis of the Rutinose-Conjugates Flavonoids Rutin and Hesperidin by the Gut Microbiota and Bifidobacteria
Abstract
:1. Introduction

2. Experimental Section
2.1. Chemicals and Bacterial Strains
| Strain | Rutin → Quercetin | Hesperidin → Hesperetin |
|---|---|---|
| Bioconversion % | Bioconversion % | |
| B. animalis subsp. animalis ATCC 27536 | - | - |
| B. animalis subsp. animalis WC 0409 | - | - |
| B. animalis subsp. animalis WC 0410 | - | - |
| B. animalis subsp. animalis WC 0411 | - | - |
| B. animalis subsp. lactis WC 0412 | - | - |
| B. animalis subsp. lactis WC 0413 | - | - |
| B. animalis subsp. lactis WC 0414 | - | - |
| B. animalis subsp. lactis WC 0432 | - | - |
| B. animalis subsp. lactis WC 0471 | - | - |
| B. bifidum WC 0415 | - | - |
| B. bifidum WC 0417 | - | - |
| B. bifidum WC 0418 | - | - |
| B. breve WC 0420 | - | - |
| B. breve WC 0421 | - | - |
| B. breve WC 0422 | - | 4a |
| B. breve WC 0423 | - | - |
| B. breve WC 0424 | - | - |
| B. breve WC 0473 | - | - |
| B. catenulatum ATCC 27539 | - | 6a |
| B. longum subsp. infantis ATCC 15697 | - | - |
| B. longum subsp. infantis WC 0433 | - | - |
| B. longum subsp. infantis WC 0434 | - | - |
| B. longum subsp. longum WC 0436 | - | - |
| B. longum subsp. longum WC 0438 | - | - |
| B. longum subsp. longum WC 0439 | - | - |
| B. longum subsp. longum WC 0440 | - | - |
| B. longum subsp. longum WC 0443 | - | - |
| B. pseudocatenulatum WC 0400 | - | 9a |
| B. pseudocatenulatum WC 0401 | - | 6a |
| B. pseudocatenulatum WC 0402 | - | - |
| B. pseudocatenulatum WC 0403 | - | 46c |
| B. pseudocatenulatum WC 0407 | - | 5a |
| B. pseudocatenulatum WC 0408 | - | 16b |
2.2. Bioconversion of Hesperidin and Rutin by Fecal Bacteria
2.3. Bioconversion of Hesperidin and Rutin by Bifidobacteria
2.4. Fermentation Experiments
2.5. Analysis of Flavonols and Flavanones
2.6. Statistical Analysis
3. Results
3.1. Bioconversion of Rutin and Hesperidin by Resting Cells of Human Gut Microbiota

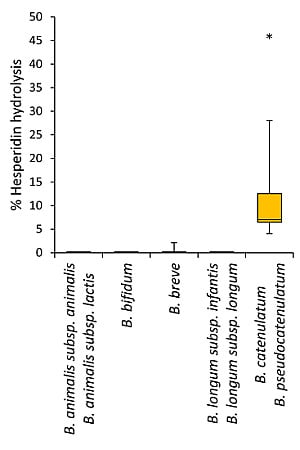
3.2. Bioconversion of Rutin and Hesperidin by Bifidobacteria
3.3. Kinetics of Hesperidin Hydrolysis by B. Pseudocatenulatum WC 0403


4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [PubMed]
- Graf, B.A.; Milbury, P.E.; Blumberg, J.B. Flavonols, flavones, flavanones, and human health: Epidemiological evidence. J. Med. Food 2005, 8, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Chidambara Murthy, K.N.; Kim, J.; Vikram, A.; Patil, B.S. Differential inhibition of human colon cancer cells by structurally similar flavonoids of citrus. Food Chem. 2012, 132, 27–34. [Google Scholar]
- Roohbakhsh, A.; Parhiz, H.; Soltani, F.; Rezaee, R.; Iranshahi, M. Molecular mechanisms behind the biological effects of hesperidin and hesperetin for the prevention of cancer and cardiovascular diseases. Life Sci. 2015, 124, 64–74. [Google Scholar] [CrossRef]
- DuPont, M.S.; Day, A.J.; Bennett, R.N.; Mellon, F.A.; Kroon, P.A. Absorption of kaempferol from endive, a source of kaempferol-3-glucuronide, in humans. Eur. J. Clin. Nutr. 2004, 58, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.; de Vries, J.H.; van Leeuwen, S.D.; Mengelers, M.J.; Katan, M.B. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am. J. Clin. Nutr. 1995, 62, 1276–1282. [Google Scholar] [PubMed]
