Effect of Oat β-Glucan Intake on Glycaemic Control and Insulin Sensitivity of Diabetic Patients: A Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection
2.4. Methodological Assessment
2.5. Data Extraction
2.6. Statistical Analysis
3. Results
3.1. Literature Search
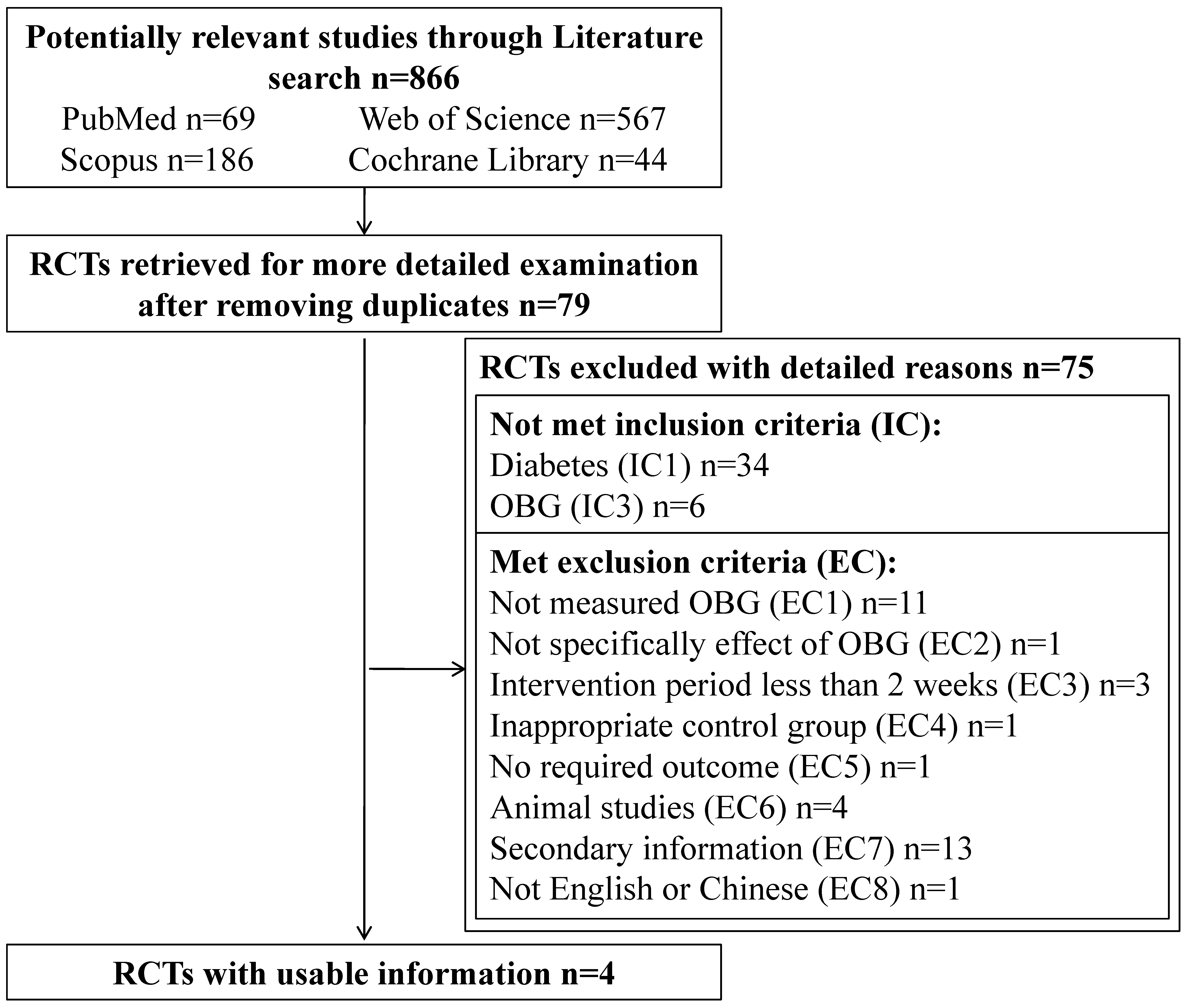
3.2. Study Characteristics
| Author (Publication Year) | No. of Subjects | Healthy Status | Male (%) | Age (Years) Mean (or Range) | BMI (kg/m2) Mean (or Range) | Molecular Weight of OBG (kDa) | Trial Design | Treatment Group | Control Group | Run-in/Washout Period | Intervention Period |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cugnet-Anceau, C., et al. (2010) [13] | 53 | T2DM | 60 | 61.9 (44–75) | 29.82 | 80 | Parallel | Soluble oat soups (3.5 g/day β-glucan) | Soups without β-glucan | 3 weeks | 8 weeks |
| Kabir, M., et al. (2002) [14] | 13 | T2DM | 100 | 59 (41–67) | 28 (23–36) | Not reported | Cross-over | Oat bran concentrate (3 g/day β-glucan) | Whole wheat grains without β-glucan | 1 month/15 days | Two periods of 4 weeks |
| Liatis, S., et al. (2009) [9] | 41 | T2DM | 56 | 63 | 28.47 | Not reported | Parallel | Bread (3 g/day β-glucan) | White bread without β-glucan | 3 weeks | 3 weeks |
| Ma, X., et al. (2013) [15] | 243 | T2DM | 43 | 59.4 (50–65) | 26.69 | Not reported | Parallel | Organic naked oat with whole germ (2.5 or 5 g/day β-glucan) | Diet without β-glucan | 1 week | 30 days |
3.3. Effect of OBG Intake on Fasting Plasma Glucose (FPG) Concentration


3.4. Effect of OBG Intake on Glycosylated Hemoglobin (HbA1c) Concentration


3.5. Effect of OBG Intake on Fasting Plasma Insulin (FPI) Concentration

4. Discussion
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2014, 37, S81–S90. [Google Scholar] [CrossRef] [PubMed]
- Guariguata, L.; Whiting, D.R.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Cai, X.; Xu, M.; Li, Y. Effect of oat intake on glycaemic control and insulin sensitivity: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2014, 112, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Andrade, E.F.; Lobato, R.V.; de Araújo, T.V.; Zangerônimo, M.G.; de Sousa, R.V.; Pereira, L.J. Effect of beta-glucans in the control of blood glucose levels of diabetic patients: A systematic review. Nutr. Hosp. 2015, 31, 170–177. [Google Scholar]
