Exogenous Glutamine in Respiratory Diseases: Myth or Reality?
Abstract
:1. Introduction
2. Is Glutamine Depleted in Critical Illness and Respiratory Diseases?
3. The Role of the Lungs in the Glutamine Pool
4. Glutamine Therapy in Respiratory Diseases
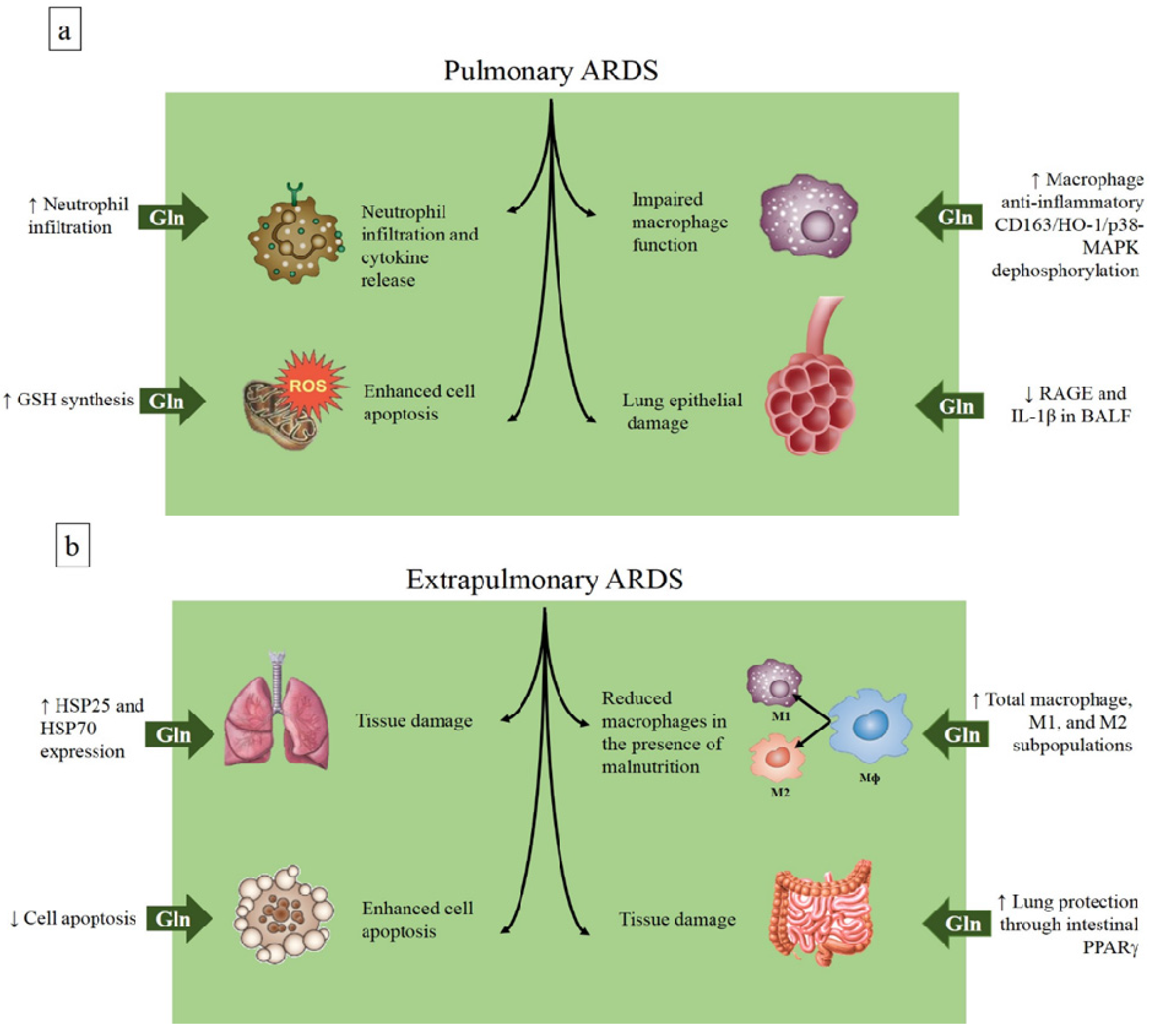
4.1. Glutamine in the Acute Respiratory Distress Syndrome
| Reference | Animal Model | Form Administered | Time, Route of Administration | Dose | Main Effects |
|---|---|---|---|---|---|
| Extrapulmonary | |||||
| Singleton et al. 2005 [46] | CLP (Sprague-Dawley rats) | Alanyl-glutamine | 1 h after injury, i.v. | 0.75 g·kg−1 | Reduced mortality, attenuated occurrence of lung injury |
| Oliveira et al. 2009 [47] | CLP (Wistar rats) | Alanyl-glutamine | 1 h after injury, i.v. | 0.75 g·kg−1 | Attenuated lung, diaphragm, and distal organ injury |
| Oliveira et al. 2014 [48] | CLP + malnourishment (Wistar rats) | Alanyl-glutamine | 1 h after injury, i.v. | 0.75 g·kg−1 | Attenuated lung and distal organ injury |
| Shih et al. 2015 [49] | Limb IR (C57BL/6 mice) | Alanyl-glutamine | Immediately after the injury, i.v. | 0.75 g·kg−1 | Reduced systemic inflammation, minimized lung injury |
| Peng et al. 2015 [50] | Gut IR (C57BL/6J mice) | Glutamine | 1 h after the ischemia, enteral | 60 mM | Improved survival and protected lung against injury and inflammation |
| Pulmonary | |||||
| Hou et al. 2009 [52] | LPS i.t. (C57BL/6 mice) | Glutamine | Pretreatment, oral (supplemented diet) | 25% total nitrogen | Increased lung inflammation |
| Zhang et al. 2009 [53] | LPS i.t. (Sprague-Dawley rats) | Alanyl-glutamine | Immediately after the injury, i.v. | 0.75 g·kg−1 | Protected alveolar barrier and attenuated inflammatory injury |
| Chuang et al. 2014 [54] | Hydrochloric acid + LPS i.t. (BALB/c mice) | Glutamine | Pretreatment, oral (supplemented diet) | 0.8 g·kg−1 | Inhibited RAGE expression and minimized lung injury |
| Lai et al. 2014 [55] | Hydrochloric acid + injurious mechanical ventilation (MV) (Sprague-Dawley rats) | Alanyl-glutamine | Immediately after the injury induced by hydrochloric acid and 30 min before MV, i.v. | 0.75 g·kg−1 | Improved oxygenation and lung mechanics, decreased tissue damage and inflammation |
| Fernandez-Bustamante et al. 2015 [56] | IL-1 + LPS i.t. (Sprague-Dawley rats) | Alanyl-glutamine | Pretreatment, oral gavage | 0.75 g·kg−1 | Decreased lung capillary damage |
| ARDS |
| Enhances HSP-70 and HSP-25 expression |
| Inhibits apoptosis |
| Improves macrophage function |
| Reduces pro-inflammatory cytokine release |
| Decreases neutrophil infiltration |
| Enhances GSH synthesis |
| Reduces RAGE expression |
| Activates CD163/heme-oxygenase-1/p-38 MAPK dephosphorylation |
| Asthma |
| Suppresses cPLA2 activity |
| Reduces activation of p38 MAPK |
| COPD |
| Modulates IL-6, IL-8, and TNF-α release |
| Increases citrulline and arginine production |
| CF |
| No significant regulatory actions |
| Cancer |
| Improves immune function |
| Preserves GSH levels |
4.2. Glutamine in Asthma

4.3. Glutamine and COPD
4.4. Glutamine in Cystic Fibrosis
4.5. Paradoxical Effects of Glutamine in Lung Cancer
4.6. The Lungs in Randomized Clinical Trials of Glutamine Supplementation in Critically Ill Patients