- Hollman, P.C.; van Trijp, J.M.; Buysman, M.N.; van der Gaag, M.S.; Mengelers, M.J.; de Vries, J.H.; Katan, M.B. Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett. 1997, 418, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Morand, C.; Demigné, C.; Texier, O.; Régérat, F.; Rémésy, C. Bioavailability of rutin and quercetin in rats. FEBS Lett. 1997, 409, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.; Bijsman, M.N.; van Gameren, Y.; Cnossen, E.P.; de Vries, J.H.; Katan, M.B. The sugar moiety is a major determinant of the absorption of dietary flavonoid glycosides in man. Free Radic. Res. 1999, 31, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Carbonaro, M.; Grant, G. Absorption of quercetin and rutin in rat small intestine. Ann. Nutr. MeTable 2005, 49, 178–182. [Google Scholar] [CrossRef]
- Olthof, M.R.; Hollman, P.C.; Buijsman, M.N.; van Amelsvoort, J.M.; Katan, M.B. Chlorogenic acid, quercetin-3-rutinoside and black tea phenols are extensively metabolized in humans. J. Nutr. 2003, 133, 1806–1814. [Google Scholar] [PubMed]
- Rechner, A.R.; Smith, M.A.; Kuhnle, G.; Gibson, G.R.; Debnam, E.S.; Srai, S.K.; Moore, K.P.; Rice-Evans, C.A. Colonic metabolism of dietary polyphenols: Influence of structure on microbial fermentation products. Free Radic. Biol. Med. 2004, 36, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, F.; Dinan, T.G.; Ross, P.; Hill, C. Probiotics in transition. Clin. Gastroenterol. Hepatol. 2012, 10, 1220–1224. [Google Scholar] [CrossRef] [PubMed]
- Chong, E.S. A potential role of probiotics in colorectal cancer prevention: Review of possible mechanisms of action. World J. Microbiol. Biotechnol. 2014, 30, 351–374. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Tomaro-Duchesneau, C.; Saha, S.; Rodes, L.; Kahouli, I.; Malhotra, M. Probiotics for the prevention and treatment of allergies, with an emphasis on mode of delivery and mechanism of action. Curr. Pharm. Des. 2014, 20, 1025–1037. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Amaretti, A. Probiotic Properties of Bifidobacteria. In Bifidobacteria: Genomics and Molecular Aspects; van Synderen, D., Mayo, B., Eds.; Horizon Scientific Press: Rowan House, UK, 2010; pp. 97–123. [Google Scholar]
- Rossi, M.; Amaretti, A.; Leonardi, A.; Raimondi, S.; Simone, M.; Quartieri, A. Potential impact of probiotic consumption on the bioactivity of dietary phytochemicals. J. Agric. Food Chem. 2013, 61, 9551–6558. [Google Scholar] [PubMed]
- Lee, J.H.; O’Sullivan, D.J. Genomic insights into bifidobacteria. Microbiol. Mol. Biol. Rev. 2010, 74, 378–416. [Google Scholar] [CrossRef] [PubMed]
- Amaretti, A.; Bernardi, T.; Leonardi, A.; Raimondi, S.; Zanoni, S.; Rossi, M. Fermentation of xylo-oligosaccharides by Bifidobacterium adolescentis DSMZ 18350: Kinetics, metabolism, and β-xylosidase activities. Appl. Microbiol. Biotechnol. 2013, 97, 3109–3117. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, S.; Roncaglia, L.; de Lucia, M.; Amaretti, A.; Leonardi, A.; Pagnoni, U.M.; Rossi, M. Bioconversion of soy isoflavones daidzin and daidzein by Bifidobacterium strains. Appl. Microbiol. Biotechnol. 2009, 81, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Roncaglia, L.; Amaretti, A.; Raimondi, S.; Leonardi, A.; Rossi, M. Role of bifidobacteria in the activation of the lignan secoisolariciresinol diglucoside. Appl. Microbiol. Biotechnol. 