Nitrogen Balance and Protein Requirements for Critically Ill Older Patients
Abstract
:1. Introduction
2. Nutritional Assessment of Older Patients
3. Determination of Protein Requirements in Clinical Practice
4. Protein Requirements for Older Adults
4.1. Requirements of Healthy Older Subjects
4.2. Hypocaloric, High Protein Nutrition Therapy for Critically Ill Older Patients with Obesity
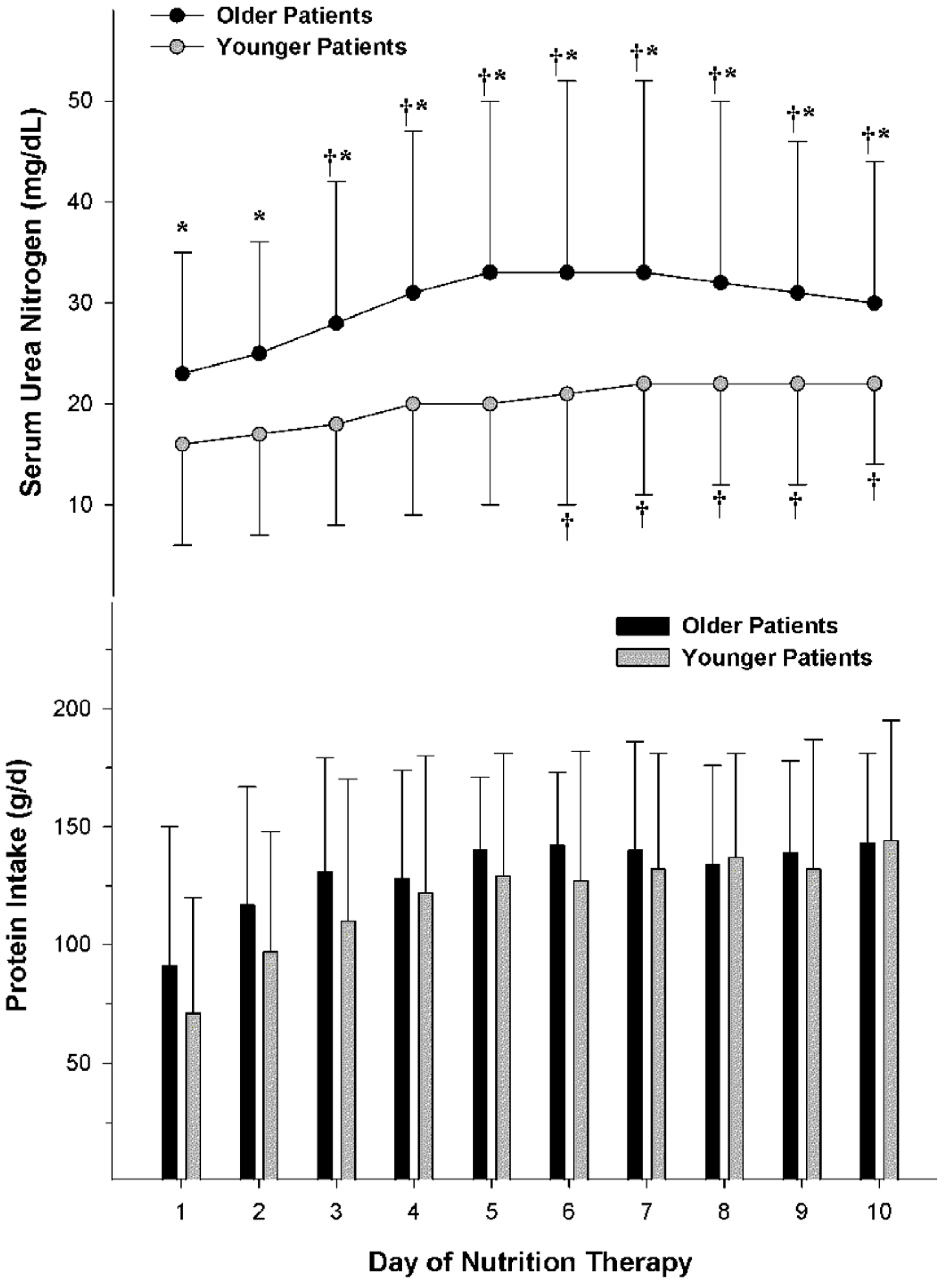
4.3. Comparative Nitrogen Accretion Response to Protein Intake in Older vs. Younger Non-Obese Patients with Severe Traumatic Injuries
5. Impact of Protein Intake upon Renal Function in Older Patient
5.1. Glomerular Filtration Rate, Creatinine Clearance and Renal Functional Reserve
5.2. Ureagenesis and Azotemia in Obese Patients during Hypocaloric High Protein Nutrition Therapy
5.3. Ureagenesis and Azotemia in Non-Obese Patients
6. Higher Protein Dosing without Overfeeding in Older Critically Ill Patients
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Dickerson, R.N.; Pitts, S.L.; Maish, G.O., 3rd; Schroeppel, T.J.; Magnotti, L.J.; Croce, M.A.; Minard, G.; Brown, R.O. A reappraisal of nitrogen requirements for patients with critical illness and trauma. J. Trauma Acute Care Surg. 2012, 73, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Monk, D.N.; Plank, L.D.; Franch-Arcas, G.; Finn, P.J.; Streat, S.J.; Hill, G.L. Sequential changes in the metabolic response in critically injured patients during the first 25 days after blunt trauma. Ann. Surg. 1996, 223, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Doig, G.S.; Heighes, P.T.; Simpson, F.; Sweetman, E.A. Early enteral nutrition reduces mortality in trauma patients requiring intensive care: A meta-analysis of randomised controlled trials. Injury 2011, 42, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Rapp, R.P.; Young, B.; Twyman, D.; Bivins, B.A.; Haack, D.; Tibbs, P.A.; Bean, J.R. The favorable effect of early parenteral feeding on survival in head-injured patients. J. Neurosurg. 1983, 58, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Sacks, G.S.; Brown, R.O.; Teague, D.; Dickerson, R.N.; Tolley, E.A.; Kudsk, K.A. Early nutrition support modifies immune function in patients sustaining severe head injury. JPEN J. Parenter. Enter. Nutr. 1995, 19, 387–392. [Google Scholar] [CrossRef]
- Hartl, R.; Gerber, L.M.; Ni, Q.; Ghajar, J. Effect of early nutrition on deaths due to severe traumatic brain injury. J. Neurosurg. 2008, 109, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Moisey, L.L.; Mourtzakis, M.; Cotton, B.A.; Premji, T.; Heyland, D.K.; Wade, C.E.; Bulger, E.; Kozar, R.A.; Nutrition and Rehabilitation Investigators Consortium (NUTRIC). Skeletal muscle predicts ventilator-free days, ICU-free days, and mortality in elderly ICU patients. Crit. Care 2013, 17, R206. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Karvellas, C.J.; Baracos, V.; Williams, D.C.; Khadaroo, R.G.; Acute Care and Emergency Surgery (ACES) Group. Sarcopenia is a predictor of outcomes in very elderly patients undergoing emergency surgery. Surgery. 2014, 156, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.R.; Miller, S.L.; Miller, K.B. Optimal protein intake in the elderly. Clin. Nutr. 2008, 27, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-Age study group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef] [PubMed]
