Hypoglycemic Effect of Opuntia ficus-indica var. saboten Is Due to Enhanced Peripheral Glucose Uptake through Activation of AMPK/p38 MAPK Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. α-Glucosidase Activity
2.3. Na+-Dependent Glucose Uptake in Brush-Border-Membrane Vesicle (BBMV)
2.4. Cell Culture
2.5. Cell Viability
2.6. Glucose Uptake
2.7. Immunoblot Analysis
2.8. Plasma Membrane Fractionation and GLUT4 Translocation Analysis
2.9. In Vivo Hypoglycemic Activity Evaluation in db/db Mice
2.9.1. Metabolic Parameters
2.9.2. Histological Examination
2.10. Statistical Analysis
3. Results
3.1. Effects on α-Glucosidase Activity and Intestinal Glucose Uptake
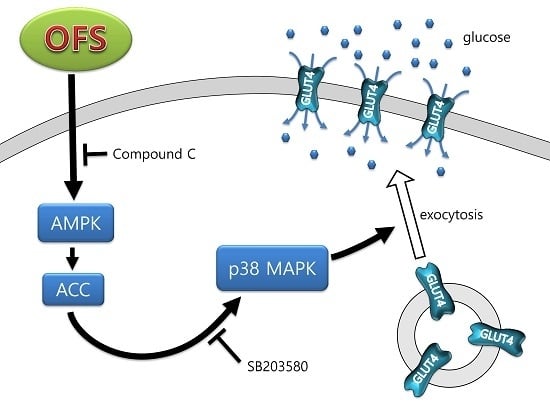
3.2. Effect on Glucose Uptake and AMPK Activation in L6 Myoblasts
3.3. Effect on MAPK Activation in L6 Myoblasts
3.4. Effect on GLUT4 Translocation to the Plasma Membrane in L6 Myoblasts
3.5. Preventive Effect on Progression of Diabetes in db/db Mice
3.6. Effect on Regeneration of β-Cells in db/db Mice
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gorboulev, V.; Schürmann, A.; Vallon, V.; Kipp, H.; Jaschke, A.; Klessen, D.; Friedrich, A.; Scherneck, S.; Rieg, T.; Cunard, R.; et al. Na(+)-d-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 2012, 61, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Fedorak, R.N.; CheesemanI, C.I.; Thomson, A.B.; Porter, V.M. Altered glucose carrier expression: Mechanism of intestinal adaptation during streptozocin-induced diabetes in rats. Am. J. Physiol. 1991, 261, G585–G591. [Google Scholar] [PubMed]
- Dyer, J.; Wood, I.S.; Palejwala, A.; Ellis, A.; Shirazi-Beechey, S.P. Expression of monosaccharide transporters in intestine of diabetic humans. Am. J. Physiol. 2002, 282, G241–G248. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.I. Deconstructing type 2 diabetes. Cell 1999, 97, 9–12. [Google Scholar] [CrossRef]
- Kaneto, H. Pancreatic β-cell glucose toxicity in type 2 diabetes mellitus. Curr. Diabetes Rev. 2015, 11, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Alvim, R.O.; Cheuhen, M.R.; Machado, S.R.; Sousa, A.G.; Santos, P.C. General aspects of muscle glucose uptake. An. Acad. Bras. Cienc. 2015, 87, 351–368. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A. Lilly lecture 1987. The triumvirate: Beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 1988, 37, 667–687. [Google Scholar] [CrossRef] [PubMed]
- Eid, H.M.; Martineau, L.C.; Saleem, A.; Muhammad, A.; Vallerand, D.; Benhaddou-Andaloussi, A.; Nistor, L.; Afshar, A.; Arnason, J.T.; Haddad, P.S. Stimulation of AMP-activated protein kinase and enhancement of basal glucose uptake in muscle cells by quercetin and quercetin glycosides, active principles of the antidiabetic medicinal plant Vaccinium vitis-idaea. Mol. Nutr. Food Res. 2010, 54, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Sawada, K.; Ikeda, K.; Fukuda, I.; Kawasaki, K.; Yamamoto, N.; Ashida, H. Prenylated chalcones 4-hydroxyderricin and xanthoangelol stimulate glucose uptake in skeletal muscle cells by inducing GLUT4 translocation. Mol. Nutr. Food Res. 2011, 55, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Czech, M.P. The GLUT4 glucose transporter. Cell Metab. 2007, 5, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.R., 3rd; Bergeron, R.; Shulman, G.I.; Young, L.H. Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am. J. Physiol. 1999, 277, H643–H649. [Google Scholar] [PubMed]
