Quercetin, Inflammation and Immunity
Abstract
:1. Introduction
2. Physicochemical Properties of Quercetin
3. Dietary Sources of Quercetin
4. Absorption, Bioavailability and Metabolism of Quercetin
4.1. Absorption
4.2. Transformation and Transportation
4.3. Excretion
5. Effect of Quercetin on Inflammation and Immune Function
5.1. In Vitro
5.1.1. Anti-Inflammation and Promotion of Immunity
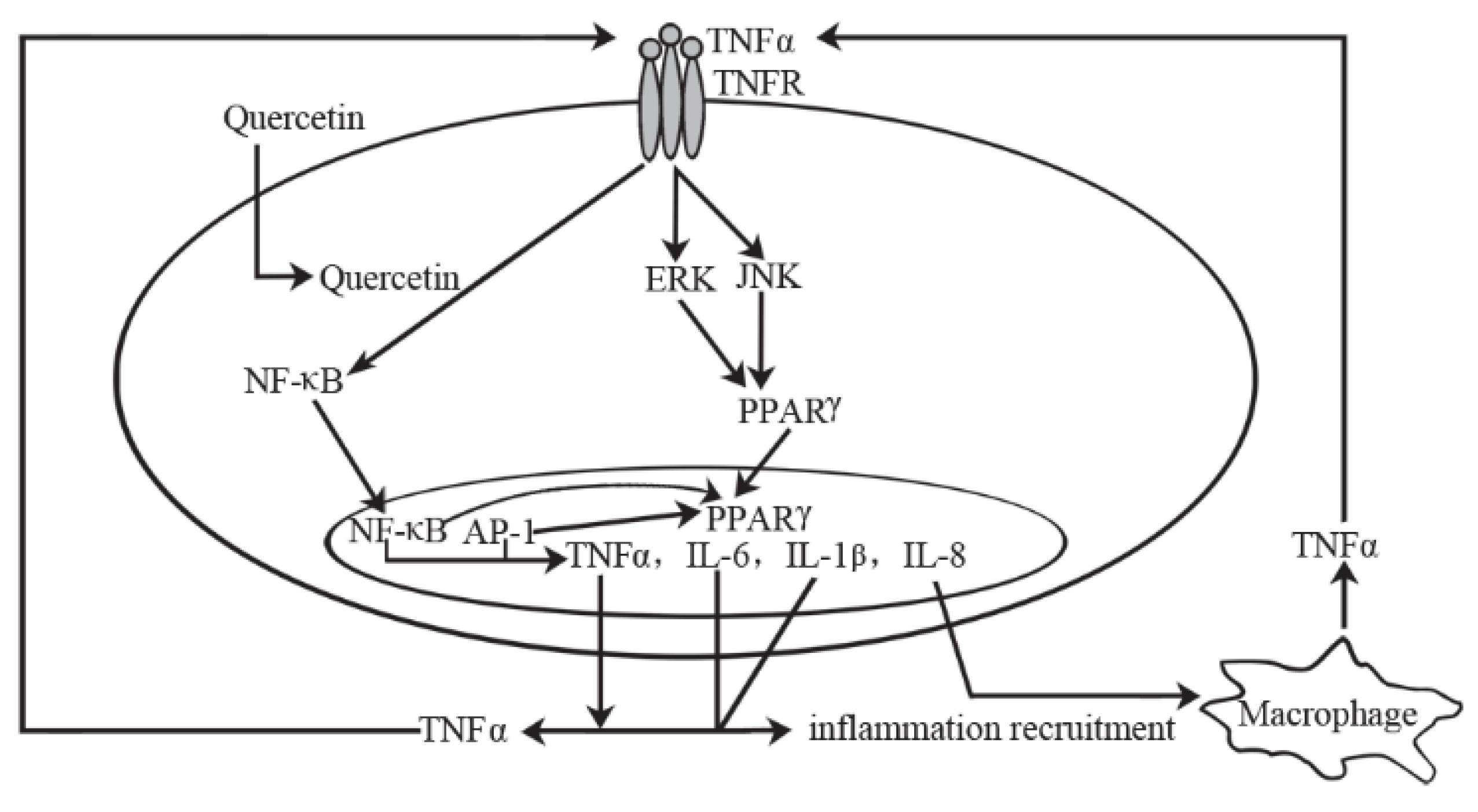
5.1.2. Mechanism of Action
5.2. In Vivo
5.2.1. Animal Models
5.2.2. Mechanism of Action in Animal
5.2.3. Clinical Studies
6. Summary
Acknowledgments
Conflicts of Interest
References
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D. Effects of the dietary flavonoid quercetin upon performance and health. Curr. Sports Med. Rep. 2009, 8, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, L.; Arias, N.; Macarulla, M.T.; Gracia, A.; Portillo, M.P. Beneficial effects of quercetin on obesity and diabetes. Open Nutraceuticals J. 2011, 4, 189–198. [Google Scholar]
- Fischer, C.; Speth, V.; Fleig-Eberenz, S.; Neuhaus, G. Induction of zygotic polyembryos in wheat: Influence of Auxin Polar Transport. Plant Cell 1997, 9, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.; Bijsman, M.N.; van Gameren, Y.; Cnossen, E.P.; de Vries, J.H.; Katan, M.B. The sugar moiety is a major determinant of the absorption of dietary flavonoid glycosides in man. Free Radic. Res. 1999, 31, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, S.H.; Kärenlampi, S.O.; Heinonen, I.M.; Mykkänen, H.M.; Törrönen, A.R. Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J. Agric. Food Chem. 1999, 47, 2274–2279. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81 (Suppl. S1), 243S–255S. [Google Scholar] [PubMed]
