Protective Effect of Proanthocyanidins from Sea Buckthorn (Hippophae Rhamnoides L.) Seed against Visible Light-Induced Retinal Degeneration in Vivo
Abstract
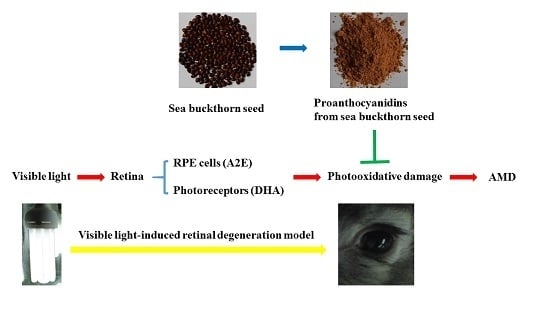
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animal Care
2.3. Treatment with Proanthocyanidins from Sea Buckthorn Seed and Exposure to Visible Light
2.4. Electroretinographic Analysis
2.5. Hematoxylin and Eosin Staining and Measurement at the Full Thickness Retina and Outer Nuclear Layer Thickness
2.6. Determination of MDA and T-AOC Levels and GSH-Px and CAT Activities
2.7. Determination of TNF-α, IL-1β, IL-6 and VEGF Levels
2.8. Western Blot Analysis
2.9. Immunohistochemistry
2.10. Statistical Analysis
3. Results
3.1. Effect of Sea Buckthorn Seed PACs on Visual Function
3.2. Effect of Sea Buckthorn Seed PACs on Full Thickness Retina and ONL Thickness
3.3. Effect of Sea Buckthorn Seed PACs on CAT and GSH-Px Activities and MDA and T-AOC Levels
3.4. Effect of Sea Buckthorn Seed PACs on TNF-α, IL-1β, IL-6 and VEGF Levels
3.5. Effect of Sea Buckthorn Seed PACs on the Expression of Apoptosis-Related Proteins in the Retina
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wu, J.; Seregard, S.; Algvere, P.V. Photochemical damage of the retina. Surv. Ophthalmol. 2006, 51, 461–481. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, J.R.; Zhou, J.; Ben-Shabat, S.; Vollmer, H.; Itagaki, Y.; Nakanishi, K. Involvement of oxidative mechanisms in blue-light-induced damage to a2e-laden rpe. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1222–1227. [Google Scholar]
- Sparrow, J.R.; Gregory-Roberts, E.; Yamamoto, K.; Blonska, A.; Ghosh, S.K.; Ueda, K.; Zhou, J. The bisretinoids of retinal pigment epithelium. Prog. Retin. Eye Res. 2012, 31, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, D.; Liu, Y.X.; Wang, D.; Liu, J.; Ji, B.P. The protective effects of berry-derived anthocyanins against visible light-induced damage in human retinal pigment epithelial cells. J. Sci. Food Agric. 2015, 95, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huo, Y.Z.; Zhao, L.; Lu, F.; Wang, O.; Yang, X.; Ji, B.P.; Zhou, F. Cyanidin-3-glucoside and its phenolic acid metabolites attenuate visible light-induced retinal degeneration in vivo via activation of Nrf2/HO-1 pathway and NF-κB suppression. Mol. Nutr. Food Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.J.; Morgan, J.I.W.; Merigan, W.H.; Sliney, D.H.; Sparrow, J.R.; Williams, D.R. The susceptibility of the retina to photochemical damage from visible light. Prog. Retin. Eye Res. 2012, 31, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.R.; Lawrenson, J.G. A review of the evidence for dietary interventions in preventing or slowing the progression of age-related macular degeneration. Ophthalmic Physiol. Opt. 2014, 34, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.S.M.; Akhtar, H.; Zaheer, K.; Ali, R. Dietary sources of lutein and zeaxanthin carotenoids and their role in eye health. Nutrients 2013, 5, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- Koushan, K.; Rusovici, R.; Li, W.; Ferguson, L.R.; Chalam, K.V. The role of lutein in eye-related disease. Nutrients 2013, 5, 1823–1839. [Google Scholar] [CrossRef] [PubMed]
- Augood, C.A.; Vingerling, J.R.; de Jong, P.T.; Chakravarthy, U.; Seland, J.; Soubrane, G.; Tomazzoli, L.; Topouzis, F.; Bentham, G.; Rahu, M.; et al. Prevalence of age-related maculopathy in older Europeans—The European Eye Study (EUREYE). Arch. Ophthalmol. 2006, 124, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Suryakumar, G.; Gupta, A. Medicinal and therapeutic potential of sea buckthorn (hippophae rhamnoides l.). J. Ethnopharmacol. 2011, 138, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-J.; Kaur, M.; Dhillon, R.S.; Tappia, P.S.; Dhalla, N.S. Health benefits of sea buckthorn for the prevention of cardiovascular diseases. J. Funct. Foods 2011, 3, 2–12. [Google Scholar] [CrossRef]
- Sea Buckthorn. Available online: https://examine.com/supplements/sea-buckthorn/ (accessed on 11 April 2016).