- Schultze, S.M.; Hemmings, B.A.; Niessen, M.; Tschopp, O. PI3K/AKT, MAPK and AMPK signalling: Protein kinases in glucose homeostasis. Expert Rev. Mol. Med. 2012, 14, e1. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.; Han, J.; Zhang, J.Z. Stimulation of glucose transport by AMP-activated protein kinase via activation of p38 mitogen-activated protein kinase. J. Biol. Chem. 2001, 276, 41029–41034. [Google Scholar] [CrossRef] [PubMed]
- Konrad, D.; Somwar, R.; Sweeney, G.; Yaworsky, K.; Hayashi, M.; Ramlal, T.; Klip, A. The antihyperglycemic drug a-lipoic acid stimulates glucose uptake via both GLUT4 translocation and GLUT4 activation: Potential role of p38 mitogen-activated protein kinase in GLUT4 activation. Diabetes 2001, 50, 1464–1471. [Google Scholar] [CrossRef] [PubMed]
- Somwar, R.; Perreault, M.; Kapur, S.; Taha, C.; Sweeney, G.; Ramlal, T.; Kim, D.Y.; Keen, J.; Côte, C.H.; Klip, A. Activation of p38 mitogen-activated protein kinase alpha and beta by insulin and contraction in rat skeletal muscle: Potential role in the stimulation of glucose transport. Diabetes 2000, 49, 1794–1800. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Nag, P.; Rahuja, N.; Srivastava, R.; Chaurasia, S.; Gautam, S.; Chandra, S.; Siddiqi, M.I.; Srivastava, A.K. Discovery of biaryl-4-carbonitriles as antihyperglycemic agents that may act through AMPK-p38 MAPK pathway. Mol. Cell Endocrinol. 2014, 394, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.O.; Kim, N.; Lee, H.J.; Lee, Y.W.; Kim, J.K.; Kim, H.I.; Lee, S.K.; Kim, S.J.; Park, S.H.; Kim, H.S. Visfatin, a novel adipokine, stimulates glucose uptake through the Ca2+ -dependent AMPK-p38 MAPK pathway in C2C12 skeletal muscle cells. J. Mol. Endocrinol. 2015, 54, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Lopez, D. Review: Use of the fruits and stems of the prickly pear cactus (Opuntia spp.) into human food. Food Sci. Tech. Int. 1995, 1, 65–74. [Google Scholar] [CrossRef]
- Ahn, D.K. Illustrated Book of Korean Medicinal Herbs; Kyohaksa: Seoul, Korea, 1988; p. 497. [Google Scholar]
- Antunes-Ricardo, M.; Moreno-García, B.E.; Gutiérrez-Uribe, J.A.; Aráiz-Hernández, D.; Alvarez, M.M.; Serna-Saldivar, S.O. Induction of apoptosis in colon cancercells treated with isorhamnetin glycosides from Opuntia ficus-indica pads. Plant Foods Hum. Nutr. 2014, 69, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Reynoso-Camacho, R.; Gonzalez de Mejia, E. Nopal (Opuntia spp.) and other traditional Mexican plants. In Nutraceuticals, Glycemic Health & Type 2 Diabetes; Pasupuleti, V.K., Anderson, J.W., Eds.; Wiley-Blackwell: Ames, LA, USA, 2008; pp. 379–399. [Google Scholar]
- Park, E.-H.; Kahng, J.H.; Lee, S.H.; Shin, K.H. An anti-inflammatory principle from cactus. Fitoterapia 2001, 72, 288–290. [Google Scholar] [CrossRef]
- Godard, M.P.; Ewing, B.A.; Pischel, I.; Ziegler, A.; Benedek, B.; Feistel, B. Acute blood glucose lowering effects and long-term safety of OpunDia supplementation in pre-diabetic males and females. J. Ethnopharmacol. 2010, 130, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Butterweck, V.; Semlin, L.; Feistel, B.; Pischel, I.; Bauer, K.; Verspohl, E.J. Comparative evaluation of two different Opuntia ficus-indica extracts for blood sugar lowering effects in rats. Phytother. Res. 2011, 25, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Halmi, S.; Benlakssira, B.; Bechtarzi, K.; Djerrou, Z.; Djeaalab, H.; Riachi, F.; Hamdi Pacha, Y. Antihyperglycemic activity of prickly pear (Opuntia ficus-indica) aqueous extract. Int. J. Med. Arom. Plants 2012, 2, 540–543. [Google Scholar]
