Regulation of Intestinal Glucose Absorption by Ion Channels and Transporters
Abstract
:1. Introduction
2. Mechanisms of Glucose Absorption in the Intestine

3. Detection Methods of Glucose Absorption in Vitro
4. Regulation of Glucose Absorption by Potassium Channels
4.1. Potassium Channels in Small Intestinal Epithelial Cells
4.2. Regulatory Mechanisms of Glucose Absorption by Potassium Channels
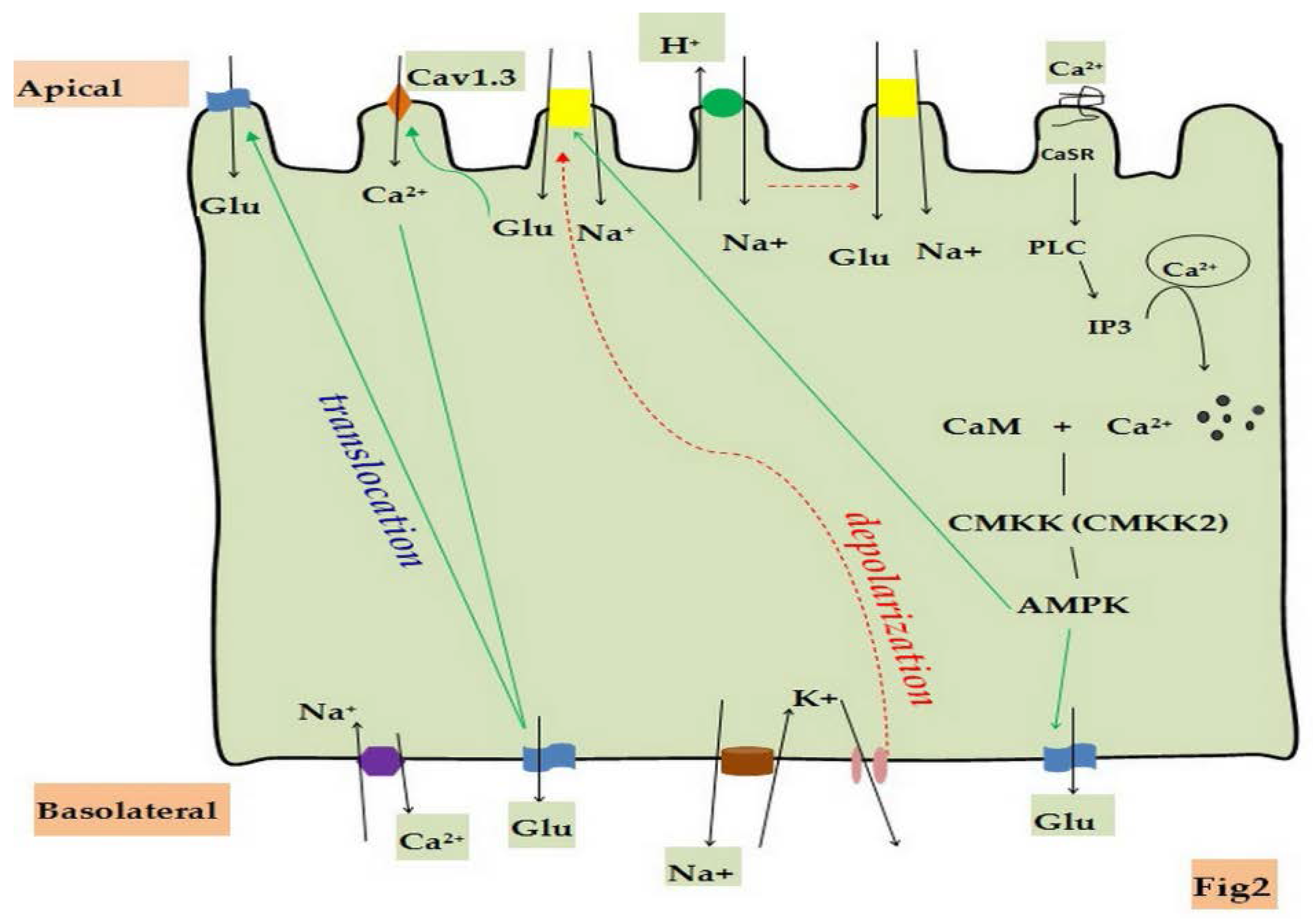
5. Regulation of Glucose Absorption by Calcium Channels and Calcium-Sensing Receptors
5.1. Calcium Channels and Calcium-Sensing Receptors(CaSR) in Small Intestinal Epithelial Cells
5.2. Regulatory Mechanisms of Glucose Absorption by Calcium Channels and CaSR
6. Regulation of Glucose Absorption by Transporters
6.1. Transporters in Small Intestinal Epithelial Cells
6.2. Regulatory Mechanisms of Glucose Absorption by Transporters
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- An, R. Prevalence and trends of adult obesity in the US, 1999–2012. ISRN Obes. 2014, 2014, 185132. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Ahn, H.S.; Choi, B.H.; Jang, H.J.; Kim, M.J.; Rhie, D.J.; Yoon, S.H.; Jo, Y.H.; Kim, M.S.; Sung, K.W.; et al. Open channel block of a-type, Kv4.3, and delayed rectifier k+ channels, Kv1.3 and Kv3.1, by sibutramine. J. Pharmacol. Exp. Ther. 2007, 321, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Crane, R.K. Na+ -dependent transport in the intestine and other animal tissues. Fed. Proc. 1965, 24, 1000–1006. [Google Scholar] [PubMed]
- Gorboulev, V.; Schürmann, A.; Vallon, V.; Kipp, H.; Jaschke, A.; Klessen, D.; Friedrich, A.; Scherneck, S.; Rieg, T.; Cunard, R. Na+-D-glucose cotransporter SGLT11 is pivotal for intestinal glucose absorption and glucose dependent incretin secretion. Diabetes 2011, 61, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, J.R.; Loo, D.D.; Wright, E.M. Regulation of Na+/glucose cotransporter expression by protein kinases in xenopus laevis oocytes. J. Biol. Chem. 1996, 271, 14740–14746. [Google Scholar] [PubMed]
- Alexander, A.N.; Carey, H.V. Involvement of PI 3-kinase in IGF-I stimulation of jejunal na+-k+-atpase activity and nutrient absorption. Am. J. Physiol. Gastrointest. L. 2001, 280, G222–G228. [Google Scholar]
- Casselbrant, A.; Malinauskas, M.; Marschall, H.U.; Wallenius, V.; Fändriks, L. Angiotensin II exerts dual actions on sodium-glucose transporter 1-mediated transport in the human jejunal mucosa. Scand. J. Gastroentero. 2015, 50, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Umbach, J.A.; Coady, M.J.; Wright, E.M. Intestinal Na+-glucose cotransporter expressed in xenopus oocytes is electrogenic. Biophys. J. 1990, 57, 1217–1224. [Google Scholar] [CrossRef]
- Wang, J.Y.; Wang, J.; Golovina, V.A.; Li, L.; Platoshyn, O.; Yuan, J.X. Role of K+ channel expression in polyamine-dependent intestinal epithelial cell migration. Am. J. Physiol. Cell Physiol. 2000, 278, C303–C314. [Google Scholar] [PubMed]