5. Conclusions
Acknowledgments
Author contributions
Conflicts of Interest
References
- Newsholme, P.; Procopio, J.; Lima, M.M.; Pithon-Curi, T.C.; Curi, R. Glutamine and glutamate—Their central role in cell metabolism and function. Cell Biochem. Funct. 2003, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Van Hall, G.; van der Vusse, G.J.; Söderlund, K.; Wagenmakers, A.J. Deamination of amino acids as a source for ammonia production in human skeletal muscle during prolonged exercise. J. Physiol. 1995, 489, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.K.; Yang, K.D.; Shaio, M.F. Lymphocyte proliferation modulated by glutamine: Involved in the endogenous redox reaction. Clin. Exp. Immunol. 1999, 117, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Conjard, A.; Komaty, O.; Delage, H.; Boghossian, M.; Martin, M.; Ferrier, B.; Baverel, G. Inhibition of glutamine synthetase in the mouse kidney: A novel mechanism of adaptation to metabolic acidosis. J. Biol. Chem. 2003, 278, 38159–38166. [Google Scholar] [CrossRef] [PubMed]
- Busque, S.M.; Stange, G.; Wagner, C.A. Dysregulation of the glutamine transporter Slc38a3 (SNAT3) and ammoniagenic enzymes in obese, glucose-intolerant mice. Cell. Physiol. Biochem. 2014, 34, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Rowbottom, D.G.; Keast, D.; Goodman, C.; Morton, A.R. The haematological, biochemical and immunological profile of athletes suffering from the overtraining syndrome. Eur. J. Appl. Physiol. Occup. Physiol. 1995, 70, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Roth, E.; Funovics, J.; Mühlbacher, F.; Schemper, M.; Mauritz, W.; Sporn, P.; Fritsch, A. Metabolic disorders in severe abdominal sepsis: Glutamine deficiency in skeletal muscle. Clin. Nutr. 1982, 1, 25–41. [Google Scholar] [CrossRef]
- Oudemans-van Straaten, H.M.; Bosman, R.J.; Treskes, M.; van der Spoel, H.J.; Zandstra, D.F. Plasma glutamine depletion and patient outcome in acute ICU admissions. Intensive Care Med. 2001, 27, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Wischmeyer, P.E.; Dhaliwal, R.; McCall, M.; Ziegler, T.R.; Heyland, D.K. Parenteral glutamine supplementation in critical illness: A systematic review. Crit. Care 2014, 18, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Sandini, M.; Nespoli, L.; Oldani, M.; Bernasconi, D.P.; Gianotti, L. Effect of glutamine dipeptide supplementation on primary outcomes for elective major surgery: Systematic review and meta-analysis. Nutrients 2015, 7, 481–499. [Google Scholar] [CrossRef] [PubMed]
- Van Zanten, A.R.; Dhaliwal, R.; Garrel, D.; Heyland, D.K. Enteral glutamine supplementation in critically ill patients: A systematic review and meta-analysis. Crit. Care 2015, 19, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Oldani, M.; Sandini, M.; Nespoli, L.; Coppola, S.; Bernasconi, D.P.; Gianotti, L. Glutamine Supplementation in Intensive Care Patients: A Meta-Analysis of Randomized Clinical Trials. Medicine 2015, 94. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Zimmerman, G.A. Acute lung injury and the acute respiratory distress syndrome: Four decades of inquiry into pathogenesis and rational management. Am. J. Respir. Cell Mol. Biol. 2005, 33, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Locksley, R.M. Asthma and allergic inflammation. Cell 2010, 140, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Tuder, R.M.; Petrache, I. Pathogenesis of chronic obstructive pulmonary disease. J. Clin. Investig. 2012, 122, 2749–2755. [Google Scholar] [CrossRef] [PubMed]
