Extract of Polygonum cuspidatum Attenuates Diabetic Retinopathy by Inhibiting the High-Mobility Group Box-1 (HMGB1) Signaling Pathway in Streptozotocin-Induced Diabetic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Plant Material and Preparation of PCE
2.3. HPLC Analysis of PCE
2.4. Induction of Diabetic Animals and Experimental Design
2.5. Measurement of Retinal Vascular Permeability
2.6. Immunohistochemical and Immunofluorescence Staining
2.7. Western Blot Analysis
2.8. Measurement of NF-κB Activity
2.9. Chromatin Immunoprecipitation Assay
2.10. Enzymatic Linked Immunosorbent Assay
2.11. Statistical Analysis
3. Results
3.1. Analysis of PCE by HPLC
3.2. Blood Glucose Levels and Body Weight
3.3. Effect of PCE on HMGB1 Expression in the Retina
3.4. Effect of PCE on RAGE Expression in the Retina
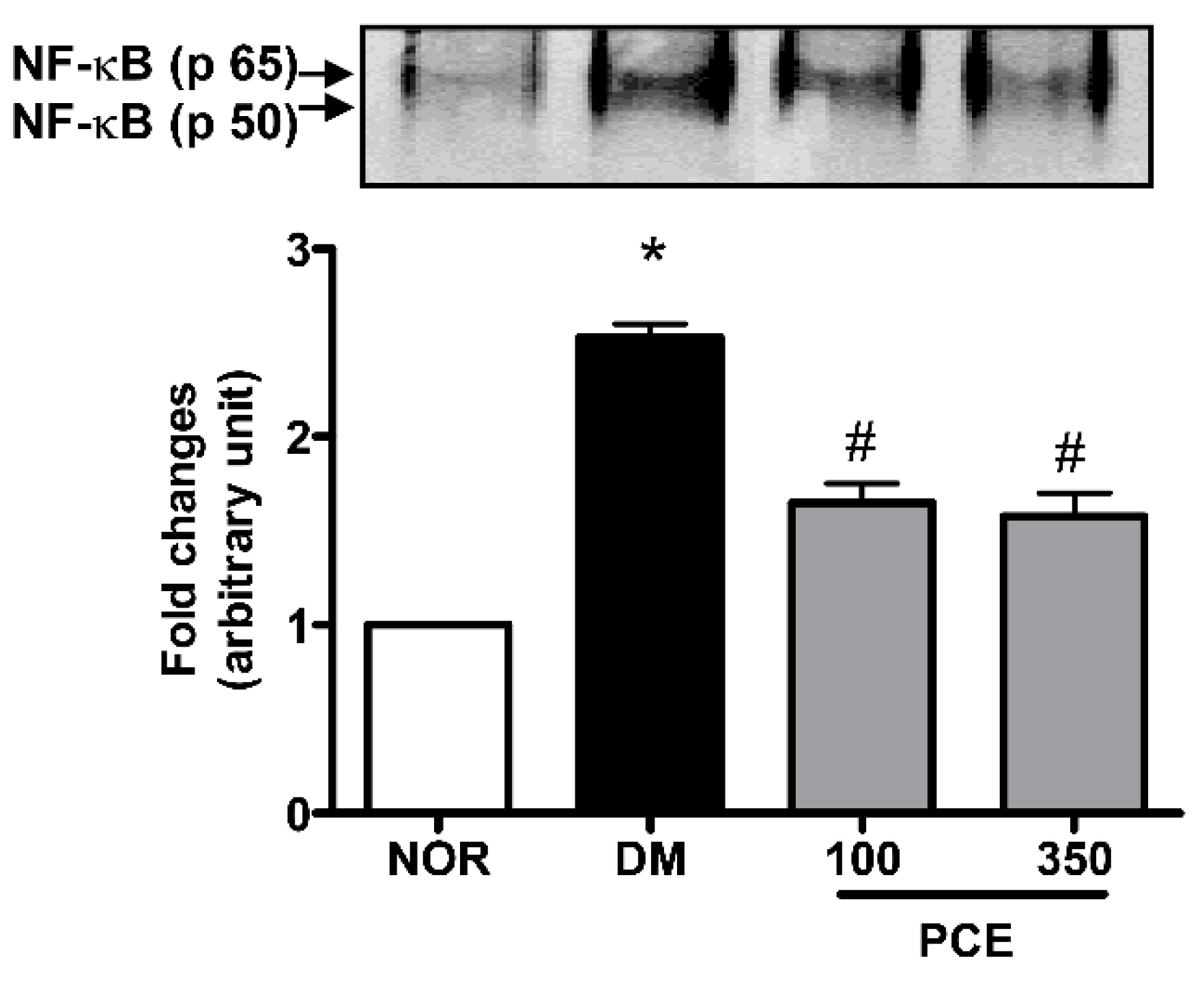
3.5. Effect of PCE on the Activation of NF-κB in the Retina
3.6. Effect of PCE on the Activation of NF-κB in RAGE mRNA Expression
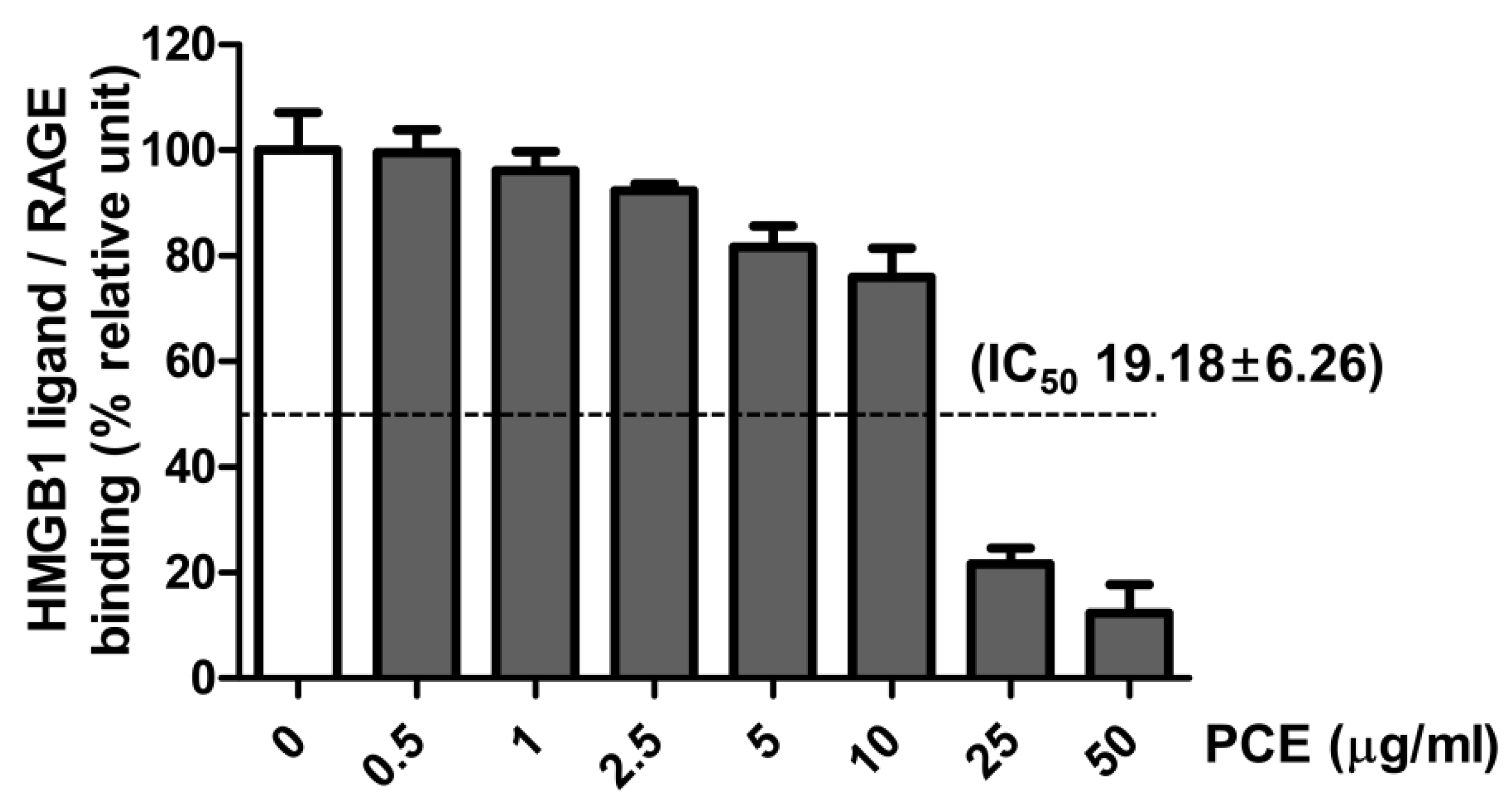
3.7. Effect of PCE on HMGB1 Ligand to Receptor Binding
3.8. Effect of PCE on Diabetes-Induced Vascular Hyperpermeability
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Adamis, A.P. Is diabetic retinopathy an inflammatory disease? Br. J. Ophthalmol. 2002, 86, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.R.; Dvoriantchikova, G.; Li, Y.; Mohammad, G.; Abu El-Asrar, A.M.; Wen, R.; Ivanov, D. Cellular mechanisms of high mobility group 1 (HMGB-1) protein action in the diabetic retinopathy. PLoS ONE 2014, 9, e87574. [Google Scholar] [CrossRef] [PubMed]
- Voll, R.E.; Urbonaviciute, V.; Herrmann, M.; Kalden, J.R. High mobility group box 1 in the pathogenesis of inflammatory and autoimmune diseases. Isr. Med. Assoc. J. 2008, 10, 26–28. [Google Scholar] [PubMed]
- Lee, Y.M.; Kim, J.; Jo, K.; Shin, S.D.; Kim, C.S.; Sohn, E.J.; Kim, S.G.; Kim, J.S. Ethyl pyruvate inhibits retinal pathogenic neovascularization by downregulating HMGB1 expression. J. Diabetes Res. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, G.; Siddiquei, M.M.; Othman, A.; Al-Shabrawey, M.; Abu El-Asrar, A.M. High-mobility group box-1 protein activates inflammatory signaling pathway components and disrupts retinal vascular-barrier in the diabetic retina. Exp. Eye Res. 2013, 107, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Luan, Z.G.; Zhang, H.; Yang, P.T.; Ma, X.C.; Zhang, C.; Guo, R.X. HMGB1 activates nuclear factor-kappaB signaling by RAGE and increases the production of TNF-alpha in human umbilical vein endothelial cells. Immunobiology 2010, 215, 956–962. [Google Scholar] [CrossRef] [PubMed]
- El-Asrar, A.M.; Nawaz, M.I.; Kangave, D.; Geboes, K.; Ola, M.S. High-mobility group box-1 and biomarkers of inflammation in the vitreous from patients with proliferative diabetic retinopathy. Mol. Vis. 2011, 17, 1829–1838. [Google Scholar] [PubMed]
- Kim, J.; Sohn, E.; Kim, C.S.; Jo, K.; Kim, J.S. The role of high-mobility group box-1 protein in the development of diabetic nephropathy. Am. J. Nephrol. 2011, 33, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.O.; Lee, J.H.; Ko, N.Y.; Mun, S.H.; Kim, J.W.; Kim do, K.; Kim, J.D.; Kim, B.K.; Kim, H.S.; Her, E.; et al. Polygoni cuspidati radix inhibits the activation of Syk kinase in mast cells for antiallergic activity. Exp. Biol. Med. 2007, 232, 1425–1431. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Qin, R.; Li, X.; Zhou, H. Botany, phytochemistry, pharmacology, and potential application of Polygonum cuspidatum Sieb.et Zucc.: A review. J. Ethnopharmacol. 2013, 148, 729–745. [Google Scholar] [CrossRef] [PubMed]
- Kirino, A.; Takasuka, Y.; Nidhi, A.; Kawabe, S.; Yamashita, H.; Kimoto, M.; Ito, H.; Tsuji, H. Analysis and functionality of major polypenolic components of Polugonum cuspidatum (Itadory). J. Nutr. Sci. Vitaminol. 2012, 58, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Bob, F.; Philippe, S. The treatment of modern medical diseases with Chinese Medicine. In Text Book and Clinal Manual; Flaws, B., Sionneau, P., Eds.; Blue Poppy: Colorado, CO, USA, 1897. [Google Scholar]