- Wiczkowski, W.; Romaszko, J.; Bucinski, A.; Szawara-Nowak, D.; Honke, J.; Zielinski, H.; Piskula, M.K. Quercetin from shallots (Allium cepa L. var. aggregatum) is more bioavailable than its glucosides. J. Nutr. 2008, 138, 885–888. [Google Scholar] [PubMed]
- Smith, C.; Lombard, K.A.; Peffley, E.B.; Liu, W. Genetic analysis of quercetin in onion (Allium cepa L.) Lady Raider. Tex. J. Agric. Natl. Resour. 2003, 16, 24–28. [Google Scholar]
- Mitchell, A.E.; Hong, Y.J.; Koh, E.; Barrett, D.M.; Bryant, D.E.; Denison, R.F.; Kaffka, S. Ten-year comparison of the influence of organic and conventional crop management practices on the content of flavonoids in tomatoes. J. Agric. Food Chem. 2007, 55, 6154–6159. [Google Scholar] [CrossRef] [PubMed]
- Petrus, K.; Schwartz, H.; Sontag, G. Analysis of flavonoids in honey by HPLC coupled with coulometric electrode array detection and electrospray ionization mass spectrometry. Anal. Bioanal. Chem. 2011, 400, 2555–2563. [Google Scholar] [CrossRef] [PubMed]
- Tutelian, V.A.; Lashneva, N.V. Biologically active substances of plant origin. Flavonols and flavones: Prevalence, dietary sources and consumption. Vopr. Pitan. 2013, 82, 4–22. [Google Scholar]
- Bhagwat, S.; Haytowits, D.B.; Holden, J.M. USDA Database for the Flavonoid Content of Selected Foods, Release 3; U.S. Department of Agriculture: Beltsville, MD, USA, 2011.