- Deldicque, L.; Van Proeyen, K.; Ramaekers, M.; Pischel, I.; Sievers, H.; Hespel, P. Additive insulinogenic action of Opuntia ficus-indica cladode and fruit skin extract and leucine after exercise in healthy males. J. Int. Soc. Sports Nutr. 2013, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- López-Romero, P.; Pichardo-Ontiveros, E.; Avila-Nava, A.; Vázquez-Manjarrez, N.; Tovar, A.R.; Pedraza-Chaverri, J.; Torres, N. The effect of nopal (Opuntiaficus indica) on postprandial blood glucose, incretins, and antioxidant activityin Mexican patients with type 2 diabetes after consumption of two different composition breakfasts. J. Acad. Nutr. Diet. 2014, 114, 1811–1818. [Google Scholar] [CrossRef] [PubMed]
- Debnam, E.S.; Sharp, P.A. Acute and chronic effects of pancreatic glucagon on sugar transport across the brush-border and basolateral membranes of rat jejunal enterocytes. Exp. Physiol. 1993, 78, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Kasugai, S.; Hasegawa, N.; Ogura, H. A simple in vito cytotoxicity test using the MTT (3-(4,5)-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) colorimetric assay: Analysis of eugenol toxicity on dental pulp cells (RPC-C2A). Jpn. J. Pharmacol. 1990, 52, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Nakata, M.; Horimoto, N.; Saito, M.; Matsuoka, H.; Inagaki, N. Measurement of glucose uptake and intracellular calcium concentration in single, living pancreatic beta-cells. J. Biol. Chem. 2000, 275, 22278–22283. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follmann, D.A.; Sullivan, G.; Quon, M.J. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J. Clin. Endocrinol. Metab. 2000, 85, 2402–2410. [Google Scholar] [CrossRef] [PubMed]
- Lyon, H.; Prentø, P. Aldehyde Fuchsin staining of pancreatic B cells. Reproducible high-contrast staining of formalin-fixed and paraffin-embedded material. Histochem. J. 1980, 12, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Fröjdö, S.; Vidal, H.; Pirola, L. Alterations of insulin signaling in type 2 diabetes: A review of the current evidence from humans. Biochim. Biophys. Acta. 2009, 1792, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Govers, R. Cellular regulation of glucose uptake by glucose transporter GLUT4. Adv. Clin. Chem. 2014, 66, 173–240. [Google Scholar] [PubMed]
- Fryer, L.G.; Parbu-Patel, A.; Carling, D. The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J. Biol. Chem. 2002, 277, 25226–25232. [Google Scholar] [CrossRef] [PubMed]
- Nawrocki, A.R.; Rajala, M.W.; Tomas, E.; Pajvani, U.B.; Saha, A.K.; Trumbauer, M.E.; Pang, Z.; Chen, A.S.; Ruderman, N.B.; Chen, H.; et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J. Biol. Chem. 2006, 281, 2654–2660. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Tripathy, D. Skeletal Muscle Insulin Resistance Is the Primary Defect in Type 2 Diabetes. Diab. Care 2009, 32 (Suppl. S2), S157–S163. [Google Scholar] [CrossRef] [PubMed]
- Katsuda, Y.; Ohta, T.; Shinohara, M.; Bin, T.; Yamada, T. Diabetic mouse models. Open J. Anim. Sci. 2013, 3, 334–342. [Google Scholar] [CrossRef]
- Kaneto, H.; Matsuoka, T.A.; Kimura, T.; Obata, A.; Shimoda, M.; Kamei, S.; Mune, T.; Kaku, K. Appropriate therapy for type 2 diabetes mellitus in view of pancreatic β-cell glucose toxicity: “The earlier, the better”. Diabetes 2016, 8, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Bensadón, S.; Hervert-Hernández, D.; Sáyago-Ayerdi, S.G.; Goñi, I. By-products of Opuntia ficus-indica as a source of antioxidant dietary fiber. Plant Foods Hum. Nutr. 2010, 65, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Hannan, J.M.; Ali, L.; Rokeya, B.; Khaleque, J.; Akhter, M.; Flatt, P.R.; Abdel-Wahab, Y.H. Soluble dietary fibre fraction of Trigonella foenum-graecum (fenugreek) seed improves glucose homeostasis in animal models of type 1 and type 2 diabetes by delaying carbohydrate digestion and absorption, and enhancing insulin action. Br. J. Nutr. 2007, 97, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, S.; Khan, A. Antioxidants and diabetes. Indian J. Endocrinol. Metab. 2012, 16 (Suppl. S2), S267–S271. [Google Scholar] [PubMed]
