Xylobiose, an Alternative Sweetener, Ameliorates Diabetes-Related Metabolic Changes by Regulating Hepatic Lipogenesis and miR-122a/33a in db/db Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. XB Extraction
2.2. Animals and Diet
2.3. Oral Glucose Tolerance Test (OGTT)
2.4. Biochemical Analysis of Blood Samples
2.5. Western Blotting Analysis
2.6. RNA Isolation and Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) Analysis
2.7. MicroRNA Quantification by Real-Time qRT-PCR
2.8. Histopathological Evaluation of Liver Lesions
2.9. Statistical Analyses
3. Results
3.1. Body Weight, Food and Water Intake, and Plasma Lipid Profiles
3.2. OGTT, FBG, and Related Biochemical Profiles
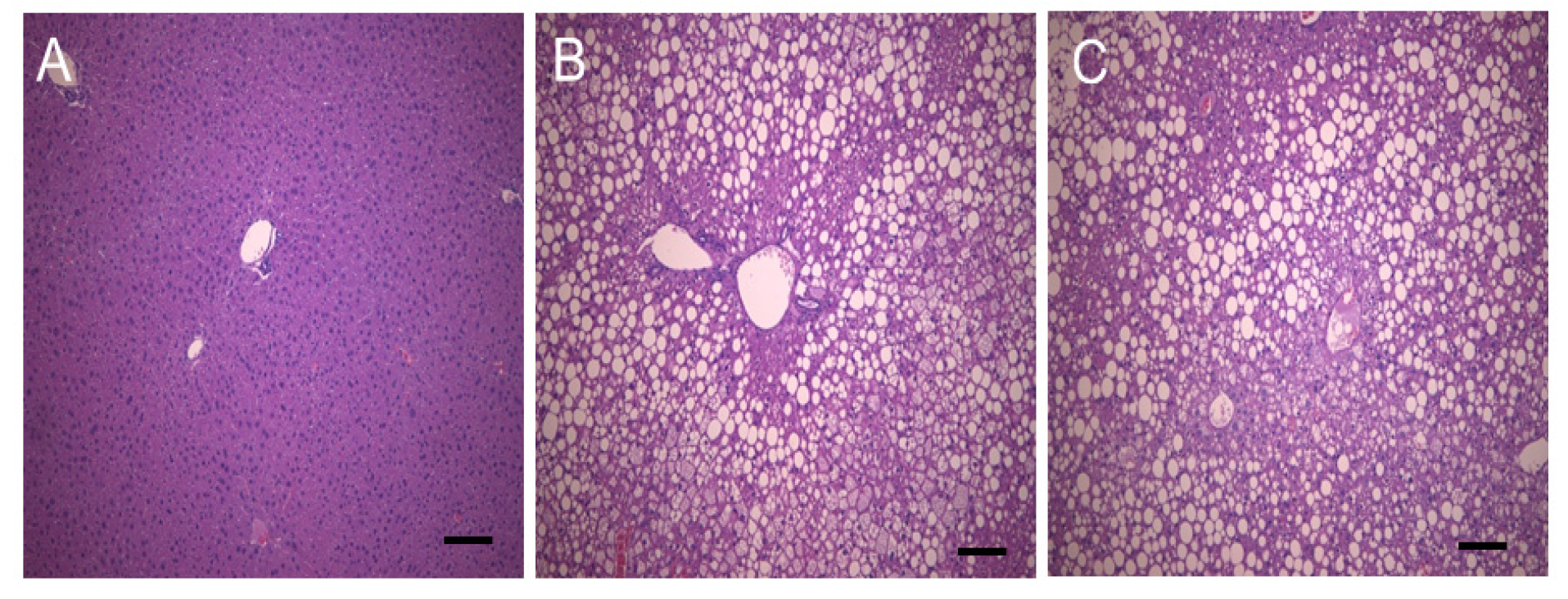
3.3. Hepatic Histology
3.4. Expression of Hepatic miRNAs Associated with Lipid Metabolism
3.5. Expression Profiles of Genes Involved in Hepatic Lipogenesis and Cholesterol Homeostasis
3.6. Hepatic Oxidative Stress
3.7. The Expressions of Genes Related to Inflammatory Response
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zimmet, P.; Alberti, K.G.; Shaw, J. Global and societal implications of the diabetes epidemic. Nature 2001, 414, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Stahlman, M.; Fagerberg, B.; Adiels, M.; Ekroos, K.; Chapman, J.M.; Kontush, A.; Boren, J. Dyslipidemia, but not hyperglycemia and insulin resistance, is associated with marked alterations in the hdl lipidome in type 2 diabetic subjects in the diwa cohort: Impact on small hdl particles. Biochim. Biophys. Acta 2013, 1831, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Cerf, M.E. Beta cell dysfunction and insulin resistance. Front. Endocrinol. 2013, 4, 37. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; Tripathy, D.; DeFronzo, R.A. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 2006, 29, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Williamson, D.F.; Vinicor, F.; Bowman, B.A.; Centers for Disease Control and Prevention Primary Prevention Working Group. Primary prevention of type 2 diabetes mellitus by lifestyle intervention: Implications for health policy. Ann. Intern. Med. 2004, 140, 951–957. [Google Scholar] [PubMed]
- Almdal, T.; Scharling, H.; Jensen, J.S.; Vestergaard, H. The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: A population-based study of 13,000 men and women with 20 years of follow-up. Arch. Intern. Med. 2004, 164, 1422–1426. [Google Scholar] [CrossRef] [PubMed]
- Mooradian, A.D. Dyslipidemia in type 2 diabetes mellitus. Nat. Clin. Pract. Endocrinol. Metab. 2009, 5, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Neuschwander-Tetri, B.A.; Caldwell, S.H. Nonalcoholic steatohepatitis: Summary of an AASLD single topic conference. Hepatology 2003, 37, 1202–1219. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, H.J.; Lee, K.E.; Kim, D.J.; Kim, S.K.; Ahn, C.W.; Lim, S.K.; Kim, K.R.; Lee, H.C.; Huh, K.B.; et al. Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch. Intern. Med. 2004, 164, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Ryysy, L.; Hakkinen, A.M.; Goto, T.; Vehkavaara, S.; Westerbacka, J.; Halavaara, J.; Yki-Jarvinen, H. Hepatic fat content and insulin action on free fatty acids and glucose metabolism rather than insulin absorption are associated with insulin requirements during insulin therapy in type 2 diabetic patients. Diabetes 2000, 49, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Filipowicz, W.; Bhattacharyya, S.N.; Sonenberg, N. Mechanisms of post-transcriptional regulation by micrornas: Are the answers in sight? Nat. Rev. Genet. 2008, 9, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Rottiers, V.; Naar, A.M. Micrornas in metabolism and metabolic disorders. Nat. Rev. Mol. Cell Biol. 2012, 13, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Esau, C.; Davis, S.; Murray, S.F.; Yu, X.X.; Pandey, S.K.; Pear, M.; Watts, L.; Booten, S.L.; Graham, M.; McKay, R.; et al. Mir-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006, 3, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.C.; Hsu, S.D.; Hsu, C.S.; Lai, T.C.; Chen, S.J.; Shen, R.; Huang, Y.; Chen, H.C.; Lee, C.H.; Tsai, T.F.; et al. Microrna-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. J. Clin. Investig. 2012, 122, 2884–2897. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhang, Z.R.; Zhang, J.L.; Zhu, X.B.; He, L.; Shi, Z.; Gao, L.; Li, Y.; Hu, B.; Feng, F.M. Microrna-122 is involved in oxidative stress in isoniazid-induced liver injury in mice. Genet. Mol. Res. 2015, 14, 13258–13265. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.H.; Wang, B.; Kota, J.; Yu, J.; Costinean, S.; Kutay, H.; Yu, L.; Bai, S.; La Perle, K.; Chivukula, R.R.; et al. Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of mir-122 in liver. J. Clin. Investig. 2012, 122, 2871–2883. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.M.; Seo, S.Y.; Kim, T.H.; Kim, S.G. Decrease of microrna-122 causes hepatic insulin resistance by inducing protein tyrosine phosphatase 1b, which is reversed by licorice flavonoid. Hepatology 2012, 56, 2209–2220. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Francl, J.M.; Boehme, S.; Chiang, J.Y. Regulation of cholesterol and bile acid homeostasis by the cholesterol 7alpha-hydroxylase/steroid response element-binding protein 2/microrna-33a axis in mice. Hepatology 2013, 58, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.; Lee, H.B. Reactive oxygen species as glucose signaling molecules in mesangial cells cultured under high glucose. Kidney Int. Suppl. 2000, 77, S19–S25. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; dello Russo, P.; Amstad, P.; Cerutti, P. High glucose induces antioxidant enzymes in human endothelial cells in culture. Evidence linking hyperglycemia and oxidative stress. Diabetes 1996, 45, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Loria, P.; Lonardo, A.; Bellentani, S.; Day, C.P.; Marchesini, G.; Carulli, N. Non-alcoholic fatty liver disease (NAFLD) and cardiovascular disease: An open question. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 684–698. [Google Scholar] [CrossRef] [PubMed]