- Chun, O.K.; Chung, S.J.; Song, W.O. Estimated dietary flavonoid intake and major food sources of U.S. adults. J. Nutr. 2007, 137, 1244–1252. [Google Scholar] [PubMed]
- Sun, C.; Wang, H.; Wang, D.; Chen, Y.; Zhao, Y.; Xia, W. Using an FFQ to assess intakes of dietary flavonols and flavones among female adolescents in the Suihua area of northern China. Public Health Nutr. 2015, 18, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Cao, C.; Cao, J.; Chen, W.; Zhang, Y.; Wang, C.; Wang, J.; Zhang, X.; Zhao, X. Dietary flavonol and flavone intakes and their major food sources in Chinese adults. Nutr. Cancer 2010, 62, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Sampson, L.; Rimm, E.; Hollman, P.C.; de Vries, J.H.; Katan, M.B. Flavonol and flavone intakes in US health professionals. J. Am. Diet. Assoc. 2002, 102, 1414–1420. [Google Scholar] [CrossRef]
- Nishimuro, H.; Ohnishi, H.; Sato, M.; Ohnishi-Kameyama, M.; Matsunaga, I.; Naito, S.; Ippoushi, K.; Oike, H.; Nagata, T.; Akasaka, H.; et al. Estimated daily intake and seasonal food sources of quercetin in Japan. Nutrients 2015, 7, 2345–2358. [Google Scholar] [CrossRef] [PubMed]
- Somerset, S.M.; Johannot, L. Dietary flavonoid sources in Australian adults. Nutr. Cancer 2008, 60, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Andres-Lacueva, C.; Lamuela-Raventos, R.M.; Berenguer, T.; Jakszyn, P.; Barricarte, A.; Ardanaz, E.; Amiano, P.; Dorronsoro, M.; Larrañaga, N.; et al. Estimation of dietary sources and flavonoid intake in a Spanish adult population (EPIC-Spain). J. Am. Diet. Assoc. 2010, 110, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Scholz, S.; Williamson, G. Interactions affecting the bioavailability of dietary polyphenols in vivo. Int. J. Vitam. Nutr. Res. 2007, 77, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Ader, P.; Wessmann, A.; Wolffram, S. Bioavailability and metabolism of the flavonol quercetin in the pig. Free Radic. Biol. Med. 2000, 28, 1056–1067. [Google Scholar] [CrossRef]
- Guo, Y.; Mah, E.; Davis, C.G.; Jalili, T.; Ferruzzi, M.G.; Chun, O.K.; Bruno, R.S. Dietary fat increases quercetin bioavailability in overweight adults. Mol. Nutr. Food Res. 2013, 57, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Crespy, V.; Morand, C.; Manach, C. Part of quercetin absorbed in the small intestine is conjugated and further secreted in the intestinal l: Umen. Am. J. Physiol. 1999, 277, G120–G126. [Google Scholar] [PubMed]
- Manach, C.; Morand, C.; Texier, O.; Favier, M.L.; Agullo, G.; Demigné, C.; Régérat, F.; Rémésy, C. Quercetin metabolites in plasma of rats fed diets containing rutin or quercetin. J. Nutr. 1995, 125, 1911–1922. [Google Scholar] [PubMed]
- Hollman, P.C.; Katan, M.B. Absorption, metabolism and bioavailability of flavonoids. In Flavonoids in Health and Disease; Rice-Evans, C.A., Packer, L., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1998; pp. 483–522. [Google Scholar]
- Day, A.J.; Bao, Y.; Morgan, M.R.; Williamson, G. Conjugation position of quercetin glucuronides and effect on biological activity. Free Radic. Biol. Med. 2000, 29, 1234–1243. [Google Scholar] [CrossRef]
- De Boer, V.C.; Dihal, A.A.; van der Woude, H.; Arts, L.C.; Wolffram, S.; Alink, G.M.; Rietjens, I.M.; Keijer, J.; Hollman, P.C. Tissue distribution of quercetin in rats and pigs. J. Nutr. 2005, 135, 1718–1725. [Google Scholar] [PubMed]