- Flodin, L.; Svensson, S.; Cederholm, T. Body mass index as a predictor of 1 year mortality in geriatric patients. Clin. Nutr. 2000, 19, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.; Cai, J.; Pamuk, E.R.; Williamson, D.F.; Thun, M.J.; Wood, J.L. The effect of age on the association between body-mass index and mortality. N. Engl. J. Med. 1998, 338, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.E.; MacInnis, R.J.; Wattanapenpaiboon, N.; Nowson, C.A. BMI and all-cause mortality in older adults: A meta-analysis. Am. J. Clin. Nutr. 2014, 99, 875–890. [Google Scholar] [CrossRef] [PubMed]
- Al Snih, S.; Ottenbacher, K.J.; Markides, K.S.; Kuo, Y.F.; Eschbach, K.; Goodwin, J.S. The effect of obesity on disability vs. mortality in older Americans. Arch. Intern. Med. 2007, 167, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Potter, J.F.; Schafer, D.F.; Bohi, R.L. In-hospital mortality as a function of body mass index: An age-dependent variable. J. Gerontol. 1988, 43, M59–M63. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, K.L.; Volpi, E. Amino acid metabolism and regulatory effects in aging. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.T.; Plank, L.D.; Hill, G.L. Prolonged overexpansion of extracellular water in elderly patients with sepsis. Arch. Surg. 1998, 133, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Luckey, A.E.; Parsa, C.J. Fluid and electrolytes in the aged. Arch. Surg. 2003, 138, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Dennis, R.A.; Johnson, L.E.; Roberson, P.K.; Heif, M.; Bopp, M.M.; Cook, J.; Sullivan, D.H. Changes in prealbumin, nutrient intake, and systemic inflammation in elderly recuperative care patients. J. Am. Geriatr. Soc. 2008, 56, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, S.; Gougeon, R.; Nayar, K.; Morais, J.A. Frailty amplifies the effects of aging on protein metabolism: Role of protein intake. Am. J. Clin. Nutr. 2003, 78, 422–429. [Google Scholar] [PubMed]
- Mackenzie, T.A.; Clark, N.G.; Bistrian, B.R.; Flatt, J.P.; Hallowell, E.M.; Blackburn, G.L. A simple method for estimating nitrogen balance in hospitalized patients: A review and supporting data for a previously proposed technique. J. Am. Coll. Nutr. 1985, 4, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, R.N.; Tidwell, A.C.; Minard, G.; Croce, M.A.; Brown, R.O. Predicting total urinary nitrogen excretion from urinary urea nitrogen excretion in multiple-trauma patients receiving specialized nutrition support. Nutrition 2005, 21, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Shaw-Delanty, S.N.; Elwyn, D.H.; Jeejeebhoy, K.N.; Askanazi, J.; Schwarz, Y.; Iles, M.; Kinney, J.M. Components of nitrogen excretion in hospitalised adult patients on intravenous diets. Clin. Nutr. 1987, 6, 257–266. [Google Scholar] [CrossRef]
- Waxman, K.; Rebello, T.; Pinderski, L.; O’Neal, K.; Khan, N.; Tourangeau, S.; Himes, E.; Cordill, K. Protein loss across burn wounds. J. Trauma 1987, 27, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Allingstrup, M.G.; Esmailzadeh, N.; Wilkens Knudsen, A.; Espersen, K.; Hartvig Jensen, T.; Wiis, J.; Perner, A.; Kondrup, J. Provision of protein and energy in relation to measured requirements in intensive care patients. Clin. Nutr. 2012, 31, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Scheinkestel, C.D.; Kar, L.; Marshall, K.; Bailey, M.; Davies, A.; Nyulasi, I.; Tuxen, D.V. Prospective randomized trial to assess caloric and protein needs of critically ill, anuric, ventilated patients requiring continuous renal replacement therapy. Nutrition 2003, 19, 909–916. [Google Scholar] [CrossRef]