- Silver, K.; Littlejohn, A.; Thomas, L.; Marsh, E.; Lillich, J.D. Inhibition of Kv channel expression by nsaids depolarizes membrane potential and inhibits cell migration by disrupting calpain signaling. Biochem. Pharmacol. 2015, 98, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Sheptitskiĭ, V.A.; Guska, N.I. Ca2+-dependent glucose absorption in the small intestine of rats under head-down tilt stress. Fiziologicheskiĭ Zhurnal Imeni I.M. Sechenova 1996, 82, 125–130. [Google Scholar] [PubMed]
- Gurman, E.G. The characteristics of the action of calcium antagonists on glucose transport in the rat small intestine. Fiziologicheskiĭ Zhurnal Imeni I.M. Sechenova 1990, 76, 509–514. [Google Scholar]
- Christakos, S.; Dhawan, P.; Ajibade, D.; Benn, B.S.; Feng, J.; Joshi, S.S. Mechanisms involved in vitamin D mediated intestinal calcium absorption and in non-classical actions of vitamin D. J. Steroid. Biochem. Mol. Biol. 2010, 121, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Mace, O.J.; Morgan, E.L.; Affleck, J.A.; Lister, N.; Kellett, G.L. Calcium absorption by Cav1.3 induces terminal web myosin II phosphorylation and apical GLUT2 insertion in rat intestine. J. Physiol. 2007, 580, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Röder, P.V.; Geillinger, K.E.; Zietek, T.S.; Thorens, B.; Koepsell, H.; Daniel, H. The role of SGLT1 and GLUT2 in intes tinal glucose transportand sensing. PLoS ONE 2014, 26, e89977. [Google Scholar] [CrossRef] [PubMed]
- Boudry, G.; Cheeseman, C.I.; Perdue, M.H. Psychological stress impairs Na+-dependent glucose absorption and increases GLUT2 expression in the rat jejunal brush-border membrane. Am. J. Physiol. Regul. 2007, 292, R862–R867. [Google Scholar] [CrossRef] [PubMed]
- Ait-Omar, A.; Monteiro-Sepulveda, M.; Poitou, C.; le Gall, M.; Cotillard, A.; Gilet, J.; Garbin, K.; Houllier, A.; Château, D.; Lacombe, A. GLUT2 accumulation in enterocyte apical and intracellular membranes. Diabetes 2011, 60, 2598–2607. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, R.M.; Scow, J.S.; Madhavan, S.; Duenes, J.A.; Sarr, M.G. Acute enterocyte adaptation to luminal glucose: A posttranslational mechanism for rapid apical recruitment of the transporter glut2. J. Gastrointest. Surg. 2012, 16, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Affleck, J.A.; Helliwell, P.A.; Kellett, G.L. Immunocytochemical detection of GLUT2 at the rat intestinal brush-border membrane. J. Histochem. Cytochem. 2003, 51, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Scow, J.S.; Duenes, J.A.; Sarr, M.G. Mechanisms of glucose uptake in intestinal cell lines: Role of GLUT2. Surgery 2012, 151, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Grefner, N.M.; Gromova, L.V.; Gruzdkov, A.A.; Komissarchik, I. Interaction of glucose transporters SGLT1 and GLUT2 with cytoskeleton in enterocytes and caco2 cells during hexose absorption. Cell Tissue Biol. 2015, 9, 45–52. [Google Scholar] [CrossRef]
- Schultz, S.G.; Zalusky, R. Ion transport in isolated rabbit ileum I. Short-circuit current and na fluxes. J. Gen. Physiol. 1964, 47, 567–584. [Google Scholar] [CrossRef] [PubMed]