- Kim, D.H.; Kim, S.Y.; Park, S.Y.; Han, M.J. Metabolism of quercetin by human intestinal bacteria and its relation to some biological activities. Biol. Pharm. Bull. 1999, 22, 749–751. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Texier, O.; Morand, C.; Crespy, V.; Régérat, F.; Demigné, C.; Rémésy, C. Comparison of the bioavailability of quercetin and catechin in rats. Free Radic. Biol. Med. 1999, 27, 1259–1266. [Google Scholar] [CrossRef]
- Oliveira, E.J.; Watson, D.G. In vitro glucuronidation of kaempferol and quercetin by human UGT-1A9 microsomes. FEBS Lett. 2000, 471, 1–6. [Google Scholar] [CrossRef]
- Koli, R.; Erlund, I.; Jula, A.; Marniemi, J.; Mattila, P.; Alfthan, G. Bioavailability of various polyphenols from a diet containing moderate amounts of berries. J. Agric. Food Chem. 2010, 58, 3927–3932. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.A.; Edwards, C.A.; Lean, M.E.; Crozier, A. Absorption and excretion of conjugated flavonols, including quercetin-4′-O-beta-glucoside and isorhamnetin-4′-O-beta-glucoside by human volunteers after the consumption of onions. Free Radic. Res. 1998, 29, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Morand, C.; Crespy, V.; Manach, C.; Besson, C.; Demigné, C.; Rémésy, C. Plasma metabolites of quercetin and their antioxidant properties. Am. J. Physiol. 1998, 275, R212–R219. [Google Scholar] [PubMed]
- Boulton, D.W.; Walle, U.K.; Walle, T. Extensive binding of the bioflavonoid quercetin to human plasma proteins. J. Pharm. Pharmacol. 1998, 50, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Young, J.F.; Nielsen, S.E.; Haraldsdóttir, J.; Daneshvar, B.; Lauridsen, S.T.; Knuthsen, P.; Crozier, A.; Sandström, B.; Dragsted, L.O. Effect of fruit juice intake on urinary quercetin excretion and biomarkers of antioxidative status. Am. J. Clin. Nutr. 1999, 69, 87–94. [Google Scholar] [PubMed]
- Graefe, E.U.; Derendorf, H.; Veit, M. Pharmacokinetics and bioavailability of the flavonol quercetin in humans. Int. J. Clin. Pharmacol. Ther. 1999, 37, 219–233. [Google Scholar] [PubMed]
- Manach, C.; Mazur, A.; Scalbert, A. Polyphenols and prevention of cardiovascular diseases. Curr. Opin. Lipidol. 2005, 16, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Konrad, M.; Nieman, D.C. Evaluation of quercetin as a countermeasure to exercise-induced physiological stress. In Source Antioxidants in Sport Nutrition; Lamprecht, M., Ed.; CRC Press: Boca Raton, FL, USA, 2015; Chapter 10. [Google Scholar]
- Moon, Y.J.; Wang, L.; DiCenzo, R.; Morris, M.E. Quercetin pharmacokinetics in humans. Biopharm. Drug Dispos. 2008, 29, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Walle, U.K.; Halushka, P.V. Carbon dioxide is the major metabolite of quercetin in humans. J. Nutr. 2001, 131, 2648–2652. [Google Scholar] [PubMed]
- Harwood, M.; Danielewska-Nikiel, B.; Borzelleca, J.F.; Flamm, G.W.; Williams, G.M.; Lines, T.C. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem. Toxicol. 2007, 45, 2179–2205. [Google Scholar] [CrossRef] [PubMed]
- Read, M.A. Flavonoids: Naturally occurring anti-inflammatory agents. Am. J. Pathol. 1995, 147, 235–237. [Google Scholar] [PubMed]
- Orsolic, N.; Knezevic, A.H.; Sver, L.; Terzic, S.; Basic, I. Immunomodulatory and antimetastatic action of propolis and related polyphenolic compounds. J. Ethnopharmacol. 2004, 94, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Manjeet, K.R.; Ghosh, B. Quercetin inhibits LPS-induced nitric oxide and tumor necrosis factor-alpha production in murine macrophages. Int. J. Immunopharmocol. 1999, 21, 435–443. [Google Scholar]
- Gerates, L.; Moonen, H.J.J.; Brauers, K.; Wouters, E.F.M.; Bast, A.; Hageman, G.J. Dietary flavones and flavonols are inhibitor of poly (ADP-ribose) polymerase-1 in pulmonary epithelial cells. J. Nutr. 2007, 137, 2190–2195. [Google Scholar]