- Maki, K.; Nagai, K.; Suzuki, M.; Inomata, T.; Yoshida, T.; Nishimura, M. Temporal changes in glutaredoxin 1 and protein s-glutathionylation in allergic airway inflammation. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Steinkamp, G.; Wiedemann, B. Relationship between nutritional status and lung function in cystic fibrosis: Cross sectional and longitudinal analyses from the German CF quality assurance (CFQA) project. Thorax 2002, 57, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Celli, B.R. Systemic manifestations and comorbidities of COPD. Eur. Respir. J. 2009, 33, 1165–1185. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Vinnars, E.; Bergstöm, J.; Fürst, P. Influence of the postoperative state on the intracellular free amino acids in human muscle tissue. Ann. Surg. 1975, 182, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Rodas, P.C.; Rooyackers, O.; Hebert, C.; Norberg, Å.; Wernerman, J. Glutamine and glutathione at ICU admission in relation to outcome. Clin. Sci. 2012, 122, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Heyland, D.; Muscedere, J.; Wischmeyer, P.E.; Cook, D.; Jones, G.; Albert, M.; Elke, G.; Berger, M.M.; Day, A.G. A randomized trial of glutamine and antioxidants in critically ill patients. N. Engl. J. Med. 2013, 368, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Smedberg, M.; Grass, J.N.; Pettersson, L.; Norberg, Å.; Rooyackers, O.; Wernerman, J. Plasma glutamine concentration after intensive care unit discharge: An observational study. Crit. Care 2014, 18, 677. [Google Scholar] [CrossRef] [PubMed]
- Morrison, W.L.; Gibson, J.N.; Scrimgeour, C.; Rennie, M.J. Muscle wasting in emphysema. Clin. Sci. 1988, 75, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Engelen, M.P.; Schols, A.M.; Does, J.D.; Deutz, N.E.; Wouters, E.F. Altered glutamate metabolism is associated with reduced muscle glutathione levels in patients with emphysema. Am. J. Respir. Crit. Care Med. 2000, 161, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Pouw, E.M.; Schols, A.M.; Deutz, N.E.; Wouters, E.F. Plasma and muscle amino acid levels in relation to resting energy expenditure and inflammation in stable chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1998, 158, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Kutsuzawa, T.; Shioya, S.; Kurita, D.; Haida, M. Plasma branched-chain amino acid levels and muscle energy metabolism in patients with chronic obstructive pulmonary disease. Clin. Nutr. 2009, 28, 203–208. [Google Scholar] [CrossRef] [PubMed]
- D’Eufemia, P.; Finocchiaro, R.; Celli, M.; Tote, J.; Ferrucci, V.; Zambrano, A.; Troiani, P.; Quattrucci, S. Neutrophil glutamine deficiency in relation to genotype in children with cystic fibrosis. Pediatr. Res. 2006, 59, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Curi, T.C.; De Melo, M.P.; De Azevedo, R.B.; Zorn, T.M.; Curi, R. Glutamine utilization by rat neutrophils: Presence of phosphate-dependent glutaminase. Am. J. Physiol. 1997, 273, C1124–C1129. [Google Scholar] [PubMed]