- Schultz, S.G.; Zalusky, R. Ion transport in isolated rabbit II. The interaction between active active sugar transport. J. Gen. Physiol. 1964, 47, 1043–1059. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, K.L.; Butt, A.G. Glucose transport into everted sacs of the small intestine of mice. Adv. Physiol. Educ. 2013, 37, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Johnston, K.; Sharp, P.; Clifford, M.; Morgan, L. Dietary polyphenols decrease glucose uptake by human intestinal caco-2 cells. FEBS Lett. 2005, 579, 1653–1657. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Y.; Wang, W.F.; Yu, Y.X.; Hou, Y.T.; Ren, G.P.; Li, D.S. FGF-21 improves glucose uptake and glycogen synthesis of insulin-resistant liver cells. Prog. Biochem. Biophys. 2009, 36, 1327–1333. [Google Scholar] [CrossRef]
- Carrisoza-Gaytán, R.; Salvador, C.; Diaz-Bello, B.; Escobar, L. Differential expression of the Kv1 voltage-gated potassium channel family in the rat nephron. J. Mol. Histol. 2014, 45, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Kirchheim, F.; Tinnes, S.; Haas, C.A.; Stegen, M.; Wolfart, J. Regulation of action potential delays via voltage-gated potassium Kv1.1 channels in dentate granule cells during hippocampal epilepsy. Front. Cell. Neurosci. 2013, 7, 248. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Ma, J.; Zhou, Q.; Li, J.; Tang, Y.; Liu, Y.; Yang, Y.; Xiao, J.; Peng, L. KCNQ1 loss-of-function mutation impairs gastric acid secretion in mice. Mol. Biol. Rep. 2010, 37, 1329–1333. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.E.; Song, M.S.; Ryu, P.D.; Lee, S.Y. The role of voltage-gated potassium channels in osteogenic differentiation. FASEB J. 2015, 29, 1. [Google Scholar]
- Hristov, K.L.; Chen, M.; Soder, R.P.; Parajuli, S.P.; Cheng, Q.; Kellett, W.F.; Petkov, G.V. Kv2.1 and electrically silent Kv channel subunits control excitability and contractility of guinea pig detrusor smooth muscle. Am. J. Physiol. Cell. Physiol. 2011, 302, C360–C372. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Zhuo, Y.H.; Bi, W.N.; Bai, Y.J.; Li, Y.N.; Wang, Z.J. Voltage-gated potassium channel Kv1.3 in rabbit ciliary epithelium regulates the membrane potential via coupling intracellular calcium. Chin. Med. J. 2008, 121, 2272–2277. [Google Scholar] [PubMed]
- Xie, R.; Dong, X.; Wong, C.; Vallon, V.; Tang, B.; Sun, J.; Yang, S.; Dong, H. Molecular mechanisms of calcium-sensing receptor-mediated calcium signaling in the modulation of epithelial ion transport and bicarbonate secretion. J. Biol. Chem. 2014, 289, 34642–34653. [Google Scholar] [CrossRef] [PubMed]
- Nanda Kumar, N.S.; Singh, S.K.; Rajendran, V.M. Mucosal potassium efflux mediated via Kcnn4 channels provides the driving force for electrogenic anion secretion in colon. Am. J. Physiol. Gastrointest. 2010, 299, G707–G714. [Google Scholar] [CrossRef] [PubMed]
- Lionetto, M.G.; Giordano, M.E.; de Nuccio, F.; Nicolardi, G.; Hoffmann, E.K.; Schettino, T. Hypotonicity induced K+ and anion conductive pathways activation in eel intestinal epithelium. J. Exp. Biol. 2005, 208, 749–760. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, S.S.; Platoshyn, O.; Yu, Y.; Sweeney, M.; Miriel, V.A.; Golovina, V.A.; Krick, S.; Lapp, B.R.; Wang, J.Y.; Yuan, J.X. Anorexic effect of k+ channel blockade in mesenteric arterial smooth muscle and intestinal epithelial cells. J. Appl. Physiol. 2001, 91, 2322–2333. [Google Scholar] [PubMed]