- Bureau, G.; Longpre, F.; Martinoli, M.G. Resveratrol and quercetin, two natural polyphenols, reduce apoptotic neuronal cell death induced by neuroinflammation. J. Neurosci. Res. 2008, 86, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.P.; Mani, I.; Iversen, L.; Ziboh, V.A. Effects of naturally-occurring flavonoids and bioflavonoids on epidermal cyclooxygenase and lipoxygenase from guinea-pigs. Prostaglandins Leukot. Essent. Fat. Acids 1998, 58, 17–24. [Google Scholar] [CrossRef]
- Lee, K.M.; Hwang, M.K.; Lee, D.E.; Lee, K.W.; Lee, H.J. Protective effect of quercetin against arsenite-induced COX-2 expression by targeting PI3K in rat liver epithelial cells. J. Agric. Food Chem. 2010, 58, 5815–5820. [Google Scholar] [CrossRef] [PubMed]
- Endale, M.; Park, S.C.; Kim, S.; Kim, S.H.; Yang, Y.; Cho, J.Y.; Rhee, M.H. Quercetin disrupts tyrosine-phosphorylated phosphatidylinositol 3-kinase and myeloid differentiation factor-88 association, and inhibits MAPK/AP-1 and IKK/NF-κB-induced inflammatory mediators production in RAW 264.7 cells. Immunobiology 2013, 218, 1452–1467. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Madhappan, B.; Christodoulou, S.; Boucher, W.; Cao, J.; Papadopoulou, N.; Cetrulo, C.L.; Theoharides, T.C. Flavonols inhibit proinflammatory mediator release, intracellular calcium ion levels and protein kinase C theta phosphorylation in human mast cells. Br. J. Pharmacol. 2005, 145, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Liu, X.; Liu, M.; Chi, H.; Liu, J.; Han, H. Protective effects of quercetin and taraxasterol against H2O2-induced human umbilical vein endothelial cell injury in vitro. Exp. Ther. Med. 2015, 10, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Chirumbolo, S. The role of quercetin, flavonols and flavones in modulating inflammatory cell function. Inflamm. Allergy Drug Targets 2010, 9, 263–285. [Google Scholar]
- Penissi, A.B.; Rudolph, M.I.; Piezzi, R.S. Role of mast cells in gastrointestinal mucosal defense. Biocell 2003, 27, 163–172. [Google Scholar] [PubMed]
- Huang, R.Y.; Yu, Y.L.; Cheng, W.C.; OuYang, C.N.; Fu, E.; Chu, C.L. Immunosuppressive effect of quercetin on dendritic cell activiation and function. J. Immunol. 2010, 184, 6815–6821. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.P.N.; Kandaswami, C.; Mahajan, S.; Chadha, K.C.; Chawda, R.; Nair, H.; Kumar, N.; Nair, R.E.; Schwartz, S.A. The flavonoid, quercetin, differentially regulates Th-1 (IFNg) and Th-2 (IL4) cytokine gene expression by normal peripheral blood mononuclear cells. Biochim. Biophys. Acta 2002, 1593, 29–36. [Google Scholar] [CrossRef]
- Lim, H.; Kim, H.P. Inhibition of mammalian collagenase, matrix metalloproteinase-1, by naturally-occurring flavonoids. Planta Med. 2007, 73, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Kandere-Grzybowska, K.; Kempuraj, D.; Cao, J.; Cetrulo, C.L.; Theoharides, T.C. Regulation of IL-1-induced selective IL-6 release from human mast cells and inhibition by quercetin. Br. J. Pharmacol. 2006, 148, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Muthian, G.; Bright, J.J. Quercetin, a flavonoid phytoestrogen, ameliorates experimental allergic encephalomyelitis by blocking IL-12 signaling through JAK-STAT pathway in T lymphocyte. J. Clin. Immunol. 2004, 24, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, K.; Nonaka, M.; Narahara, M.; Torii, I.; Kawaguchi, K.; Yoshikawa, T.; Kumazawa, Y.; Morikawa, S. Inhibitory effect of quercetin on carrageenan-induced inflammation in rats. Life Sci. 2003, 74, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Stewart, L.K.; Soileau, J.L.; Ribnicky, D.; Wang, Z.Q.; Raskin, I.; Poulev, A.; Majewski, M.; Cefalu, W.T.; Gettys, T.W. Quercetin transiently increases energy expenditure but persistently decreases circulating markers of inflammation in C57BL/6J mice fed a high-fat diet. Metabolism 2008, 57, S39–S46. [Google Scholar] [CrossRef] [PubMed]