- Fogarty, A.; Broadfield, E.; Lewis, S.; Lawson, N.; Britton, J. Amino acids and asthma: A case-control study. Eur. Respir. J. 2004, 23, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Sackesen, C.; Ercan, H.; Dizdar, E.; Soyer, O.; Gumus, P.; Tosun, B.N.; Büyüktuncer, Z.; Karabulut, E.; Besler, T.; Kalayci, O. A comprehensive evaluation of the enzymatic and nonenzymatic antioxidant systems in childhood asthma. J. Allergy Clin. Immunol. 2008, 122, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Kim, S.-H.H.; Lee, H.-S.S.; Choi, G.S.; Jung, Y.-S.S.; Ryu, D.H.; Park, H.-S.S.; Hwang, G.-S.S. Serum metabolomics reveals pathways and biomarkers associated with asthma pathogenesis. Clin. Exp. Allergy 2013, 43, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.K.; Salloum, R.M.; Austgen, T.R.; Bland, J.B.; Bland, K.I.; Copeland, E.M.; Souba, W.W. Tumor regulation of hepatic glutamine metabolism. J. Parenter. Enter. Nutr. 1991, 15, 159–164. [Google Scholar] [CrossRef]
- Brower, M.; Carney, D.N.; Oie, H.K.; Gazdar, A.F.; Minna, J.D. Growth of cell lines and clinical specimens of human non-small cell lung cancer in a serum-free defined medium. Cancer Res. 1986, 46, 798–806. [Google Scholar] [PubMed]
- Pacitti, A.J.; Chen, M.K.; Bland, K.I.; Copeland, E.M.; Souba, W.W. Mechanisms of accelerated hepatic glutamine efflux in the tumour-bearing rat. Surg. Oncol. 1992, 1, 173–182. [Google Scholar] [CrossRef]
- Chen, M.K.; Espat, N.J.; Bland, K.I.; Copeland, E.M.; Souba, W.W. Influence of progressive tumor growth on glutamine metabolism in skeletal muscle and kidney. Ann. Surg. 1993, 217, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Souba, W.W.; Herskowitz, K.; Plumley, D.A. Lung glutamine metabolism. J. Parenter. Enter. Nutr. 1990, 14, 68S–70S. [Google Scholar] [CrossRef]
- Plumley, D.A.; Souba, W.W.; Hautamaki, R.D.; Martin, T.D.; Flynn, T.C.; Rout, W.R.; Copeland, E.M. Accelerated lung amino acid release in hyperdynamic septic surgical patients. Arch. Surg. 1990, 125, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Hulsewé, K.W.; van der Hulst, R.R.R.; Ramsay, G.; van Berlo, C.L.; Deutz, N.E.; Soeters, P.B. Pulmonary glutamine production: Effects of sepsis and pulmonary infiltrates. Intensive Care Med. 2003, 29, 1833–1836. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Wasa, M.; Ryan, U.; Souba, W. Inhibition of pulmonary microvascular endothelial glutamine transport by glucocorticoids and endotoxin. J. Parenter. Enter. Nutr. 1995, 19, 477–481. [Google Scholar] [CrossRef]
- Labow, B.I.; Abcouwer, S.F.; Lin, C.M.; Souba, W.W. Glutamine synthetase expression in rat lung is regulated by protein stability. Am. J. Physiol. 1998, 275, L877–L886. [Google Scholar] [PubMed]