- Millan, J.; Pinto, X.; Munoz, A.; Zuniga, M.; Rubies-Prat, J.; Pallardo, L.F.; Masana, L.; Mangas, A.; Hernandez-Mijares, A.; Gonzalez-Santos, P.; et al. Lipoprotein ratios: Physiological significance and clinical usefulness in cardiovascular prevention. Vasc. Health Risk Manag. 2009, 5, 757–765. [Google Scholar] [PubMed]
- Brenner, R.R.; Rimoldi, O.J.; Lombardo, Y.B.; Gonzalez, M.S.; Bernasconi, A.M.; Chicco, A.; Basabe, J.C. Desaturase activities in rat model of insulin resistance induced by a sucrose-rich diet. Lipids 2003, 38, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Dekker, M.J.; Su, Q.; Baker, C.; Rutledge, A.C.; Adeli, K. Fructose: A highly lipogenic nutrient implicated in insulin resistance, hepatic steatosis, and the metabolic syndrome. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E685–E694. [Google Scholar] [CrossRef] [PubMed]
- Basaranoglu, M.; Basaranoglu, G.; Sabuncu, T.; Sentürk, H. Fructose as a key player in the development of fatty liver disease. World J. Gastroenterol. 2013, 19, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Han, H.J.; Kim, A.H.; Choi, J.Y.; Cho, S.J.; Park, Y.B.; Jung, U.J.; Choi, M.S. d-allulose supplementation normalized the body weight and fat-pad mass in diet-induced obese mice via the regulation of lipid metabolism under isocaloric fed condition. Mol. Nutr. Food Res. 2016, 60, 1695–1706. [Google Scholar] [CrossRef] [PubMed]
- Young, H.; Benton, D. The effect of using isomaltulose (palatinose) to modulate the glycaemic properties of breakfast on the cognitive performance of children. Eur. J. Nutr. 2015, 54, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Arai, H.; Mizuno, A.; Fukaya, M.; Sato, T.; Koganei, M.; Sasaki, H.; Yamamoto, H.; Taketani, Y.; Doi, T.; et al. Dietary palatinose and oleic acid ameliorate disorders of glucose and lipid metabolism in zucker fatty rats. J. Nutr. 2007, 137, 1908–1915. [Google Scholar] [PubMed]
- Lakshmi, G.S.; Rajeswari, B.U.; Prakasham, R.S. Biosynthesis of xylobiose: A strategic way to enrich the value of oil palm empty fruit bunch fiber. J. Microbiol. Biotechnol. 2012, 22, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, K.; Nakatsu, Y.; Sato, M.; Sedarnawati, Y.; Sugano, M. Effects of xylooligosaccharides on blood glucose, serum and liver lipids and cecum short-chain fatty acids in diabetic rats. Agric. Biol. Chem. 1991, 55, 199–205. [Google Scholar]
- Lee, O.S.; Rhee, I.K. The production of xylooligosaccharides with microbial xylanase. Food Nutr. 2001, 6, 21–24. [Google Scholar]
- Ministry of Food and Drug Safety. Nutrition Labeling Information Legistration; Ministry of Food and Drug Safety: Cheongwon County, Korea, 2016; pp. 1–164.