- Rivera, L.; Morón, R.; Sánchez, M.; Zarzuelo, A.; Galisteo, M. Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese Zucker rats. Obesity (Silver Spring) 2008, 16, 2081–2087. [Google Scholar] [CrossRef] [PubMed]
- Mamani-Matsuda, M.; Kauss, T.; Al-Kharrat, A.; Rambert, J.; Fawaz, F.; Thiolat, D.; Moynet, D.; Coves, S.; Malvy, D.; Mossalayi, M.D. Therapeutic and preventive properties of quercetin in experimental arthritis correlate with decreased macrophage inflammatory mediators. Biochem. Pharmacol. 2006, 72, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Schültke, E.; Kendall, E.; Kamencic, H.; Ghong, Z.; Griebel, R.W.; Juurlink, B.H. Quercetin promotes functional recovery following acute spinal cord injury. J. Neurotrauma 2003, 20, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Schültke, E.; Kamencic, H.; Skihar, V.M.; Griebel, R.; Juurlink, B. Quercetin in an animal model of spinal cord compression injury: Correlation of treatment duration with recovery of motor function. Spinal Cord 2010, 48, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Kang, J.I.; Kim, H.S. Effect of quercetin on impaired immune function in mice exposed to irradiation. Nutr. Res. Pract. 2012, 6, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, L.; Lu, S.P. Evaluation of antioxidant and immunity activities of quercetin in isoproterenol-treated rats. Molecules 2012, 17, 4281–4291. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.S.; Wang, J.L.; Feng, D.Y.; Qin, H.Z.; Wen, H.; Yin, Z.M.; Gao, G.D.; Li, C. Protective effect of quercetin against oxidative stress and brain edema in an experimental rat model of subarachnoid hemorrhage. Int. J. Med. Sci. 2014, 11, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Milenković, M.; Arsenović-Ranin, N.; Stojić-Vukanić, Z.; Bufan, B.; Vučićević, D.; Jančić, I. Quercetin ameliorates experimental autoimmune myocarditis in rats. J. Pharm. Pharm. Sci. 2010, 13, 311–319. [Google Scholar] [PubMed]
- Kobori, M.; Takahashi, Y.; Sakurai, M.; Akimoto, Y.; Tsushida, T.; Oike, H.; Ippoushi, K. Quercetin suppresses immune cell accumulation and improves mitochondrial gene expression in adipose tissue of diet-induced obese mice. Mol. Nutr. Food Res. 2016, 60, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.A.; Khan, D.A.; Mahjabeen, W.; Papasian, C.J.; Qureshi, N. Suppression of nitric oxide production and cardiovascular risk factors in healthy seniors and hypercholesterolemic subjects by a combination of polyphenols and vitamins. J. Clin. Exp. Cardiol. 2012, S5, 8. [Google Scholar]
- Heinz, S.A.; Henson, D.A.; Austin, M.D.; Jin, F.; Nieman, D.C. Quercetin supplementation and upper respiratory tract infection: A randomized community clinical trial. Pharmacol. Res. 2010, 62, 237–242. [Google Scholar] [CrossRef]