- Han, J.H.; Koh, W.; Lee, H.J.; Lee, E.O.; Lee, S.J.; Khilc, J.H.; Tae, K.J.; Jeonga, S.J.; Kim, S.H. Analgesic and anti-inflammatory effects of ethyl acetate fraction of Polygonum cuspidatum in experimental animals. Immunopharmacol. Immunotoxicol. 2012, 34, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yu, C.H.; Jiang, Y.P.; Peng, C.; He, K.; Tang, J.Y.; Xin, H.L. Protective effects of polydatin from Polygonum cuspidatum against carbon tetrachloride-induced liver injury in mice. PLoS ONE 2012, 7, e46574. [Google Scholar] [CrossRef] [PubMed]
- Fei, H.H.; Xiao, Z.D.; Gao, W. Effects of giant knotweed rhizome medicine on the expressions of RAGE and VEGF of rats with diabetic nephropathy. J. Shandong Univ. Health Sci. 2008, 46, 43–47. [Google Scholar]
- Zhang, W.; Liu, H.; Rojas, M.; Caldwell, R.W.; Caldwell, R.B. Anti-inflammatory therapy for diabetic retinopathy. Immunotherapy 2011, 3, 609–628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, Q.; Wu, J.; Zhou, X.; Weng, J.; Xu, J.; Wang, W.; Huang, Q.; Guo, X. Role of Src in vascular hyperpermeability induced by advanced glycation end products. Sci. Rep. 2015, 18. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Xu, Z.; Gui, Q.; Wu, W.; Yang, Y. Gliquidone versus metformin: Differential effects on aorta in streptozotocin induced diabetic rats. Chin. Med. J. 2014, 127, 1298–1303. [Google Scholar] [PubMed]
- Liu, W.J.; Xie, S.H.; Liu, Y.N.; Kim, W.; Jin, H.Y.; Park, S.K.; Shao, Y.M.; Park, T.S. Dipeptidyl peptidase IV inhibitor attenuates kidney injury in streptozotocin-induced diabetic rats. J. Pharmacol. Exp. Ther. 2012, 340, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.; Marques, C.; Leal, E.; Ribeiro, C.F.; Reis, F.; Ambrósio, A.F.; Fernandes, R. Dipeptidyl peptidase-IV inhibition prevents blood-retinal barrier breakdown, inflammation and neuronal cell death in the retina of type 1 diabetic rats. Biochim. Biophys. Acta 2014, 1842, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.P.; Coppey, L.J.; Dake, B.; Yorek, M.A. Treatment of streptozotocin-induced diabetic rats with alogliptin: Effect on vascular and neural complications. Exp. Diabetes Res. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Karuppagounder, V.; Arumugam, S.; Thandavarayan, R.A.; Pitchaimani, V.; Sreedhar, R.; Afrin, R.; Harima, M.; Suzuki, H.; Nomoto, M.; Miyashita, S.; et al. Resveratrol attenuates HMGB1 signaling and inflammation in house dust mite-induced atopic dermatitis in mice. Int. Immunopharmacol. 