- Okazaki, M.; Koda, H.; Izumi, R.; Fujikawa, S.; Matsumoto, N. In vitro digestibility and in vivo utilization of xylobiose. J. Jpn. Soc. Nutr. Food Sci. (Jpn.) 1991, 9, 77–86. [Google Scholar] [CrossRef]
- Aachary, A.A.; Prapulla, S.G. Corncob-induced endo-1,4-beta-d-xylanase of aspergillus oryzae mtcc 5154: Production and characterization of xylobiose from glucuronoxylan. J. Agric. Food Chem. 2008, 56, 3981–3988. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.-G.; Mun, S. Optimal design and experimental validation of a three-zone simulated moving bed process based on the amberchrom-cg161c adsorbent for continuous removal of acetic acid from biomass hydrolyzate. Process Biochem. 2012, 42, 725–734. [Google Scholar] [CrossRef]
- Wolever, T.M.; Jenkins, D.J.; Jenkins, A.L.; Josse, R.G. The glycemic index: Methodology and clinical implications. Am. J. Clin. Nutr. 1991, 54, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Sabine, J.R.; James, M.J. The intracellular mechanism responsible for dietary feedback control of cholesterol synthesis. Life Sci. 1976, 18, 1185–1192. [Google Scholar] [CrossRef]
- Hsu, C.K.; Liao, J.W.; Chung, Y.C.; Hsieh, C.P.; Chan, Y.C. Xylooligosaccharides and fructooligosaccharides affect the intestinal microbiota and precancerous colonic lesion development in rats. J. Nutr. 2004, 134, 1523–1528. [Google Scholar] [PubMed]
- Sheu, W.H.; Lee, I.T.; Chen, W.; Chan, Y.C. Effects of xylooligosaccharides in type 2 diabetes mellitus. J. Nutr. Sci. Vitaminol. (Tokyo) 2008, 54, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Gobinath, D.; Madhu, A.N.; Prashant, G.; Srinivasan, K.; Prapulla, S.G. Beneficial effect of xylo-oligosaccharides and fructo-oligosaccharides in streptozotocin-induced diabetic rats. Br. J. Nutr. 2010, 104, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.; Lim, J.Y.; Shin, J.H.; Seok, P.R.; Jung, S.; Yoo, S.H.; Kim, Y. d-xylose suppresses adipogenesis and regulates lipid metabolism genes in high-fat diet-induced obese mice. Nutr. Res. 2015, 35, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.L.K.; Kyung, M.; Jung, S.; Park, Y.; Yang, C. Study on the proper d-xylose concentration in sugar mixture to reduce glycemic index (gi) value in the human clinical model. Korean J. Food Nutr. 2012, 25, 787–792. [Google Scholar] [CrossRef]
- Bae, Y.J.; Bak, Y.K.; Kim, B.; Kim, M.S.; Lee, J.H.; Sung, M.K. Coconut-derived d-xylose affects postprandial glucose and insulin responses in healthy individuals. Nutr. Res. Pract. 2011, 5, 533–539. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Screening for type 2 diabetes. Diabetes Care 2004, 27, S11–S14. [Google Scholar]
- Ozcan, S. Micrornas in pancreatic beta-cell physiology. Adv. Exp. Med. Biol. 2015, 887, 101–117. [Google Scholar] [PubMed]
- Li, S.; Chen, X.; Zhang, H.; Liang, X.; Xiang, Y.; Yu, C.; Zen, K.; Li, Y.; Zhang, C.Y. Differential expression of micrornas in mouse liver under aberrant energy metabolic status. J. Lipid Res. 2009, 50, 1756–1765. [Google Scholar] [CrossRef] [PubMed]
- Murase, T.; Misawa, K.; Minegishi, Y.; Aoki, M.; Ominami, H.; Suzuki, Y.; Shibuya, Y.; Hase, T. Coffee polyphenols suppress diet-induced body fat accumulation by downregulating srebp-1c and related molecules in c57bl/6j mice. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E122–E133. [Google Scholar] [CrossRef] [PubMed]