- Arimboor, R.; Arumughan, C. Sea buckthorn (Hippophae rhamnoides) proanthocyanidins inhibit in vitro enzymatic hydrolysis of protein. J. Food Sci. 2011, 76, T130–T137. [Google Scholar] [CrossRef] [PubMed]
- Arimboor, R.; Arumughan, C. Effect of polymerization on antioxidant and xanthine oxidase inhibitory potential of sea buckthorn (H. rhamnoides) proanthocyanidins. J. Food Sci. 2012, 77, C1036–C1041. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.H.; Zhang, X.; Huang, L.Q.; Gong, H.; Cheng, B.; Sun, Y.; Li, Y.X.; Liu, Q.; Zheng, L.; Huang, K. Proanthocyanidins are the major anti-diabetic components of cinnamon water extract. Food Chem. Toxicol. 2013, 56, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Josepa, S.M.; Casanova, E.; Fernandez-Iglesias, A.; Arola, L.; Blade, C. Roles of proanthocyanidin rich extracts in obesity. Food Funct. 2015, 6, 1053–1071. [Google Scholar]
- Prasad, R.; Vaid, M.; Katiyar, S.K. Grape proanthocyanidin inhibit pancreatic cancer cell growth in vitro and in vivo through induction of apoptosis and by targeting the PI3K/Akt pathway. PLoS ONE 2012, 7, e43064. [Google Scholar] [CrossRef] [PubMed]
- Pallares, V.; Fernandez-Iglesias, A.; Cedo, L.; Castell-Auvi, A.; Pinent, M.; Ardevol, A.; Josepa, S.M.; Garcia-Vallve, S.; Blay, M. Grape seed procyanidin extract reduces the endotoxic effects induced by lipopolysaccharide in rats. Free Radic. Biol. Med. 2013, 60, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Quinones, M.; Miguel, M.; Aleixandre, A. Beneficial effects of polyphenols on cardiovascular disease. Pharmacol. Res. 2013, 68, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chung, S.J.; Song, W.O.; Chun, O.K. Estimation of daily proanthocyanidin intake and major food sources in the U.S. diet. J. Nutr. 2011, 141, 447–452. [Google Scholar] [CrossRef] [PubMed]
- De la lglesia, R.; Milagro, F.I.; Campion, J.; Boque, N.; Martinez, J.A. Healthy properties of proanthocyanidins. Biofactors 2010, 36, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Matito, C.; Agell, N.; Sanchez-Tena, S.; Torres, J.L.; Cascante, M. Protective effect of structurally diverse grape procyanidin fractions against UV-induced cell damage and death. J. Agric. Food Chem. 2011, 59, 4489–4495. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.R.; Zhang, S.J.; Li, Y.F.; Tsoi, B.; Huang, W.S.; Yao, N.; Hong, M.; Zhai, Y.J.; Mao, Z.F.; Tang, L.P.; et al. Proanthocyanidins prevent high glucose-induced eye malformation by restoring Pax6 expression in chick embryo. Nutrients 2015, 7, 6567–6581. [Google Scholar] [CrossRef] [PubMed]
- Muthenna, P.; Raghu, G.; Akileshwari, C.; Sinha, S.N.; Suryanarayana, P.; Reddy, G.B. Inhibition of protein glycation by procyanidin-B2 enriched fraction of cinnamon: Delay of diabetic cataract in rats. IUBMB Life 2013, 65, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, C.; Lu, D.; Shu, X.; Zhu, L.; Qi, R.; So, K.F.; Lu, D.; Xu, Y. Oligomeric proanthocyanidin protects retinal ganglion cells against oxidative stress-induced apoptosis. Neural Regen. Res. 2013, 8, 2317–2326. [Google Scholar] [PubMed]
- Yang, H.; Lee, B.K.; Kook, K.H.; Jung, Y.S.; Ahn, J. Protective effect of grape seed extract against oxidative stress-induced cell death in a staurosporine-differentiated retinal ganglion cell line. Curr. Eye Res. 2012, 37, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Yamakoshi, J.; Saito, M.; Kataoka, S.; Tokutake, S. Procyanidin-rich extract from grape seeds prevents cataract formation in hereditary cataractous (ICR/f) rats. J. Agric. Food Chem. 2002, 50, 4983–4988. [Google Scholar] [CrossRef] [PubMed]