- Leite-Junior, J.H.H.; Garcia, C.S.; Souza-Fernandes, A.B.; Silva, P.L.; Ornellas, D.S.; Larangeira, A.P.; Castro-Faria-Neto, H.C.; Morales, M.M.; Negri, E.M.; Capelozzi, V.L.; et al. Methylprednisolone improves lung mechanics and reduces the inflammatory response in pulmonary but not in extrapulmonary mild acute lung injury in mice. Crit. Care Med. 2008, 36, 2621–2628. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.P.; Silva, J.D.; Marques, P.S.; Gonçalves-de-Albuquerque, C.F.; Santos, H.L.L.; Vascocellos, A.P.; Takiya, C.M.; Morales, M.M.; Pelosi, P.; Mócsai, A.; et al. The Effects of Dasatinib in Experimental Acute Respiratory Distress Syndrome Depend on Dose and Etiology. Cell. Physiol. Biochem. 2015, 36, 1644–1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calfee, C.S.; Janz, D.R.; Bernard, G.R.; May, A.K.; Kangelaris, K.N.; Matthay, M.A.; Ware, L.B. Distinct Molecular Phenotypes of Direct Versus Indirect ARDS in Single and Multi-Center Studies. Chest J. 2015, 147, 1539–1548. [Google Scholar] [CrossRef] [PubMed]
- Ware, L.B.; Matthay, M.A. The acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1334–1349. [Google Scholar] [CrossRef] [PubMed]
- Singleton, K.D.; Serkova, N.; Beckey, V.E.; Wischmeyer, P.E. Glutamine attenuates lung injury and improves survival after sepsis: Role of enhanced heat shock protein expression. Crit. Care Med. 2005, 33, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.P.; Oliveira, M.B.; Santos, R.S.; Lima, L.D.D.; Dias, C.M.; Ab’Saber, A.M.; Teodoro, W.R.; Capelozzi, V.L.; Gomes, R.N.; Bozza, P.T.; et al. Intravenous glutamine decreases lung and distal organ injury in an experimental model of abdominal sepsis. Crit. Care 2009, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Oliveira, G.P.; Silva, J.D.; de Araújo, C.C.; Prota, L.F.; Abreu, S.C.; Madeira, C.; Morales, M.M.; Takiya, C.M.; Diaz, B.L.; Capelozzi, V.L.; et al. Intravenous glutamine administration reduces lung and distal organ injury in malnourished rats with sepsis. Shock 2014, 41, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-M.M.; Shih, J.-M.M.; Pai, M.-H.H.; Hou, Y.-C.C.; Yeh, C.-L.L.; Yeh, S.-L.L. Glutamine Administration After Sublethal Lower Limb Ischemia Reduces Inflammatory Reaction and Offers Organ Protection in Ischemia/Reperfusion Injury. J. Parenter. Enter. Nutr. 2015. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Ban, K.; Wawrose, R.A.; Gover, A.G.; Kozar, R.A. Protection by enteral glutamine is mediated by intestinal epithelial cell peroxisome proliferator-activated receptor-γ during intestinal ischemia/reperfusion. Shock 2015, 43, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Melis, G.C.; Boelens, P.G.; van der Sijp, J.R.; Popovici, T.; De Bandt, J.-P.P.; Cynober, L.; van Leeuwen, P.A. The feeding route (enteral or parenteral) affects the plasma response of the dipetide Ala-Gln and the amino acids glutamine, citrulline and arginine, with the administration of Ala-Gln in preoperative patients. Br. J. Nutr. 2005, 94, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.-C.C.; Pai, M.-H.H.; Chiu, W.-C.C.; Hu, Y.-M.M.; Yeh, S.-L.L. Effects of dietary glutamine supplementation on lung injury induced by lipopolysaccharide administration. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 296, L288–L295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, X.; Pan, L.; Wang, W.; Li, N.; Li, J. Glutamine attenuates lipopolysaccharide-induced acute lung injury. Nutrition 2009, 25, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.-C.C.; Shaw, H.