- Heinz, S.A.; Henson, D.A.; Nieman, D.C.; Austin, M.D.; Jin, F. A 12-week supplementation with quercetin does not affect natural killer cell activity, granulocyte oxidative burst activity or granulocyte phagocytosis in female human subjects. Br. J. Nutr. 2010, 104, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Henson, D.A.; Gross, S.J.; Jenkins, D.P.; Davis, J.M.; Murphy, E.A.; Carmichael, M.D.; Dumke, C.L.; Utter, A.C.; McAnulty, S.R.; et al. Quercetin reduces illness but not immune perturbations after intensive exercise. Med. Sci. Sports Exerc. 2007, 39, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Henson, D.; Nieman, D.; Davis, J.M.; Dumke, C.; Gross, S.; Murphy, A.; Carmichael, M.; Jenkins, D.P.; Quindry, J.; McAnulty, S.; et al. Post-160-km race illness rates and decreases in granulocyte respiratory burst and salivary IgA output are not countered by quercetin ingestion. Int. J. Sports Med. 2008, 29, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Henson, D.A.; Maxwell, K.R.; Williams, A.S.; McAnulty, S.R.; Jin, F.; Shanely, R.A.; Lines, T.C. Effects of quercetin and EGCG on mitochondrial biogenesis and immunity. Med. Sci. Sports Exerc. 2009, 41, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Williams, A.S.; Shanely, R.A.; Jin, F.; McAnulty, S.R.; Triplett, N.T.; Austin, M.D.; Henson, D.A. Quercetin’s influence on exercise performance and muscle mitochondrial biogenesis. Med. Sci. Sports Exerc. 2010, 42, 338–345. [Google Scholar] [CrossRef] [PubMed]

| Dosage | Cell Lines | Effect | Mechanism | Reference |
|---|---|---|---|---|
| Cells from animals | ||||
| 100 μmol/L | Pulmonary Epithelial Cell (A549) | Anti-inflammation | PARP-1 inhibition and preservation of cellular NAD1 and energy production | [46] |
| 100 μmol/L | N9 microglial cells | Inhibition of TNFα and IL-1α; Reduce of apoptotic neuronal cell death induced by microglial activation | [47] | |
| 3 μmol/L | Gunea pig epithelial cells | Inhibition of both cyclooxygenase and lipoxygenase | [48] | |
| 15–30 μmol/L | Rat liver epithelial (RLE) cells | Inhibition of arsenite-induced COX-2 expression mainly by blocking the activation of the PI3K signaling pathway | [49] | |
| - | RAW 264.7 cells | Inhibition of Src- and Syk-mediated PI3K-(p85) tyrosine phosphorylation and subsequent TLR4/MyD88/PI3K complex formation that limits activation of downstream signaling pathways | [50] | |
| Cells from human | ||||
| 10 μmol/L | Human umbilical cord blood-derived cultured mast cells (hCBMCs) | Anti-allergic and anti-inflammation; Protective effects against cell injury; Gastrointestinal cytoprotective action | Inhibition of intracellular calcium influx and PKC theta signaling | [51] |
| 50 or 100 µg | T lymphocyte | Blockage of interleukin-12 signaling through JAK-STAT pathway | [52] | |
| - | Mast cell | Stabilization of mast cell and gastrointestinal cytoprotection via lactone stimulating mucus production, and inhibiting histamine and serotonin release from intestinal mast cells | ||
| 12.5–25.0 mmol/L | Human inflamed/UV-irradiated skin | Inhibition of MMP-1 and down-regulation of MMP-1 expression via an inhibition of the AP-1 activation | [54] | |
| 0–210 μmol/L | Human umbilical vein endothelial cells (HUVECs) | Downregulation of VCAM-1 and CD80 expression | [56] | |
| 0.5–50 mmol/L | Human normal peripheral blood mononuclear cells | Beneficial immuno-stimulatory effects | Induction of Th-1 derived cytokine, IFNgamma, and inhibition of Th-2 derived cytokine, IL-4 | [57] |
| 1–100 mmol/L | Human umbilical cord blood-derived cultured mast cells (hCBMCs) | Inhibition of IL-1-induced IL-6 secretion, p38 and PKC-theta phosphorylation | [58] | |
| ≥100 mmol/L or ≤50 mmol/L | Mouse endritic cells (mDCs) | Immunosuppression | Inhibition of DC activation; DC apoptosis; Downregulation of the cytokines and chemokines, disturbance of immunoregulation; Attenuation of LPS-induced DC maturation and limitation of immunostimulatory activity; downregulate of endocytosis and impairment of Ag loading; suppression of DC migration and disconnection of the induction of adaptive immune responses | [55] |