- Campbell, W.W.; Crim, M.C.; Dallal, G.E.; Young, V.R.; Evans, W.J. Increased protein requirements in elderly people: New data and retrospective reassessments. Am. J. Clin. Nutr. 1994, 60, 501–509. [Google Scholar] [PubMed]
- Morse, M.H.; Haub, M.D.; Evans, W.J.; Campbell, W.W. Protein requirement of elderly women: Nitrogen balance responses to three levels of protein intake. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M724–M730. [Google Scholar] [CrossRef] [PubMed]
- Volpi, E.; Mittendorfer, B.; Rasmussen, B.B.; Wolfe, R.R. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J. Clin. Endocrinol. Metab. 2000, 85, 4481–4490. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, C.S.; Kobayashi, H.; Sheffield-Moore, M.; Aarsland, A.; Wolfe, R.R. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am. J. Clin. Nutr. 2005, 82, 1065–1073. [Google Scholar] [PubMed]
- Volpi, E.; Mittendorfer, B.; Wolf, S.E.; Wolfe, R.R. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am. J. Physiol. 1999, 277, E513–E520. [Google Scholar] [PubMed]
- Volpi, E.; Ferrando, A.A.; Yeckel, C.W.; Tipton, K.D.; Wolfe, R.R. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J. Clin. Investig. 1998, 101, 2000–2007. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, B.B.; Fujita, S.; Wolfe, R.R.; Mittendorfer, B.; Roy, M.; Rowe, V.L.; Volpi, E. Insulin resistance of muscle protein metabolism in aging. FASEB J. 2006, 20, 768–769. [Google Scholar] [CrossRef] [PubMed]
- Koopman, R.; Verdijk, L.; Manders, R.J.; Gijsen, A.P.; Gorselink, M.; Pijpers, E.; Wagenmakers, A.J.; van Loon, L.J. Co-ingestion of protein and leucine stimulates muscle protein synthesis rates to the same extent in young and elderly lean men. Am. J. Clin. Nutr. 2006, 84, 623–632. [Google Scholar] [PubMed]
- Katsanos, C.S.; Kobayashi, H.; Sheffield-Moore, M.; Aarsland, A.; Wolfe, R.R. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E381–E387. [Google Scholar] [CrossRef] [PubMed]
- Deutz, N.E.; Matheson, E.M.; Matarese, L.E.; Luo, M.; Baggs, G.E.; Nelson, J.L.; Hegazi, R.A.; Tappenden, K.A.; Ziegler, T.R.; NOURISH Study Group. Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: A randomized clinical trial. Clin. Nutr. 2016, 35, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Bos, C.; Benamouzig, R.; Bruhat, A.; Roux, C.; Mahé, S.; Valensi, P.; Gaudichon, C.; Ferrière, F.; Rautureau, J.; Tomé, D. Short-term protein and energy supplementation activates nitrogen kinetics and accretion in poorly nourished elderly subjects. Am. J. Clin. Nutr. 2000, 71, 1129–1137. [Google Scholar] [PubMed]
- Guillet, C.; Prod’homme, M.; Balage, M.; Gachon, P.; Giraudet, C.; Morin, L.; Grizard, J.; Boirie, Y. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. FASEB J. 2004, 18, 1586–1587. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, R.N.; Maish, G.O., 3rd; Croce, M.A.; Minard, G.; Brown, R.O. Influence of aging on nitrogen accretion during critical illness. JPEN J. Parenter. Enter. Nutr. 2015, 39, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, R.N.; Medling, T.L.; Smith, A.C.; Maish, G.O., 3rd; Croce, M.A.; Minard, G.; Brown, R.O. Hypocaloric, high-protein nutrition therapy in older vs. younger critically ill patients with obesity. JPEN J. Parenter. Enter. Nutr. 2013, 37, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.J.; Cho, M.J.; Atten, M.J.; Panizales, E.; Walter, R.; Hawkins, D.; Donahue, P.A. Hypocaloric parenteral nutrition support in elderly obese patients. Am. Surg. 2000, 66, 394–399. [Google Scholar] [PubMed]