-M.M.; Chen, C.-C.C.; Pan, H.-J.J.; Lai, W.-C.C.; Huang, H.-L.L. Short-term glutamine supplementation decreases lung inflammation and the receptor for advanced glycation end-products expression in direct acute lung injury in mice. BMC Pulm. Med. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-C.C.; Liu, W.-L.L.; Chen, C.-M.M. Glutamine attenuates acute lung injury caused by acid aspiration. Nutrients 2014, 6, 3101–3116. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Bustamante, A.; Agazio, A.; Wilson, P.; Elkins, N.; Domaleski, L.; He, Q.; Baer, K.A.; Moss, A.F.; Wischmeyer, P.E.; Repine, J.E. Brief Glutamine Pretreatment Increases Alveolar Macrophage CD163/Heme Oxygenase-1/p38-MAPK Dephosphorylation Pathway and Decreases Capillary Damage but Not Neutrophil Recruitment in IL-1/LPS-Insufflated Rats. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Reddel, H.K.; Hurd, S.S.; FitzGerald, J.M. World Asthma Day. GINA 2014: A global asthma strategy for a global problem. Int. J. Tuberc. Lung Dis. 2014, 18, 505–506. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.M.; Kang, N.I.; Kim, Y.S.; Lee, Y.M.; Jin, Z.W.; Jung, Y.J.; Im, S.Y.; Kim, J.H.; Shin, Y.H.; Cho, B.H.; et al. Glutamine preferentially inhibits T-helper type 2 cell-mediated airway inflammation and late airway hyperresponsiveness through the inhibition of cytosolic phospholipase A(2) activity in a murine asthma model. Clin. Exp. Allergy 2008, 38, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Triggiani, M.; Granata, F.; Giannattasio, G.; Marone, G. Secretory phospholipases A2 in inflammatory and allergic diseases: Not just enzymes. J. Allergy Clin. Immunol. 2005, 116, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Pniewska, E.; Sokolowska, M.; Kupryś-Lipińska, I.; Przybek, M.; Kuna, P.; Pawliczak, R. The step further to understand the role of cytosolic phospholipase A2 alpha and group X secretory phospholipase A2 in allergic inflammation: Pilot study. Biomed. Res. Int. 2014, 2014, 670814. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.H.; Kim, H.-K.K.; Kim, J.-M.M.; Ayush, O.; Im, S.-Y.Y.; Oh, D.-K.K.; Lee, H.-K.K. Glutamine suppresses airway neutrophilia by blocking cytosolic phospholipase A(2) via an induction of MAPK phosphatase-1. J. Immunol. 2012, 189, 5139–5146. [Google Scholar] [CrossRef] [PubMed]
- Bruijnzeel, P.L.; Uddin, M.; Koenderman, L. Targeting neutrophilic inflammation in severe neutrophilic asthma: Can we target the disease-relevant neutrophil phenotype? J. Leukoc. Biol. 2015, 98, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.H.; Kim, H.-K.K.; Jeong, J.-S.S.; Lee, Y.-D.D.; Jin, Z.W.; Im, S.-Y.Y.; Lee, H.-K.K. Mechanism of glutamine inhibition of cytosolic phospholipase a2 (cPLA2 ): Evidence of physical interaction between glutamine-Induced mitogen-activated protein kinase phosphatase-1 and cPLA2. Clin. Exp. Immunol. 2015, 180, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Lange, P.; Marott, J.L.; Vestbo, J.; Olsen, K.R.; Ingebrigtsen, T.S.; Dahl, M.; Nordestgaard, B.G. Prediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: A study of the general population. Am. J. Respir. Crit. Care Med. 2012, 186, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Marwood, S.; Jack, S.; Patel, M.; Walker, P.; Bowtell, J.; Calverley, P. No effect of glutamine ingestion on indices of oxidative metabolism in stable COPD. Respir. Physiol. Neurobiol. 2011, 177, 41–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Memiş, D.; Turan, A.; Karamanlioglu, B.; Koyuncu, O.; Pamukçu, Z. Glutamine and chronic obstructive pulmonary disease. Eur. J. Anaesthesiol. 2006, 23, 621–623. [Google Scholar] [CrossRef] [PubMed]