| Dosage | Subjects | Effect | Mechanism | Reference |
|---|---|---|---|---|
| Animals | ||||
| 10 mg/kg diet | Rat | Anti-inflammation | Modulation of prostanoid synthesis and cytokine production | [60] |
| 0.8% diet | C57BL/6J mouse | Increase of energy expenditure; Decrease of interferon-γ, interleukin-1α, and interleukin-4 | [61] | |
| 10 mg/kg of body weight | Zucker rat | Downregulation of visceral adipose tissue inducible nitric oxide synthase expression, increase of endothelial nitric oxide synthase expression | [62] | |
| 160 mg/kg body weight (oral administration) 60 mg/kg (intra-cutaneous injections) | Lewis rat | Inhibition of macrophage-derived cytokines and nitric oxide | [63] | |
| 10 and 40 mg/kg body weight | Mouse | Increase of cytokine (interleukin-1β and interleukin-6) secretion | [66] | |
| 5–100 micromoles /kg body weight (administered intraperitoneally) 25 µmol/kg | Wistar rat | Functional recovery of acute spinal cord injury and motor function | Decrease of secondary damage through iron chelation, No effect | [64,65] |
| 0.05% diet | C57BL/6J mouse | Suppression of the accumulation and activation of immune cells, Suppression of oxidative stress and NFκB activity | [70] | |
| 50, 100, 150 mg/kg body weight | Wistar rat | Amelioration of immunity function impairment induced by isoproterenol; Amelioration of brain damage and neuroprotection, experimental allergic encephalomyelitis, experimental autoimmune myocarditis | Increase of activity in aspartate transaminase, creatine kinase, nitric oxide, nitric oxide synthase, interleukin-10, interleukin-1, interleukin-8 and lactate dehydrogenase | [59] |
| 50 mg/kg | Sprague-Dawley (SD) rat | Increase of activity of endogenous antioxidant enzymes and inhibition of free radical generation | [67] | |
| 50 or 100 μg | SJL/J mice | Blockage of interleukin-12 signaling and Th1 differentiation | [68] | |
| 10 or 20 mg/kg (oral administration) | Dark Agouti rat | Interference of pro-inflammatory (TNF-α and IL-17) and/or anti-inflammatory (IL-10) cytokines production | [69] | |
| Human | ||||
| 50 and 100 mg/person | Elderly Human subject | Anti-inflammatory properties | Inhibition of proteasome (nitric oxide, C-reactive protein, γ-glutamyltransferase) activity | [71] |
| 500 and 1000 mg/day | Human subject | Reduction of upper respiratory tract infection and total sick days; Improvement in 12-min treadmill time trial performance | No effect | [72] |
| 1000 mg/day | Human in treadmill | No effect | [76] | |
| 500 and 1000 mg/day | Human subject | No effect on innate immune function or inflammation, illness rates | No effect | [73] |
| 1000 mg/day | Human cyclist | No effect | [74] | |
| 1000 mg/day | Human runner | No effect | [75] | |
| 1000 mg/day | Human cyclist | No effect | [77] | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. https://doi.org/10.3390/nu8030167
Li Y, Yao J, Han C, Yang J, Chaudhry MT, Wang S, Liu H, Yin Y. Quercetin, Inflammation and Immunity. Nutrients. 2016; 8(3):167. https://doi.org/10.3390/nu8030167
Chicago/Turabian StyleLi, Yao, Jiaying Yao, Chunyan Han, Jiaxin Yang, Maria Tabassum Chaudhry, Shengnan Wang, Hongnan Liu, and Yulong Yin. 2016. "Quercetin, Inflammation and Immunity" Nutrients 8, no. 3: 167. https://doi.org/10.3390/nu8030167
APA StyleLi, Y., Yao, J., Han, C., Yang, J., Chaudhry, M. T., Wang, S., Liu, H., & Yin, Y. (2016). Quercetin, Inflammation and Immunity. Nutrients, 8(3), 167. https://doi.org/10.3390/nu8030167