- Davalos, A.; Goedeke, L.; Smibert, P.; Ramirez, C.M.; Warrier, N.P.; Andreo, U.; Cirera-Salinas, D.; Rayner, K.; Suresh, U.; Pastor-Pareja, J.C.; et al. Mir-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 9232–9237. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, F.; Lefebvre, P.; Scheen, A. Non-alcoholic steatohepatitis: Association with obesity and insulin resistance, and influence of weight loss. Diabetes Metab. 2008, 26, 98–106. [Google Scholar] [CrossRef]
- Loftus, T.M.; Jaworsky, D.E.; Frehywot, G.L.; Townsend, C.A.; Ronnett, G.V.; Lane, M.D.; Kuhajda, F.P. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science 2000, 288, 2379–2381. [Google Scholar] [CrossRef] [PubMed]
- Halminski, M.A.; Marsh, J.B.; Harrison, E.H. Differential effects of fish oil, safflower oil and palm oil on fatty acid oxidation and glycerolipid synthesis in rat liver. J. Nutr. 1991, 121, 1554–1561. [Google Scholar] [PubMed]
- Brown, M.S.; Goldstein, J.L. The srebp pathway: Regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 1997, 89, 331–340. [Google Scholar] [CrossRef]
- Shimano, H.; Horton, J.D.; Shimomura, I.; Hammer, R.E.; Brown, M.S.; Goldstein, J.L. Isoform 1c of sterol regulatory element binding protein is less active than isoform 1a in livers of transgenic mice and in cultured cells. J. Clin. Investig. 1997, 99, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Loren, J.; Huang, Z.; Laffitte, B.A.; Molteni, V. Liver x receptor modulators: A review of recently patented compounds (2009–2012). Expert Opin. Ther. Pat. 2013, 23, 1317–1335. [Google Scholar] [CrossRef] [PubMed]
- Bilz, S.; Samuel, V.; Morino, K.; Savage, D.; Choi, C.S.; Shulman, G.I. Activation of the farnesoid x receptor improves lipid metabolism in combined hyperlipidemic hamsters. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E716–E722. [Google Scholar] [CrossRef] [PubMed]
- Biddinger, S.B.; Haas, J.T.; Yu, B.B.; Bezy, O.; Jing, E.; Zhang, W.; Unterman, T.G.; Carey, M.C.; Kahn, C.R. Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nat. Med. 2008, 14, 778–782. [Google Scholar] [CrossRef] [PubMed]
- Crespo, J.; Cayon, A.; Fernandez-Gil, P.; Hernandez-Guerra, M.; Mayorga, M.; Dominguez-Diez, A.; Fernandez-Escalante, J.C.; Pons-Romero, F. Gene expression of tumor necrosis factor alpha and tnf-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology 2001, 34, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Insulin resistance, inflammation, and non-alcoholic fatty liver disease. Trends Endocrinol. Metab. 2008, 19, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Xirouchakis, E.; Manousou, P.; Tsartsali, L.; Georgopoulos, S.; Burroughs, A. Insights into the pathogenesis of NAFLD: The role of metabolic and pro-inflammatory mediators. Ann. Gastroenterol. 2009, 22, 24–33. [Google Scholar]
- Tarantino, G.; Caputi, A. Jnks, insulin resistance and inflammation: A possible link between NAFLD and coronary artery disease. World J. Gastroenterol. 2011, 17, 3785. [Google Scholar] [CrossRef] [PubMed]
- Herlaar, E.; Brown, Z. P38 mapk signalling cascades in inflammatory disease. Mol. Med. Today 1999, 5, 439–447. [Google Scholar] [CrossRef]







| Ingredients (g) | AIN-93G | XB 5 2 |
|---|---|---|
| Casein, lactic | 200 | 200 |
| l-cystein | 3 | 3 |
| Corn starch | 397.5 | 397.5 |
| Maltodextrin | 132 | 132 |
| Sucrose | 100 | 95 |
| Xylobiose | - | 5 |
| Cellulose | 50 | 50 |
| Soybean Oil | 70 | 70 |
| Lard | - | - |
| Mineral mix 3 | 35 | 35 |
| Dicacium phosphate | - | - |
| Calcium carbonate | - | - |
| Potassium citrate H2O | - | - |
| Vitamin mix | 10 | 10 |
| Cholin Bitartrate | 2.5 | 2.5 |