- Choban, P.; Dickerson, R.; Malone, A.; Worthington, P.; Compher, C.; American Society for Parenteral and Enteral Nutrition. A.S.P.E.N. Clinical guidelines: Nutrition support of hospitalized adult patients with obesity. JPEN J. Parenter. Enter. Nutr. 2013, 37, 714–744. [Google Scholar] [CrossRef] [PubMed]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; McCarthy, M.; Roberts, P.; Taylor, B.; Ochoa, J.B.; Napolitano, L.; Cresci, G.; A.S.P.E.N. Board of Directors; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J. Parenter. Enter. Nutr. 2016, 40, 159–211. [Google Scholar]
- Dickerson, R.N.; Boschert, K.J.; Kudsk, K.A.; Brown, R.O. Hypocaloric enteral tube feeding in critically ill obese patients. Nutrition 2002, 18, 241–246. [Google Scholar] [CrossRef]
- Dickerson, R.N.; Rosato, E.F.; Mullen, J.L. Net protein anabolism with hypocaloric parenteral nutrition in obese stressed patients. Am. J. Clin. Nutr. 1986, 44, 747–755. [Google Scholar] [PubMed]
- Choban, P.S.; Dickerson, R.N. Morbid obesity and nutrition support: Is bigger different? Nutr. Clin. Pract. 2005, 20, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, R.N.; Mason, D.L.; Croce, M.A.; Minard, G.; Brown, R.O. Evaluation of an artificial neural network to predict urea nitrogen appearance for critically ill multiple-trauma patients. JPEN J. Parenter. Enter. Nutr. 2005, 29, 429–435. [Google Scholar] [CrossRef]
- Kaysen, G.A.; Myers, B.D. The aging kidney. Clin. Geriatr. Med. 1985, 1, 207–222. [Google Scholar] [PubMed]
- Lindeman, R.D.; Tobin, J.; Shock, N.W. Longitudinal studies on the rate of decline in renal function with age. J. Am. Geriatr. Soc. 1985, 33, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Fliser, D.; Zeier, M.; Nowack, R.; Ritz, E. Renal functional reserve in healthy elderly subjects. J. Am. Soc. Nephrol. 1993, 3, 1371–1377. [Google Scholar] [PubMed]
- Lew, S.W.; Bosch, J.P. Effect of diet on creatinine clearance and excretion in young and elderly healthy subjects and in patients with renal disease. J. Am. Soc. Nephrol. 1991, 2, 856–865. [Google Scholar] [PubMed]
- Bosch, J.P.; Saccaggi, A.; Lauer, A.; Ronco, C.; Belledonne, M.; Glabman, S. Renal functional reserve in humans. Effect of protein intake on glomerular filtration rate. Am. J. Med. 1983, 75, 943–950. [Google Scholar] [CrossRef]
- Klahr, S.; Levey, A.S.; Beck, G.J.; Caggiula, A.W.; Hunsicker, L.; Kusek, J.W.; Striker, G. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N. Engl. J. Med. 1994, 330, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Greene, T.; Sarnak, M.J.; Wang, X.; Beck, G.J.; Kusek, J.W.; Collins, A.J.; Kopple, J.D. Effect of dietary protein restriction on the progression of kidney disease: Long-term follow-up of the Modification of Diet in Renal Disease (MDRD) Study. Am. J. Kidney Dis. 2006, 48, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Fliser, D.; Bischoff, I.; Hanses, A.; Block, S.; Joest, M.; Ritz, E.; Mutschler, E. Renal handling of drugs in the healthy elderly. Creatinine clearance underestimates renal function and pharmacokinetics remain virtually unchanged. Eur. J. Clin. Pharmacol. 