- Durukan, A.H.; Evereklioglu, C.; Hurmeric, V.; Kerimoglu, H.; Erdurman, C.; Bayraktar, Z.; Mumcuoglu, T. Ingestion of IH636 grape seed proanthocyanidin extract to prevent selenite-induced oxidative stress in experimental cataract. J. Cataract Refract. Surg. 2006, 32, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.S.; Yu, S.Y.; Jung, S.H.; Lee, Y.R.; Lee, Y.M.; Kim, J.H.; Sun, H.; Kim, J.S. Proanthocyanidins from spenceria ramalana and their effects on AGE formation in vitro and hyaloid-retinal vessel dilation in larval zebrafish in vivo. J. Nat. Prod. 2013, 76, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Tsuruma, K.; Tanaka, J.; Kakino, M.; Kobayashi, S.; Shimazawa, M.; Hara, H. The protective effects of bilberry and lingonberry extracts against UV light-induced retinal photoreceptor cell damage in vitro. J. Agric. Food Chem. 2013, 61, 10345–10353. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Ding, X.; Gu, W. Radical-scavenging proanthocyanidins from sea buckthorn seed. Food Chem. 2007, 102, 168–177. [Google Scholar] [CrossRef]
- Kallio, H.; Yang, W.; Liu, P.; Yang, B. Proanthocyanidins in wild sea buckthorn (Hippophae rhamnoides) berries analyzed by reversed-phase, normal-phase, and hydrophilic interaction liquid chromatography with uv and ms detection. J. Agric. Food Chem. 2014, 62, 7721–7729. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, D.B.; Sen, C.K.; Ray, S.D.; Das, D.K.; Bagchi, M.; Preuss, H.G.; Vinson, J.A. Molecular mechanisms of cardioprotection by a novel grape seed proanthocyanidin extract. Mutat. Res. Fundam. Mol. Mutagen. 2003, 523, 87–97. [Google Scholar] [CrossRef]
- Prieur, C.; Rigaud, J.; Cheynier, V.; Moutounet, M. Oligomeric and polymeric procyanidins from grape seeds. Phytochemistry 1994, 36, 781–784. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Lu, F.; Yang, X.; Deng, Q.; Ji, B.; Huang, F. Retinoprotective effects of bilberry anthocyanins via antioxidant, anti-Inflammatory, and anti-Apoptotic mechanisms in a visible light-induced retinal degeneration model in pigmented rabbits. Molecules 2015, 20, 22395–22410. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, F.; Zhao, L.; Zhang, D.; Wang, O.; Guo, X.; Lu, F.; Yang, X.; Ji, B.; Deng, Q. Protective effect of total flavones from Hippophae rhamnoides L. against visible light-induced retinal degeneration in pigmented rabbits. J. Agric. Food Chem. 2016, 64, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Yuki, K.; Kurihara, T.; Miyake, S.; Noda, K.; Kobayashi, S.; Ishida, S.; Tsubota, K.; Ozawa, Y. Biological role of lutein in the light-induced retinal degeneration. J. Nutr. Biochem. 2012, 23, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Richer, S.; Patel, S.; Sockanathan, S.; Ulanski, L.J.; Miller, L.; Podella, C. Resveratrol based oral nutritional supplement produces long-term beneficial effects on structure and visual function in human patients. Nutrients 2014, 6, 4404–4420. [Google Scholar] [CrossRef] [PubMed]
- Blade, C.; Arola, L.; Salvado, M.J. Hypolipidemic effects of proanthocyanidins and their underlying biochemical and molecular mechanisms. Mol. Nutr. Food Res. 2010, 54, 37–59. [Google Scholar] [CrossRef] [PubMed]
- German, O.L.; Agnolazza, D.L.; Politi, L.E.; Rotstein, N.P. Light, lipids and photoreceptor survival: Live or let die? Photochem. Photobiol. Sci. 2015, 14, 1737–1753. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Elliott, M.H.; Kotake, Y.; Anderson, R.E. Protein modifications by 4-hydroxynonenal and 4-hydroxyhexenal in light-exposed rat retina. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3859–3868. [Google Scholar] [CrossRef] [PubMed]