- Liu, L.; Wang, F.; Lu, H.; Ren, X.; Zou, J. Chromanol 293b, an inhibitor of kcnq1 channels, enhances glucose-stimulated insulin secretion and increases glucagon-like peptide-1 level in mice. Islets 2014, 6, e962386. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.E.; Habib, A.M.; Rogers, G.J.; Gribble, F.M.; Reimann, F. Nutrient-dependent secretion of glucose-dependent insulinotropic polypeptide from primary murine k cells. Diabetologia 2009, 52, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Unoki, H.; Takahashi, A.; Kawaguchi, T.; Hara, K.; Horikoshi, M.; Andersen, G.; Ng, D.P.; Holmkvist, J.; Borch-Johnsen, K. SNPs in KCNQ1 are associated with susceptibility to type 2 diabetes in east asian and european populations. Nat. Genet. 2008, 40, 1098–1102. [Google Scholar] [CrossRef] [PubMed]
- Saif-Ali, R.; Ismail, I.S.; Al-Hamodi, Z.; Al-Mekhlafi, H.M.; Siang, L.C.; Alabsi, A.M.; Muniandy, S. KCNQ1 haplotypes associate with type 2 diabetes in malaysian chinese subjects. Int. J. Mol. Sci. 2011, 12, 5705–5718. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Koni, P.A.; Wang, P.; Li, G.; Kaczmarek, L.; Wu, Y.; Li, Y.; Flavell, R.A.; Desir, G.V. The voltage-gated potassium channel Kv1.3 regulates energy homeostasis and body weight. Hum. Mol. Genet. 2003, 12, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.K.; Eckel-Mahan, K.L.; Mirbolooki, M.R.; Tjong, I.; Griffey, S.M.; Schmunk, G.; Koehne, A.; Halbout, B.; Iadonato, S. Selective Kv1.3 channel blocker as therapeutic for obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 2013, 110, E2239–E2248. [Google Scholar] [CrossRef] [PubMed]
- Tucker, K.; Overton, J.M.; Fadool, D.A. Diet-induced obesity resistance of Kv1.3-/- mice is olfactory bulb dependent. J. Neuroendocrinol. 2012, 24, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Müssig, K.; Staiger, H.; Machicao, F.; Kirchhoff, K.; Guthoff, M.; Schäfer, S.A.; Kantartzis, K.; Silbernagel, G.; Stefan, N. Association of type 2 diabetes candidate polymorphisms in kcnq1 with incretin and insulin secretion. Diabetes 2009, 58, 1715–1720. [Google Scholar] [CrossRef] [PubMed]
- Straub, S.V.; Perez, S.M.; Tan, B.; Coughlan, K.A.; Trebino, C.E.; Cosgrove, P.; Buxton, J.M.; Kreeger, J.M.; Jackson, V.M. Pharmacological inhibition of Kv1.3 fails to modulate insulin sensitivity in diabetic mice or human insulin-sensitive tissues. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E380–E390. [Google Scholar] [CrossRef] [PubMed]
- Ngala, R.A.; Zaibi, M.S.; Langlands, K.; Stocker, C.J.; Arch, J.R.; Cawthorne, M.A. Stimulation of glucose uptake in murine soleus muscle and adipocytes by 5-(4-phenoxybutoxy)psoralen (PAP-1) may be mediated by Kv1.5 rather than Kv1.3. Peer J. 2014, 2, e614. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Grahammer, F.; Richter, K.; Bleich, M.; Lang, F.; Barhanin, J.; Völkl, H.; Warth, R. Role of KCNE1-dependent K+ fluxes in mouse proximal tubule. J. Am. Soc. Nephrol. 2001, 12, 2003–2011. [Google Scholar] [PubMed]
- Gal-Garber, O.; Mabjeesh, S.J.; Sklan, D.; Uni, Z. Nutrient transport in the small intestine:Na+, K+-ATPase expression and activity in the small intestine of the chicken as influenced by dietary sodium. Poult. Sci. 2003, 82, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, K.; Drengstig, T.; Ruoff, P. Transepithelial glucose transport and Na+/K+ homeostasis in enterocytes: An integrative model. Am. J. Physiol. Cell Physiol. 2014, 307, C320–C337. [Google Scholar] [CrossRef] [PubMed]