2011, 92, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Tomas-Barberan, F.; García-Villalba, R.; Quartieri, A.; Raimondi, S.; Amaretti, A.; Leonardi, A.; Rossi, M. In vitro transformation of chlorogenic acid by human gut microbiota. Mol. Nutr. Food Res. 2014, 58, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, S.; Anighoro, A.; Quartieri, A.; Amaretti, A.; Tomás-Barberán, F.A.; Rastelli, G.; Rossi, M. Role of bifidobacteria in the hydrolysis of chlorogenic acid. MicrobiologyOpen 2015, 4, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Clavel, T.; Borrmann, D.; Braune, A.; Doré, J.; Blaut, M. Occurrence and activity of human intestinal bacteria involved in the conversion of dietary lignans. Anaerobe 2006, 12, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Aura, A.M.; O’Leary, K.A.; Williamson, G.; Ojala, M.; Bailey, M.; Puupponen-Pimiä, R.; Nuutila, A.M.; Oksman-Caldentey, K.M.; Poutanen, K. Quercetin derivatives are deconjugated and converted to hydroxyphenylacetic acids but not methylated by human fecal flora in vitro. J. Agric. Food Chem. 2002, 50, 1725–1730. [Google Scholar] [CrossRef] [PubMed]
- Van den Broek, L.A.; Hinz, S.W.; Beldman, G.; Vincken, J.P.; Voragen, A.G. Bifidobacterium carbohydrases-their role in breakdown and synthesis of (potential) prebiotics. Mol. Nutr. Food Res. 2008, 52, 146–163. [Google Scholar] [CrossRef] [PubMed]
- Aura, A.M.; Martin-Lopez, P.; O’Leary, K.A.; Williamson, G.; Oksman-Caldentey, K.M.; Poutanen, K.; Santos-Buelga, C. In vitro metabolism of anthocyanins by human gut microflora. Eur. J. Nutr. 2005, 44, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.H.; Hyun, Y.J.; Shim, J.; Hong, S.W.; Kim, D.H. Metabolism of Rutin and Poncirin by Human Intestinal Microbiota and Cloning of Their Metabolizing α-l-Rhamnosidase from Bifidobacterium dentium. J. Microbiol. Biotechnol. 2015, 25, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Alegría, A.; Delgado, S.; Guadamuro, L.; Flórez, A.B.; Felis, G.E.; Torriani, S.; Mayo, B. The genome of Bifidobacterium pseudocatenulatum IPLA 36007, a human intestinal strain with isoflavone-activation activity. Gut Pathog. 2014, 6, 31. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaretti, A.; Raimondi, S.; Leonardi, A.; Quartieri, A.; Rossi, M. Hydrolysis of the Rutinose-Conjugates Flavonoids Rutin and Hesperidin by the Gut Microbiota and Bifidobacteria. Nutrients 2015, 7, 2788-2800. https://doi.org/10.3390/nu7042788
Amaretti A, Raimondi S, Leonardi A, Quartieri A, Rossi M. Hydrolysis of the Rutinose-Conjugates Flavonoids Rutin and Hesperidin by the Gut Microbiota and Bifidobacteria. Nutrients. 2015; 7(4):2788-2800. https://doi.org/10.3390/nu7042788
Chicago/Turabian StyleAmaretti, Alberto, Stefano Raimondi, Alan Leonardi, Andrea Quartieri, and Maddalena Rossi. 2015. "Hydrolysis of the Rutinose-Conjugates Flavonoids Rutin and Hesperidin by the Gut Microbiota and Bifidobacteria" Nutrients 7, no. 4: 2788-2800. https://doi.org/10.3390/nu7042788
APA StyleAmaretti, A., Raimondi, S., Leonardi, A., Quartieri, A., & Rossi, M. (2015). Hydrolysis of the Rutinose-Conjugates Flavonoids Rutin and Hesperidin by the Gut Microbiota and Bifidobacteria. Nutrients, 7(4), 2788-2800. https://doi.org/10.3390/nu7042788