- Loskutova, E.; Nolan, J.; Howard, A.; Beatty, S. Macular pigment and its contribution to vision. Nutrients 2013, 5, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Sliney, D.H. Exposure geometry and spectral environment determine photobiological effects on the human eye. Photochem. Photobiol. 2005, 81, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Boulton, M.; Rozanowska, M.; Rozanowski, B. Retinal photodamage. J. Photochem. Photobiol. B 2001, 64, 144–161. [Google Scholar] [CrossRef]
- Youn, H.Y.; Chou, B.R.; Cullen, A.P.; Sivak, J.G. Effects of 400 nm, 420 nm, and 435.8 nm radiations on cultured human retinal pigment epithelial cells. J. Photochem. Photobiol. B 2009, 95, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, M.; Tanaka, M.; Tanaka, Y.; Okubo, A.; Koriyama, C.; Tsuji, M.; Akiba, S.; Miyamoto, K.; Hillebrand, G.; Yamashita, T.; et al. Age-related maculopathy and sunlight exposure evaluated by objective measurement. Br. J. Ophthalmol. 2008, 92, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.G.; Jarrar, Z.; Wormald, R.; Cook, D.G.; Fletcher, A.E.; Rudnicka, A.R. The estimated prevalence and incidence of late stage age related macular degeneration in the UK. Br. J. Ophthalmol. 2012, 96, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.S.; O’Colmain, B.; Tomany, S.C.; McCarty, C.; de Jong, P.T.; Nemesure, B.; Mitchell, P.; Kempen, J.; Congdon, N. Prevalence of age-related macular degeneration in the United States. Arch. Ophthalmol. 2004, 122, 564–572. [Google Scholar] [PubMed]
- Martinez-Micaelo, N.; Gonzalez-Abuin, N.; Ardevol, A.; Pinent, M.; Teresa, B.M. Procyanidins and inflammation: Molecular targets and health implications. Biofactors 2012, 38, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Knaze, V.; Zamora-Ros, R.; Lujan-Barroso, L.; Romieu, I.; Scalbert, A.; Slimani, N.; Riboli, E.; Rossum, C.T.M.; Bueno de Mesquita, H.B.; Trichopoulou, A. Intake estimation of total and individual flavan-3-ols, proanthocyanidins and theaflavins, their food sources and determinants in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br. J. Nutr. 2012, 108, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Andres-Lacueva, C.; Lamuela-Raventos, R.M.; Berenguer, T.; Jakszyn, P.; Barricarte, A.; Ardanaz, E.; Amiano, P.; Dorronsoro, M.; Larranaga, N.; et al. Estimation of dietary sources and flavonoid intake in a Spanish adult population (EPIC-Spain). J. Am. Diet. Assoc. 2010, 110, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.W.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Prior, R.L. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J. Nutr. 2004, 134, 613–617. [Google Scholar] [PubMed]
- Khan, N.; Khymenets, O.; Urpi-Sarda, M.; Tulipani, S.; Garcia-Aloy, M.; Monagas, M.; Mora-Cubillos, X.; Llorach, R.; Andres-Lacueva, C. Cocoa polyphenols and inflammatory markers of cardiovascular disease. Nutrients 2014, 6, 844–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Federico, A.; Morgillo, F.; Tuccillo, C.; Ciardiello, F.; Loguercio, C. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Cancer 2007, 121, 2381–2386. [Google Scholar] [CrossRef] [PubMed]
- Adriana, I.A.; Fernandez-Robredo, P.; Heras-Mulero, H.; Manuel, S.E.L.; Garcia-Garcia, L.; Fernandez-Garcia, V.; Moreno-Orduna, M.; Redondo-Exposito, A.; Recalde, S.; Garcia-Layana, A. Modifying choroidal neovascularization development with a nutritional supplement in mice. Nutrients 2015, 7, 5423–5442. [Google Scholar]