- Palanikumar, M.; Swapna, G.; Subha, A.; Balasubramanian, P.; Soudamani, S.; Gregory, M.D.; Uma, S. Chronic and selective inhibition of basolateral membrane Na-K-ATPase uniquely regulates brush border membrane na absorption in intestinal epithelial cells. Am. J. Physiol. Cell Phys. 2015, 308, C650–C656. [Google Scholar]
- Chiba, T.; Marcus, D.C. Basolateral K+ conductance establishes driving force for cation absorption by outer sulcus epithelial cells. J. Membr. Biol. 2001, 184, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, P.J.; Alioua, A.; Lee, J.W.; Straus, D.S.; Toro, L.; Lytle, C. Fenofibrate inhibits intestinal Cl− secretion by blocking basolateral KCNQ1 K+ channels. Am. J. Physiol. Gastrl. 2007, 293, G1288–G1299. [Google Scholar] [CrossRef] [PubMed]
- Dedek, K.; Waldegger, S. Colocalization of KCNQ1/KCNE channel subunits in the mouse gastrointestinal tract. Pflugers. Arch. 2001, 442, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Grahammer, F.; Volkl, H.; Sandu, C.D.; Richter, K.; Rexhepaj, R.; Gerlach, U.; Rong, Q.; Pfeifer, K.; Lang, F. KCNQ1-dependent transport in renal and gastrointestinal epithelia. Proc. Natl. Acad. Sci. USA 2005, 102, 17864–17869. [Google Scholar] [CrossRef] [PubMed]
- Pouokam, E.; Bader, S.; Brück, B.; Schmidt, B.; Diener, M. ATP-sensitive K+ channels in rat colonic epithelium. Pflug. Arch. Eur. J. Phys. 2013, 465, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, K.L.; Devor, D.C. Basolateral membrane K+ channels in renal epithelial cells. Am. J. Physiol. Renal 2012, 302, F1069–F1078. [Google Scholar] [CrossRef] [PubMed]
- Preston, P.; Wartosch, L.; Günzel, D.; Fromm, M.; Kongsuphol, P.; Ousingsawat, J.; Kunzelmann, K.; Barhanin, J.; Warth, R.; Jentsch, T. Disruption of the K+ channel β-subunit KCNE3 reveals an important role in intestinal and tracheal Cl− transport. J. Biol. Chem. 2010, 285, 7165–7175. [Google Scholar] [CrossRef] [PubMed]
- Turnheim, K.; Plass, H.; Wyskovsky, W. Basolateral potassium channels of rabbit colon epithelium: Role in sodium absorption and chloride secretion. Biochim. Biophys. Acta 2002, 1560, 51–66. [Google Scholar] [CrossRef]
- Khuzakhmetova, V.F.; Samigullin, D.V.; Bukharaeva, E.A. The role of presynaptic ryanodine receptors in regulation of the kinetics of the acetylcholine quantal release in the mouse neuromuscular junction. Biol. Membr. 2014, 30, 499–508. [Google Scholar] [CrossRef]
- Huang, W.; Lu, C.; Wu, Y.; Ouyang, S.; Chen, Y. T-type calcium channel antagonists, mibefradil and NNC-55–0396 inhibit cell proliferation and induce cell apoptosis in leukemia cell lines. J. Exp. Clin. Cancer. Res. 2015, 34. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.R.; Zhang, M.F.; Yang, N.; Liu, Q.; Wang, R.X.; Cao, Y.N.; Yang, X.R.; Sham, J.S.; Lin, M.J. Enhanced store-operated Ca2+ entry and TRPC channel expression in pulmonary arteries of monocrotaline-induced pulmonary hypertensive rats. Am. J. Physiol. Cell Physiol. 2012, 302, C77–C87. [Google Scholar] [CrossRef] [PubMed]