- Stintzing, F.C.; Schieber, A.; Carle, R. Phytochemical and nutritional significance of cactus pear. Food Res. Tech. Int. 2001, 212, 396–407. [Google Scholar] [CrossRef]
- Dok-Go, H.; Lee, K.H.; Kim, H.J.; Lee, E.H.; Lee, J.; Song, Y.S.; Lee, Y.; Jin, C.; Lee, Y.S.; Cho, J. Neuroprotective effects of antioxidative flavonoids, quercetin, (1)-dihydroquercetin and quercetin 3-methyl ether, isolated from Opuntia ficus-indica var. saboten. Brain Res. 2003, 965, 130–136. [Google Scholar] [CrossRef]
- Avila-Nava, A.; Calderón-Oliver, M.; Medina-Campos, O.N.; Zou, T.; Gu, L.; Torres, N.; Tovar, A.R.; Pedraza-Chaverri, J. Extract of cactus (Opuntia ficus indica) cladodes scavenges reactive oxygen species in vitro and enhances plasma antioxidant capacity in humans. J. Funct. Food 2014, 10, 13–24. [Google Scholar] [CrossRef]
- Williams, D.B.; Wan, Z.; Frier, B.C.; Bell, R.C.; Field, C.J.; Wright, D.C. Dietary supplementation with vitamin E and C attenuates dexamethasone-induced glucose intolerance in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R49–R58. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.; Park, S.; Kim, M.J.; Yang, W.K.; Im, D.U.; Yang, K.R.; Hong, J.; Choe, W.; Kang, I.; Kim, S.S.; et al. AMP-activated protein kinase mediates the antioxidant effects of resveratrol through regulation of the transcription factor FoxO1. FEBS J. 2014, 281, 4421–4438. [Google Scholar] [CrossRef] [PubMed]







| Groups | DC | D1 | D2 | NC | N1 |
|---|---|---|---|---|---|
| Fasting glucose (mg/dL) | 589.3 ± 29.7 a | 347.8 ± 15.4 b | 224.8 ± 19.7 c | 124.6 ± 5.8 d | 114.6 ± 2.7 d |
| Insulin (µU/mL) | 36.8 ± 4.1 a | 26.3 ± 2.2 b | 23.6 ± 2.6 b | 16.9 ± 1.9 c | 17.5 ± 4.7 c |
| AUC 1 | 3218.3 ± 337.0 | 2841.3 ± 54.3 | 1451.7 ± 72.5 | 965.0 ± 6.3 | 2022.5 ± 10.6 |
| HOMA-IR 2 | 43.95 ± 3.2 a | 25.25 ± 2.1 b | 16.43 ± 1.4 c | 5.47 ± 0.3 d | 5.88 ± 0.4 d |
| QUICKI 3 | 0.24 ± 0.005 a | 0.25 ± 0.004 a | 0.26 ± 0.004 b | 0.30 ± 0.003 c | 0.29 ± 0.004 d |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leem, K.-H.; Kim, M.-G.; Hahm, Y.-T.; Kim, H.K. Hypoglycemic Effect of Opuntia ficus-indica var. saboten Is Due to Enhanced Peripheral Glucose Uptake through Activation of AMPK/p38 MAPK Pathway. Nutrients 2016, 8, 800. https://doi.org/10.3390/nu8120800
Leem K-H, Kim M-G, Hahm Y-T, Kim HK. Hypoglycemic Effect of Opuntia ficus-indica var. saboten Is Due to Enhanced Peripheral Glucose Uptake through Activation of AMPK/p38 MAPK Pathway. Nutrients. 2016; 8(12):800. https://doi.org/10.3390/nu8120800
Chicago/Turabian StyleLeem, Kang-Hyun, Myung-Gyou Kim, Young-Tae Hahm, and Hye Kyung Kim. 2016. "Hypoglycemic Effect of Opuntia ficus-indica var. saboten Is Due to Enhanced Peripheral Glucose Uptake through Activation of AMPK/p38 MAPK Pathway" Nutrients 8, no. 12: 800. https://doi.org/10.3390/nu8120800
APA StyleLeem, K.-H., Kim, M.-G., Hahm, Y.-T., & Kim, H. K. (2016). Hypoglycemic Effect of Opuntia ficus-indica var. saboten Is Due to Enhanced Peripheral Glucose Uptake through Activation of AMPK/p38 MAPK Pathway. Nutrients, 8(12), 800. https://doi.org/10.3390/nu8120800