| t-butylhydroquinone | 0.014 | 0.014 |
| Total amount (g) | 1000 | 1000 |
| Total energy (kcal) | 4000 | 4000 |
| Gene Symbol | GenBank ID | Forward Primer (5′ to 3′) | Reverse primer (5′ to 3′) | |
|---|---|---|---|---|
| FAS | Fasn | 14104 | CTTCGCCAACTCTACCATGG | TTCCACACCCATGAGCGAGT |
| PPARγ | Pparg | 19016 | CGAGAAGGAGAAGCTGTTGG | TCAGCGGGAAGGACTTTATGTATG |
| SREBP-1C | Srebf1 | 20787 | TAGAGCATATCCCCCAGGTG | GGTACGGGCCACAAGAAGTA |
| SREBP-2 | Srebf2 | 20788 | CAAGAGAAAGTTCCTATCAAGCAAGTG | GTCCTTCAACTCTATGATTTTGTCGTT |
| HMGCR | Hmgcr | 15357 | TGACCTTTCTAGAGCGAGTGC | GTGCCAACTCCAATCACAAG |
| ACC | Acaca | 107476 | AGGATTTGCTGTTTCTCAGAGCTT | CAGGATCTACCCAGGCCACAT |
| ABCG5 | Abcg5 | 31322257 | CCTTGGTGGAACATCAAATC | TGATTTGCAGTCATGCAGTC |
| ABCG8 | Abcg8 | 553727251 | AGCTTCAAAGTGAGGAGTGG | AAGGACCAGGTCAAATAGCC |
| CYP7A1 | Cyp7a1 | 31542444 | TCAGCTCTGGAGGGAATGC | AAGTCCTCCTTAGCTGTCCG |
| CYP8B1 | Cyp8b1 | 227497651 | AGCTTCAAAGTGAGGAGTGG | AAGGACCAGGTCAAATAGCC |
| SOD1 | Sod1 | 45597446 | GAGACCTGGGCAATGTGACT | GTTTACTGCGCAATCCCAAT |
| SOD2 | Sod2 | 76253932 | CCGAGGAGAAGTACCACGAG | GCTTGATAGCCTCCAGCAAC |
| TNF-α | Tnf | 21926 | ATGAGCACAGAAAGCATGATC | TACAGGCTTGTCACTCGAATT |
| IL-1β | Il1b | 16176 | ATGGCAACTGTTCCTGAACTCAACT | CAGGACAGGTATAGATTCTTTCCTTT |
| IL-6 | Il6 | 16193 | CTCTGGGAAATCGTGGAAATG | AAGTGCATCATCGTTGTTCATACA |
| MCP-1 | Mcpt1 | 17224 | CCCACTCACCTGCTGCTACT | TCTGGACCCATTCCTTCTTG |
| GAPDH | Gapdh | 14433 | GCCTTCCGTGTTCCTACCC | TGCCTGCTTCACCACCTT |
| Ctrl | DB | XB 5 | |
|---|---|---|---|
| Final body weight (g) | 26.7 ± 0.5 a | 43.3 ± 1.1 b | 45.0 ± 0.6 b |
| Food intake (g/day) | 2.8 ± 0.1 a | 5.5 ± 0.3 b | 4.4 ± 0.2 c |
| Water intake (mL/day) | 4.6 ± 0.3 a | 18.0 ± 1.3 b | 11.5 ± 0.6 c |
| Triglyceride (mg/dL) | 133.0 ± 6.7 a | 146.5 ± 2.7 b | 109.4 ± 3.0 c |
| TC (mg/dL) | 107.7 ± 4.7 a | 163.8 ± 7.4 b | 149.0 ± 3.5 c |
| HDL-Cholesterol (mg/dL) | 47.1 ± 2.5 a | 72.7 ± 4.6 b | 75.5 ± 2.8 b |
| LDL-Cholesterol (mg/dL) | 34.0 ± 3.4 a | 61.8 ± 4.2 b | 51.6 ± 3.0 c |
| GOT (Karmen/mL) | 39.2 ± 20.4 a | 125.1 ± 31.3 b | 149.6 ± 25.0 b |
| GPT (Karmen/mL) | 6.4 ± 7.7 a | 155.7 ± 56.2 b | 145.6 ± 49.1 b |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, E.; Lim, J.Y.; Kim, E.; Kim, Y.-S.; Shin, J.-H.; Seok, P.R.; Jung, S.; Yoo, S.-H.; Kim, Y. Xylobiose, an Alternative Sweetener, Ameliorates Diabetes-Related Metabolic Changes by Regulating Hepatic Lipogenesis and miR-122a/33a in db/db Mice. Nutrients 2016, 8, 791. https://doi.org/10.3390/nu8120791
Lim E, Lim JY, Kim E, Kim Y-S, Shin J-H, Seok PR, Jung S, Yoo S-H, Kim Y. Xylobiose, an Alternative Sweetener, Ameliorates Diabetes-Related Metabolic Changes by Regulating Hepatic Lipogenesis and miR-122a/33a in db/db Mice. Nutrients. 2016; 8(12):791. https://doi.org/10.3390/nu8120791
Chicago/Turabian StyleLim, Eunjin, Ji Ye Lim, Eunju Kim, Yoo-Sun Kim, Jae-Ho Shin, Pu Reum Seok, Sangwon Jung, Sang-Ho Yoo, and Yuri Kim. 2016. "Xylobiose, an Alternative Sweetener, Ameliorates Diabetes-Related Metabolic Changes by Regulating Hepatic Lipogenesis and miR-122a/33a in db/db Mice" Nutrients 8, no. 12: 791. https://doi.org/10.3390/nu8120791
APA StyleLim, E., Lim, J. Y., Kim, E., Kim, Y.-S., Shin, J.-H., Seok, P. R., Jung, S., Yoo, S.-H., & Kim, Y. (2016). Xylobiose, an Alternative Sweetener, Ameliorates Diabetes-Related Metabolic Changes by Regulating Hepatic Lipogenesis and miR-122a/33a in db/db Mice. Nutrients, 8(12), 791. https://doi.org/10.3390/nu8120791