1999, 55, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Fliser, D.; Ritz, E. Serum cystatin C concentration as a marker of renal dysfunction in the elderly. Am. J. Kidney Dis. 2001, 37, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Beasley, J.M.; Katz, R.; Shlipak, M.; Rifkin, D.E.; Siscovick, D.; Kaplan, R. Dietary protein intake and change in estimated GFR in the Cardiovascular Health Study. Nutrition 2014, 30, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Nicolo, M.; Heyland, D.K.; Chittams, J.; Sammarco, T.; Compher, C. Clinical Outcomes Related to Protein Delivery in a Critically Ill Population: A Multicenter, Multinational Observation Study. JPEN J. Parenter. Enter. Nutr. 2016, 40, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Weijs, P.J.; Stapel, S.N.; de Groot, S.D.; Driessen, R.H.; de Jong, E.; Girbes, A.R.; van Schijndel, R.J.S.; Beishuizen, A. Optimal protein and energy nutrition decreases mortality in mechanically ventilated, critically ill patients: A prospective observational cohort study. JPEN J. Parenter. Enter. Nutr. 2012, 36, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Zurlo, F.; Larson, K.; Bogardus, C.; Ravussin, E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J. Clin. Investig. 1990, 86, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Bosy-Westphal, A.; Eichhorn, C.; Kutzner, D.; Illner, K.; Heller, M.; Muller, M.J. The age-related decline in resting energy expenditure in humans is due to the loss of fat-free mass and to alterations in its metabolically active components. J. Nutr. 2003, 133, 2356–2362. [Google Scholar] [PubMed]
- Harris, J.A.; Benedict, F.G. A Biometric Study of Human Basal Metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Melzer, K.; Laurie Karsegard, V.; Genton, L.; Kossovsky, M.P.; Kayser, B.; Pichard, C. Comparison of equations for estimating resting metabolic rate in healthy subjects over 70 years of age. Clin. Nutr. 2007, 26, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Neelemaat, F.; van Bokhorst-de van der Schueren, M.A.; Thijs, A.; Seidell, J.C.; Weijs, P.J. Resting energy expenditure in malnourished older patients at hospital admission and three months after discharge: Predictive equations versus measurements. Clin. Nutr. 2012, 31, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Soguel, L.; Charriere, M.; Theriault, B.; Pralong, F.; Schaller, M.D. Impact of the reduction of the recommended energy target in the ICU on protein delivery and clinical outcomes. Clin. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]



© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dickerson, R.N. Nitrogen Balance and Protein Requirements for Critically Ill Older Patients. Nutrients 2016, 8, 226. https://doi.org/10.3390/nu8040226
Dickerson RN. Nitrogen Balance and Protein Requirements for Critically Ill Older Patients. Nutrients. 2016; 8(4):226. https://doi.org/10.3390/nu8040226
Chicago/Turabian StyleDickerson, Roland N. 2016. "Nitrogen Balance and Protein Requirements for Critically Ill Older Patients" Nutrients 8, no. 4: 226. https://doi.org/10.3390/nu8040226
APA StyleDickerson, R. N. (2016). Nitrogen Balance and Protein Requirements for Critically Ill Older Patients. Nutrients, 8(4), 226. https://doi.org/10.3390/nu8040226