- Shalit, I.; Halperin, D.; Haite, D.; Levitov, A.; Romano, J.; Osherov, N.; Fabian, I. Anti-inflammatory effects of moxifloxacin on IL-8, IL-1 beta and TNF-alpha secretion and NF kappa B and MAP-kinase activation in human monocytes stimulated with Aspergillus fumigatus. J. Antimicrob. Chemother. 2006, 57, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Maskarinec, G. Cancer protective properties of cocoa: A review of the epidemiologic evidence. Nutr. Cancer 2009, 61, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Esposito, D.; Chen, A.; Grace, M.H.; Komarnytsky, S.; Lila, M.A. Inhibitory effects of wild blueberry anthocyanins and other flavonoids on biomarkers of acute and chronic inflammation in vitro. J. Agric. Food Chem. 2014, 62, 7022–7028. [Google Scholar] [CrossRef] [PubMed]
- Bak, M.J.; Truong, V.L.; Kang, H.S.; Jun, M.; Jeong, W.S. Anti-inflammatory effect of procyanidins from wild grape (Vitis amurensis) seeds in LPS-induced RAW 264.7 cells. Oxid. Med. Cell. Longev. 2013, 172, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Natella, F.; Belelli, F.; Gentili, V.; Ursini, F.; Scaccini, C. Grape seed proanthocyanidins prevent plasma postprandial oxidative stress in humans. J. Agric. Food Chem. 2002, 50, 7720–7725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Li, W.G.; Wu, Y.J.; Bai, D.C.; Liu, N.F. Proanthocyanidin from grape seeds enhances doxorubicin-induced antitumor effect and reverses drug resistance in doxorubicin-resistant K562/DOX cells. Can. J. Physiol. Pharmacol. 2005, 83, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Oteiza, P.I. Dietary flavonoids: Role of (−)-epicatechin and related procyanidins in cell signaling. Free Radic. Biol. Med. 2011, 51, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, K.; Bai, B.R.S.; Devaraj, S.N. Cardioprotective effect of grape seed proanthocyanidins on isoproterenol-induced myocardial injury in rats. Int. J. Cardiol. 2007, 115, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Mantena, S.K.; Katiyar, S.K. Grape seed proanthocyanidins inhibit UV-radiation-induced oxidative stress and activation of MAPK and NF-kappa B signaling in human epidermal keratinocytes. Free Radic. Biol. Med. 2006, 40, 1603–1614. [Google Scholar] [CrossRef] [PubMed]





© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhao, L.; Huo, Y.; Zhou, F.; Wu, W.; Lu, F.; Yang, X.; Guo, X.; Chen, P.; Deng, Q.; et al. Protective Effect of Proanthocyanidins from Sea Buckthorn (Hippophae Rhamnoides L.) Seed against Visible Light-Induced Retinal Degeneration in Vivo. Nutrients 2016, 8, 245. https://doi.org/10.3390/nu8050245
Wang Y, Zhao L, Huo Y, Zhou F, Wu W, Lu F, Yang X, Guo X, Chen P, Deng Q, et al. Protective Effect of Proanthocyanidins from Sea Buckthorn (Hippophae Rhamnoides L.) Seed against Visible Light-Induced Retinal Degeneration in Vivo. Nutrients. 2016; 8(5):245. https://doi.org/10.3390/nu8050245
Chicago/Turabian StyleWang, Yong, Liang Zhao, Yazhen Huo, Feng Zhou, Wei Wu, Feng Lu, Xue Yang, Xiaoxuan Guo, Peng Chen, Qianchun Deng, and et al. 2016. "Protective Effect of Proanthocyanidins from Sea Buckthorn (Hippophae Rhamnoides L.) Seed against Visible Light-Induced Retinal Degeneration in Vivo" Nutrients 8, no. 5: 245. https://doi.org/10.3390/nu8050245
APA StyleWang, Y., Zhao, L., Huo, Y., Zhou, F., Wu, W., Lu, F., Yang, X., Guo, X., Chen, P., Deng, Q., & Ji, B. (2016). Protective Effect of Proanthocyanidins from Sea Buckthorn (Hippophae Rhamnoides L.) Seed against Visible Light-Induced Retinal Degeneration in Vivo. Nutrients, 8(5), 245. https://doi.org/10.3390/nu8050245