- Rutten, E.P.; Engelen, M.P.; Wouters, E.F.; Schols, A.M.; Deutz, N.E. Metabolic effects of glutamine and glutamate ingestion in healthy subjects and in persons with chronic obstructive pulmonary disease. Am. J. Clin. Nutr. 2006, 83, 115–123. [Google Scholar] [PubMed]
- Cutting, G.R. Cystic fibrosis genetics: From molecular understanding to clinical application. Nat. Rev. Genet. 2015, 16, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Pinet, C.; Cassart, M.; Scillia, P.; Lamotte, M.; Knoop, C.; Casimir, G.; Mélot, C.; Estenne, M. Function and bulk of respiratory and limb muscles in patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2003, 168, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Hayes, V.; Schaeffer, D.; Mauras, N.; Punati, J.; Darmaun, D. Can glutamine and growth hormone promote protein anabolism in children with cystic fibrosis? Horm. Res. Paediatr. 2002, 58 (Suppl. S1), 21–23. [Google Scholar] [CrossRef]
- Darmaun, D.; Hayes, V.; Schaeffer, D.; Welch, S.; Mauras, N. Effects of glutamine and recombinant human growth hormone on protein metabolism in prepubertal children with cystic fibrosis. J. Clin. Endocrinol. Metab. 2004, 89, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Center, M.M.; DeSantis, C.; Ward, E.M. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1893–1907. [Google Scholar] [CrossRef] [PubMed]
- Antoniu, S.A.; Mihaescu, T. Hospitalizations and mortality in the Lung Health Study. Expert Rev. Pharmacoecon. Outcomes Res. 2002, 2, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.L.; Wasa, M.; Souba, W.W.; Abcouwer, S.F. Regulation of glutamine synthetase in human breast carcinoma cells and experimental tumors. Surgery 1997, 122, 451–464. [Google Scholar] [CrossRef]
- Weinberg, F.; Hamanaka, R.; Wheaton, W.W.; Weinberg, S.; Joseph, J.; Lopez, M.; Kalyanaraman, B.; Mutlu, G.M.M.; Budinger, G.R.; Chandel, N.S. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl. Acad. Sci. USA 2010, 107, 8788–8793. [Google Scholar] [CrossRef] [PubMed]
- Wise, D.R.; Ward, P.S.; Shay, J.E.; Cross, J.R.; Gruber, J.J.; Sachdeva, U.M.; Platt, J.M.; DeMatteo, R.G.; Simon, M.C.; Thompson, C.B. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of α-ketoglutarate to citrate to support cell growth and viability. Proc. Natl. Acad. Sci. USA 2011, 108, 19611–19616. [Google Scholar] [CrossRef] [PubMed]
- Klimberg, V.S.; Souba, W.W.; Salloum, R.M.; Plumley, D.A.; Cohen, F.S.; Dolson, D.J.; Bland, K.I.; Copeland, E.M. Glutamine-enriched diets support muscle glutamine metabolism without stimulating tumor growth. J. Surg. Res. 1990, 48, 319–323. [Google Scholar] [CrossRef]
- Savarese, D.M.; Savy, G.; Vahdat, L.; Wischmeyer, P.E.; Corey, B. Prevention of chemotherapy and radiation toxicity with glutamine. Cancer Treat. Rev. 2003, 29, 501–513. [Google Scholar] [CrossRef]