2014, 23, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Manigrasso, M.B.; Juranek, J.; Ramasamy, R.; Schmidt, A.M. Unlocking the biology of RAGE in diabetic microvascular complications. Trends Endocrinol. Metab. 2014, 25, 15–22. [Google Scholar] [CrossRef] [PubMed]
- McVicar, C.M.; Ward, M.; Colhoun, L.M.; Guduric-Fuchs, J.; Bierhaus, A.; Fleming, T.; Schlotterer, A.; Kolibabka, M.; Hammes, H.P.; Chen, M.; et al. Role of the receptor for advanced glycation endproducts (RAGE) in retinal vasodegenerative pathology during diabetes in mice. Diabetologia 2015, 58, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Curtis, T.M.; Stitt, A.W. Advanced glycation end products and diabetic retinopathy. Curr. Med. Chem. 2013, 20, 3234–3240. [Google Scholar] [CrossRef] [PubMed]
- Zong, H.; Ward, M.; Stitt, A.W. AGEs, RAGE, and diabetic retinopathy. Curr. Diabetes Rep. 2011, 11, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Navaratna, D.; McGuire, P.G.; Menicucci, G.; Das, A. Proteolytic degradation of VE-cadherin alters the blood-retinal barrier in diabetes. Diabetes 2007, 56, 2380–2387. [Google Scholar] [CrossRef] [PubMed]
- Nitta, T.; Hata, M.; Gotoh, S.; Seo, Y.; Sasaki, H.; Hashimoto, N.; Furuse, M.; Tsukita, S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J. Cell Biol. 2003, 161, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Leal, E.C.; Martins, J.; Voabil, P.; Liberal, J.; Chiavaroli, C.; Bauer, J.; Cunha-Vaz, J.; Ambrósio, A.F. Calcium dobesilate inhibits the alterations in tight junction proteins and leukocyte adhesion to retinal endothelial cells induced by diabetes. Diabetes 2010, 59, 2637–2645. [Google Scholar] [CrossRef] [PubMed]
- Bralley, E.E.; Greenspan, P.; Hargrove, J.L.; Wicker, L.; Hartle, D.K. Topical anti-inflammatory activity of Polygonum cuspidatum extract in the TPA model of mouse ear inflammation. J. Inflamm. 2008, 5. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Lee, Y.M.; Kim, J.H.; Kim, J.S. Polygonum cuspidatum inhibits pancreatic lipase activity and adipogenesis via attenuation of lipid accumulation. BMC Complement. Altern. Med. 2013, 13, 282. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Choi, T.W.; Kim, C.; Nam, D.; Lee, S.G.; Jang, H.J.; Lee, J.H.; Um, J.Y.; Jung, S.H.; Shim, B.S.; et al. Anti-inflammatory activities of Reynoutria elliptica through suppression of mitogen-activated protein kinases and nuclear factor-kappaB activation pathways. Immunopharmacol. Immunotoxicol. 2013, 34, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wang, Y.; Cui, W.; Yuan, H.; Sun, J.; Wu, M.; Guo, Q.; Kong, L.; Wu, H.; Miao, L. Resveratrol prevention of diabetic nephropathy is associated with the suppression of renal inflammation and mesangial cell proliferation: Possible roles of Akt/NF-kappab pathway. Int. J. Endocrinol. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhu, S.; Chang, S.; Cao, Y.; Dong, J.; Li, J.; Long, R.; Zhou, Y. Protective effects of chronic resveratrol treatment on vascular inflammatory injury in streptozotocin-induced type 2 diabetic rats: Role of NF-kappa B signaling. Eur. J. Pharmacol. 2013, 720, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lan, Z.; Lin, Q.; Mi, X.; He, Y.; Wei, L.; Lin, Y.; Zhang, Y.; Deng, X. Polydatin ameliorates renal injury by attenuating oxidative stress-related inflammatory responses in fructose-induced urate nephropathic mice. Food Chem. Toxicol. 2013, 52, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Peng, J.; Huang, K.; Huang, J.; Shen, X.; Liu, P.; Huang, H. Polydatin ameliorates experimental diabetes-induced fibronectin through inhibiting the activation of NF-kappaB signaling pathway in rat glomerular mesangial cells. Mol. Cell. Endocrinol. 2012, 362, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Shrimali, D.; Shanmugam, M.K.; Kumar, A.P.; Zhang, J.; Tan, B.K.; Ahn, K.S.; Sethi, G. Targeted abrogation of diverse signal transduction cascades by emodin for the treatment of inflammatory disorders and cancer. Cancer Lett. 2013, 341, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Ku, S.K.; Kim, T.H.; Bae, J.S. Emodin-6-O-beta-d-glucoside inhibits HMGB1-induced inflammatory responses in vitro and in vivo. Food Chem. Toxicol. 