- Woudenberg-Vrenken, T.E.; Lameris, A.L.; Weißgerber, P.; Olausson, J.; Flockerzi, V.; Bindels, R.J.; Freichel, M.; Hoenderop, J.G. Functional TRPV6 channels are crucial for transepithelial Ca2+ absorption. Am. J. Physiol. Gastrointest. 2012, 303, G879–G885. [Google Scholar] [CrossRef] [PubMed]
- Benn, B.S.; Ajibade, D.; Porta, A.; Dhawan, P.; Hediger, M.; Peng, J.B.; Jiang, Y.; Oh, G.T.; Jeung, E.B.; Lieben, L. Active intestinal calcium transport in the absence of transient receptor potential vanilloid Type 6 and calbindin-D(9k). Endocrinology 2008, 149, 3196–3205. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Jung, E.M.; Yang, H.; Choi, K.C.; Jeung, E.B. Tissue-specific expression of the calcium transporter genes TRPV5, TRPV6, NCX1, and PMCA1b in the duodenum, kidney and heart of equus caballus. J. Vet. Med. Sci. 2011, 73, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.K.; Rathor, N.; Wang, S.R.; Wang, J.Y.; Rao, J.N. Rhoa enhances store-operated Ca2+ entry and intestinal epithelial restitution by interacting with trpc1 after wounding. Am. J. Physiol. Gastrointest. 2015, 309, G759–G767. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.M. Role of the calcium-sensing receptor in extracellular calcium homeostasis. Best. Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Diaz, R.; Hurwitz, S.; Chattopadhyay, N.; Pines, M.; Yang, Y.; Kifor, O.; Einat, M.S.; Butters, R.; Hebert, S.C.; Brown, E.M. Cloning, expression, and tissue localization of the calcium-sensing receptor in chicken (gallus domesticus). Am. J. Physiol. 1997, 273, R1008–R1016. [Google Scholar] [PubMed]
- Hyson, D.A.; Thomson, A.B.; Kappagoda, C.T. Calcium channel blockers modify jejunal uptake of D-galactose in rabbits. Dig. Dis. Sci. 1996, 41, 1871–1875. [Google Scholar] [CrossRef] [PubMed]
- Tharabenjasin, P.; Douard, V.; Patel, C.; Krishnamra, N.; Johnson, R.J.; Zuo, J.; Ferraris, R.P. Acute interactions between intestinal sugar and calcium transport in vitro. Am. J. Physiol. Gastrointest. 2013, 306, G1–G12. [Google Scholar] [CrossRef] [PubMed]
- Kuhre, R.E.; Bechmann, L.E.; Albrechtsen, N.J.W.; Hartmann, B.; Holst, J.J. Glucose stimulates neurotensin secretion from the rat small intestine by mechanisms involving sglt1 and glut2 leading to cell depolarization and calcium influx. Am. J. Physiol. Endoc. Metab. 2015, 308, E1123–E1130. [Google Scholar] [CrossRef] [PubMed]
- Nakkrasae, L.I.; Thongon, N.; Thongbunchoo, J.; Krishnamra, N.; Charoenphandhu, N. Transepithelial calcium transport in prolactin-exposed intestine-like caco-2 monolayer after combinatorial knockdown of TRPV5, TRPV6 and Cav1.3. J. Physiol. Sci. 2010, 60, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Helliwell, P.A.; Rumsby, M.G.; Kellett, G.L. Intestinal sugar absorption is regulated by phosphorylation and turnover of protein kinase C βII mediated by phosphatidylinositol 3-kinase- and mammalian target of rapamycin-dependent pathways. J. Biol. Chem. 2003, 278, 28644–28650. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.L.; Mace, O.J.; Affleck, J.; Kellett, G.L. Apical GLUT2 and Cav1.3: Regulation of rat intestinal glucose and calcium absorption. J. Physiol. 