- McGeoch, S.C.; Johnstone, A.M.; Lobley, G.E.; Adamson, J.; Hickson, K.; Holtrop, G.; Fyfe, C.; Clark, L.F.; Pearson, D.W.M.; Abraham, P.; et al. A randomized crossover study to assess the effect of an oat-rich diet on glycaemic control, plasma lipids and postprandial glycaemia, inflammation and oxidative stress in Type 2 diabetes. Diabet. Med. 2013, 30, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Cloetens, L.; Ulmius, M.; Johansson-Persson, A.; Åkesson, B.; Önning, G. Role of dietary beta-glucans in the prevention of the metabolic syndrome. Nutr. Rev. 2012, 70, 444–458. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.S.; Tahir-Nadeem, M.; Khan, M.K.I.; Shabir, R.; Butt, M.S. Oat: Unique among the cereals. Eur. J. Nutr. 2008, 47, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, A.; Beck, E.J.; Tosh, S.; Wolever, T.M.S. Cholesterol-lowering effects of oat β-glucan: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2014, 100, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Liatis, S.; Tsapogas, P.; Chala, E.; Dimosthenopoulos, C.; Kyriakopoulos, K.; Kapantais, E.; Katsilambros, N. The consumption of bread enriched with betaglucan reduces LDL-cholesterol and improves insulin resistance in patients with type 2 diabetes. Diabetes Metab. 2009, 35, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, V.; Sievenpiper, J.L.; de Souza, R.J.; Jayalath, V.H.; Mirrahimi, A.; Agarwal, A.; Chiavaroli, L.; Mejia, S.B.; Sacks, F.M.; di Buono, M.; et al. Effect of dietary pulse intake on established therapeutic lipid targets for cardiovascular risk reduction: A systematic review and meta-analysis of randomized controlled trials. Can. Med. Assoc. J. 2014, 186, E252–E262. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0.; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Cugnet-Anceau, C.; Nazare, J.A.; Biorklund, M.; le Coquil, E.; Sassolas, A.; Sothier, M.; Holm, J.; Landin-Olsson, M.; Önning, G.; Laville, M.; et al. A controlled study of consumption of β-glucan-enriched soups for 2 months by type 2 diabetic free-living subjects. Br. J. Nutr. 2010, 103, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.; Oppert, J.M.; Vidal, H.; Bruzzo, F.; Fiquet, C.; Wursch, P.; Slama, G.; Rizkalla, S.W. Four-week low-glycemic index breakfast with a modest amount of soluble fibers in type 2 diabetic men. Metabolism 2002, 51, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Gu, J.; Zhang, Z.; Jing, L.; Xu, M.; Dai, X.; Jiang, Y.; Li, Y.; Bao, L.; Cal, X.; et al. Effects of Avena nuda L. on metabolic control and cardiovascular disease risk among Chinese patients with diabetes and meeting metabolic syndrome criteria: Secondary analysis of a randomized clinical trial. Eur. J. Clin. Nutr. 2013, 67, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Kobayakawa, A.; Suzuki, T.; Ikami, T.; Saito, M.; Yabe, D.; Seino, Y. Improvement of fasting plasma glucose level after ingesting moderate amount of dietary fiber in Japanese men with mild hyperglycemia and visceral fat obesity. J. Diet. Suppl. 2013, 10, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.J. Cereal β-glucans in diet and health. J. Cereal Sci. 2007, 46, 230–238. [Google Scholar] [CrossRef]
- Wood, P.; Beer, M.; Butler, G. Evaluation of role of concentration and molecular weight of oat β-glucan in determining effect of viscosity on plasma glucose and insulin following an oral glucose load. Br. J. Nutr. 2000, 84, 19–23. [Google Scholar] [PubMed]
- Daou, C.; Zhang, H. Oat Beta-Glucan: Its Role in Health Promotion and Prevention of Diseases. Compr. Rev. Food Sci. Food Saf. 2012, 11, 355–365. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, X.L.; Zhao, T.; Zhou, Y.; Shi, X.; Zou, Y.; Zhao, G. Effect of Oat β-Glucan Intake on Glycaemic Control and Insulin Sensitivity of Diabetic Patients: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2016, 8, 39. https://doi.org/10.3390/nu8010039
Shen XL, Zhao T, Zhou Y, Shi X, Zou Y, Zhao G. Effect of Oat β-Glucan Intake on Glycaemic Control and Insulin Sensitivity of Diabetic Patients: A Meta-Analysis of Randomized Controlled Trials. Nutrients. 2016; 8(1):39. https://doi.org/10.3390/nu8010039
Chicago/Turabian StyleShen, Xiao Li, Tao Zhao, Yuanzhong Zhou, Xiuquan Shi, Yan Zou, and Guohua Zhao. 2016. "Effect of Oat β-Glucan Intake on Glycaemic Control and Insulin Sensitivity of Diabetic Patients: A Meta-Analysis of Randomized Controlled Trials" Nutrients 8, no. 1: 39. https://doi.org/10.3390/nu8010039
APA StyleShen, X. L., Zhao, T., Zhou, Y., Shi, X., Zou, Y., & Zhao, G. (2016). Effect of Oat β-Glucan Intake on Glycaemic Control and Insulin Sensitivity of Diabetic Patients: A Meta-Analysis of Randomized Controlled Trials. Nutrients, 8(1), 39. https://doi.org/10.3390/nu8010039