2013, 52, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dhawan, S.; Aggarwal, B.B. Emodin (3-methyl-1,6,8-trihydroxyanthraquinone) inhibits TNF-induced NF-kappaB activation, IkappaB degradation, and expression of cell surface adhesion proteins in human vascular endothelial cells. Oncogene 1998, 17, 913–918. [Google Scholar] [CrossRef] [PubMed]





| Time | NOR | DM | PCE-100 | PCE-350 | |
|---|---|---|---|---|---|
| Non-fasting BG (mg/dL) | Initial | 145.7 ± 9.5 | 351.5 ± 101.4 * | 349.5 ± 90.2 | 347.0 ± 86.8 |
| End | 145.9 ± 19.1 | 592.8 ± 17.6 * | 520.4 ± 77.3 | 446.0 ± 132.0 | |
| Fasting BG (mg/dL) | Initial | 77.8 ± 8.4 | 292.3 ± 76.9 * | 288.6 ± 74.7 | 288.5 ± 74.8 |
| End | 162.1 ± 11.0 | 587.3 ± 43.5 * | 530.3 ± 58.1 | 474.2 ± 88.4 | |
| Body weight (g) | Initial | 307.3 ± 17.5 | 226.6 ± 18.7 * | 224.2 ± 20.6 | 219.7 ± 16.2 |
| End | 475.9 ± 10.7 | 208.5 ± 12.1 * | 205.5 ± 23.1 | 206.0 ± 16.4 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sohn, E.; Kim, J.; Kim, C.-S.; Lee, Y.M.; Kim, J.S. Extract of Polygonum cuspidatum Attenuates Diabetic Retinopathy by Inhibiting the High-Mobility Group Box-1 (HMGB1) Signaling Pathway in Streptozotocin-Induced Diabetic Rats. Nutrients 2016, 8, 140. https://doi.org/10.3390/nu8030140
Sohn E, Kim J, Kim C-S, Lee YM, Kim JS. Extract of Polygonum cuspidatum Attenuates Diabetic Retinopathy by Inhibiting the High-Mobility Group Box-1 (HMGB1) Signaling Pathway in Streptozotocin-Induced Diabetic Rats. Nutrients. 2016; 8(3):140. https://doi.org/10.3390/nu8030140
Chicago/Turabian StyleSohn, Eunjin, Junghyun Kim, Chan-Sik Kim, Yun Mi Lee, and Jin Sook Kim. 2016. "Extract of Polygonum cuspidatum Attenuates Diabetic Retinopathy by Inhibiting the High-Mobility Group Box-1 (HMGB1) Signaling Pathway in Streptozotocin-Induced Diabetic Rats" Nutrients 8, no. 3: 140. https://doi.org/10.3390/nu8030140
APA StyleSohn, E., Kim, J., Kim, C.-S., Lee, Y. M., & Kim, J. S. (2016). Extract of Polygonum cuspidatum Attenuates Diabetic Retinopathy by Inhibiting the High-Mobility Group Box-1 (HMGB1) Signaling Pathway in Streptozotocin-Induced Diabetic Rats. Nutrients, 8(3), 140. https://doi.org/10.3390/nu8030140