2007, 580, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.L.; Mace, O.J.; Helliwell, P.A.; Affleck, J.; Kellett, G.L. A role for Cav1.3 in rat intestinal calcium absorption. Biochem. Biophys. Res. Commun. 2003, 312, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Bourzac, J.F.; L’Ériger, K.; Larrivée, J.F.; Arguin, G.; Bilodeau, M.S.; Stankova, J.; Gendron, F.P. Glucose transporter 2 expression is down regulated following P2X7 activation in enterocytes. J. Cell. Physiol. 2013, 228, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.X.; Okuda, M.; Hall, A.E.; Geibel, J.P.; Hebert, S.C. Expression of calcium-sensing receptor in rat colonic epithelium: Evidence for modulation of fluid secretion. Am. J. Physiol. Gastrointest. 2002, 283, G240–G250. [Google Scholar] [CrossRef] [PubMed]
- Geibel, J.P.; Hebert, S.C. The functions and roles of the extracellular Ca2+-sensing receptor along the gastrointestinal tract. Annu. Rev. Physiol. 2009, 71, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Stahmann, N.; Woods, A.; Carling, D.; Heller, R. Thrombin activates AMP-activated protein kinase in endothelial cells via a pathway involving Ca2+/calmodulin-dependent protein kinase kinase β. Mol. Cell. Biol. 2006, 26, 5933–5945. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.; Dickerson, K.; Heath, R.; Hong, S.P.; Momcilovic, M.; Johnstone, S.R.; Carlson, M.; Carling, D. Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005, 2, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Gabler, N.K.; Radcliffe, J.S.; Spencer, J.D.; Webel, D.M.; Spurlock, M.E. Feeding long-chain n-3 polyunsaturated fatty acids during gestation increases intestinal glucose absorption potentially via the acute activation of ampk. J. Nutr. Biochem. 2009, 20, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.; Jijon, H.B.; Diaz, H.; Salehi, P.; Churchill, T.; Madsen, K.L. 5-aminoimidazole-4-carboxamide riboside (AICAR) enhances GLUT2-dependent jejunal glucose transport: A possible role for ampk. Biochem. J. 2005, 385, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Sopjani, M.; Bhavsar, S.K.; Fraser, S.; Kemp, B.E.; Foller, M.; Lang, F. Regulation of Na+-coupled glucose carrier SGLT1 by AMP-activated protein kinase. Mol. Membr. Biol. 2010, 27, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Xie, F.; Xue, M.; Xu, X.; He, S.; Lin, M.; Bai, L. Advanced oxidation protein products decrease the expression of calcium transport channels in small intestinal epithelium via the p44/42 mapk signaling pathway. Eur. J. Cell. Biol. 2015, 94, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Yao, L.; Jiang, Z.; Aschenbach, J.R.; Martens, H.; Shen, Z. Acidic PH and short-chain fatty acids activate Na transport but differentially modulate expression of Na/H exchanger isoforms 1, 2, and 3 in omasal epithelium. J. Dairy Sci. 2016, 99, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Jacob, P.; Rossmann, H.; Lamprecht, G.; Kretz, A.; Neff, C.; Lin-Wu, E.; Gregor, M.; Groneberg, D.A. Down-regulated in adenoma mediates apical Cl−/HCO3− exchange in rabbit, rat, and human duodenum. Gastroenterology 2002, 122, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Loo, S.Y.; Chang, M.K.; Chua, C.S.; Kumar, A.P.; Pervaiz, S.; Clement, M.V. NHE-1: A promising target for novel anti-cancer therapeutics. Curr. Pharm. Des. 2012, 18, 1372–1382. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Sellers, Z.M.; Smith, A.; Chow, J.Y.; Barrett, K.E. Na+/Ca2+ exchange regulates Ca2+-dependent duodenal mucosal ion transport and HCO3− secretion in mice. Am. J. Physiol. Gastrointest. 2005, 288, G457–G465. [Google Scholar] [CrossRef] [PubMed]