- Topkan, E.; Parlak, C.; Topuk, S.; Pehlivan, B. Influence of oral glutamine supplementation on survival outcomes of patients treated with concurrent chemoradiotherapy for locally advanced non-small cell lung cancer. BMC Cancer 2012, 12. [Google Scholar] [CrossRef] [PubMed]
- Tutanc, O.D.; Aydogan, A.; Akkucuk, S.; Sunbul, A.T.; Zincircioglu, S.B.; Alpagat, G.; Erden, E.S. The efficacy of oral glutamine in prevention of acute radiotherapy-induced esophagitis in patients with lung cancer. Contemp. Oncol. 2013, 17, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Déchelotte, P.; Hasselmann, M.; Cynober, L.; Allaouchiche, B.; Coëffier, M.; Hecketsweiler, B.; Merle, V.; Mazerolles, M.; Samba, D.; Guillou, Y.M.; et al. l-alanyl-l-glutamine dipeptide-supplemented total parenteral nutrition reduces infectious complications and glucose intolerance in critically ill patients: The French controlled, randomized, double-blind, multicenter study. Crit. Care Med. 2006, 34, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Estívariz, C.F.F.; Griffith, D.P.; Luo, M.; Szeszycki, E.E.; Bazargan, N.; Dave, N.; Daignault, N.M.; Bergman, G.F.; McNally, T.; Battey, C.H.; et al. Efficacy of parenteral nutrition supplemented with glutamine dipeptide to decrease hospital infections in critically ill surgical patients. J. Parenter. Enter. Nutr. 2008, 32, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, A. The effect of intravenous alanyl-glutamine supplementation on plasma glutathione levels in intensive care unit trauma patients receiving enteral nutrition: The results of a randomized controlled trial. Anesth. Analg. 2009, 109, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bárcena, J.; Crespí, C.; Regueiro, V.; Marsé, P.; Raurich, J.M.; Ibáñez, J.; García de Lorenzo-Mateos, A.; Bengoechea, J.A.A. Lack of effect of glutamine administration to boost the innate immune system response in trauma patients in the intensive care unit. Crit. Care 2010, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, T.R.; May, A.K.; Hebbar, G.; Easley, K.A.; Griffith, D.P.; Dave, N.; Collier, B.R.; Cotsonis, G.A.; Hao, L.; Leong, T.; et al. Efficacy and Safety of Glutamine-supplemented Parenteral Nutrition in Surgical ICU Patients: An American Multicenter Randomized Controlled Trial. Ann. Surg. 2015. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, G.P.; De Abreu, M.G.; Pelosi, P.; Rocco, P.R.M. Exogenous Glutamine in Respiratory Diseases: Myth or Reality? Nutrients 2016, 8, 76. https://doi.org/10.3390/nu8020076
Oliveira GP, De Abreu MG, Pelosi P, Rocco PRM. Exogenous Glutamine in Respiratory Diseases: Myth or Reality? Nutrients. 2016; 8(2):76. https://doi.org/10.3390/nu8020076
Chicago/Turabian StyleOliveira, Gisele P., Marcelo Gama De Abreu, Paolo Pelosi, and Patricia R. M. Rocco. 2016. "Exogenous Glutamine in Respiratory Diseases: Myth or Reality?" Nutrients 8, no. 2: 76. https://doi.org/10.3390/nu8020076
APA StyleOliveira, G. P., De Abreu, M. G., Pelosi, P., & Rocco, P. R. M. (2016). Exogenous Glutamine in Respiratory Diseases: Myth or Reality? Nutrients, 8(2), 76. https://doi.org/10.3390/nu8020076