- Yoo, B.K.; Yanda, M.K.; No, Y.R.; Yun, C.C. Human intestinal epithelial cell line SK-CO15 is a new model system to study Na(+)/H(+) exchanger 3. Am. J. Physiol. Gastrointest. L. 2012, 303, G180–G188. [Google Scholar] [CrossRef] [PubMed]
- Coon, S.; Kekuda, R.; Saha, P.; Sundaram, U. Reciprocal regulation of the primary sodium absorptive pathways in rat intestinal epithelial cells. Am. J. Physiol. Cell. Physiol. 2011, 300, C496–C505. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wang, Y.; Graham, W.V.; Su, L.; Musch, M.W.; Turner, J.R. Mapkapk-2 is a critical signaling intermediate in NHE3 activation following Na+-glucose cotransport. J. Biol. Chem. 2006, 281, 24247–24253. [Google Scholar] [CrossRef] [PubMed]
- Zizak, M.; Chen, T.; Bartonicek, D.; Sarker, R.; Zachos, N.C.; Cha, B.; Kovbasnjuk, O.; Korac, J.; Mohan, S.; Cole, R. Calmodulin kinase II constitutively binds, phosphorylates, and inhibits brush border Na(+)/H(+) exchanger 3 (NHE3) by a NHERF2 protein-dependent process. J. Biol. Chem. 2012, 287, 13442–13456. [Google Scholar] [CrossRef] [PubMed]
- Centeno, V.A.; Díaz de Barboza, G.E.; Marchionatti, A.M.; Alisio, A.E.; Dallorso, M.E.; Nasif, R.; Tolosa de Talamoni, N.G. Dietary calcium deficiency increases Ca2+ uptake and Ca2+ extrusion mechanisms in chick enterocytes. Comp. Biochem. Phys. 2004, 139, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Khuituan, P.; Wongdee, K.; Jantarajit, W.; Suntornsaratoon, P.; Krishnamra, N.; Charoenphandhu, N. Fibroblast growth factor-23 negates 1,25(OH)2D3-induced intestinal calcium transport by reducing the transcellular and paracellular calcium fluxes. Arch. Biochem. Biophys. 2013, 536, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Ikumi, Y.; Kida, T.; Sakuma, S.; Yamashita, S.; Akashi, M. Polymer-phloridzin conjugates as an anti-diabetic drug that inhibits glucose absorption through the Na+/glucose cotransporter (SGLT1) in the small intestine. J. Control. Release 2008, 125, 42–49. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Tuo, B.; Dong, H. Regulation of Intestinal Glucose Absorption by Ion Channels and Transporters. Nutrients 2016, 8, 43. https://doi.org/10.3390/nu8010043
Chen L, Tuo B, Dong H. Regulation of Intestinal Glucose Absorption by Ion Channels and Transporters. Nutrients. 2016; 8(1):43. https://doi.org/10.3390/nu8010043
Chicago/Turabian StyleChen, Lihong, Biguang Tuo, and Hui Dong. 2016. "Regulation of Intestinal Glucose Absorption by Ion Channels and Transporters" Nutrients 8, no. 1: 43. https://doi.org/10.3390/nu8010043
APA StyleChen, L., Tuo, B., & Dong, H. (2016). Regulation of Intestinal Glucose Absorption by Ion Channels and Transporters. Nutrients, 8(1), 43. https://doi.org/10.3390/nu8010043



