The Effect of Green Tea Ingestion and Interval Sprinting Exercise on the Body Composition of Overweight Males: A Randomized Trial
Abstract
:1. Introduction
2. Participants and Methods
2.1. Participants
2.2. Procedures
2.3. Body Composition
2.4. Interval Sprinting Exercise Protocol
2.5. Assays
2.6. Diet
2.7. Statistical Analysis
3. Results
3.1. Exercise Training Heart Rate, RPE, and Power Output
3.2. Aerobic Power
3.3. BMI and Waist Circumference
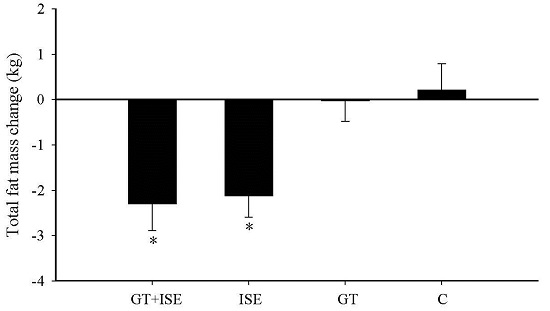
3.4. Body Composition
3.5. Blood Lipids
3.6. Diet
3.7. RER and Heart Rate during Submaximal Aerobic Exercise
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jakicic, J.M.; Clark, K.; Coleman, E.; Donnelly, J.E.; Foreyt, J.; Melanson, E.; Volek, J.; Volpe, S.L.; American College of Sports Medicine. Appropriate intervention strategies for weight loss and prevention of weight regain for adults. Med. Sci. Sports. Exerc. 2001, 33, 2145–2156. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.E. Diet, exercise or diet with exercise: Comparing the effectiveness of treatment options for weight-loss and changes in fitness for adults (18–65 years old) who are overfat, or obese; systematic review and meta-analysis. J. Diabetes Metab. Dis. 2015. [Google Scholar] [CrossRef] [PubMed]
- Mann, T.; Tomiyama, A.J.; Westling, E.; Lew, A.M.; Samuels, B.; Chapman, J. Medicare’s search for effective obesity treatments: Diets are not the answer. Am. Psychol. 2007, 62, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Saris, W.H. The role of exercise in the dietary treatment of obesity. Int. J. Obes. 1993, 17, S17–S21. [Google Scholar]
- Hursel, R.; Viechtbauer, W.; Westerterp-Plantenga, M.S. The effects of green tea on weight loss and weight maintenance: A meta-analysis. Int. J. Obes. (Lond.) 2009, 33, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Boutcher, S.H. High-intensity intermittent exercise and fat loss. J. Obes. 2011. [Google Scholar] [CrossRef] [PubMed]
- Zhanga, Y.Y.; Lia, X.; Megurod, S.; Hayashie, S.; Katashimae, M.; Yasumasue, T.; Wang, J.; Li, K. Effects of catechin-enriched green tea beverage on visceral fat loss in adults with a high proportion of visceral fat: A double-blind, placebo-controlled, randomized trial. J. Funct. Food. 2013, 4, 315–322. [Google Scholar] [CrossRef]
- Nagao, T.; Komine, Y.; Soga, S.; Meguro, S.; Hase, T.; Tanaka, Y.; Tokimitsu, I. Ingestion of a tea rich in catechins leads to a reduction in body fat and malondialdehyde-modified LDL in men. Am. J. Clin. Nutr. 2005, 81, 122–129. [Google Scholar] [PubMed]
- Wu, C.H.; Lu, F.H.; Chang, C.S.; Chang, T.C.; Wang, R.H.; Chang, C.J. Relationship among habitual tea consumption, percent body fat, and body fat distribution. Obes. Res. 2003, 11, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.H.; Tsai, T.H.; Kao, Y.H.; Hwang, K.C.; Tseng, T.Y.; Chou, P. Effect of green tea extract on obese women: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. 2008, 27, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.C.; Koo, M.W.L.; Ng, E.H.Y.; Tang, O.; Yeung, W.S.B.; Ho, P. Effects of Chinese green tea on weight, and hormonal and biochemical profiles in obese patients with polycystic ovary syndrome—A randomized placebo-controlled trial. J. Soc. Gynecol. Investig. 2006, 13, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Janssens, P.L.; Hursel, R.; Westerterp-Plantenga, M.S. Long-term green tea extract supplementation does not affect fat absorption, resting energy expenditure, and body composition in adults. J. Nutr. 2015, 145, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Bérubé-Parent, S.; Pelletier, C.; Doré, J.; Tremblay, A. Effects of encapsulated green tea and Guarana extracts containing a mixture of epigallocatechin-3-gallate and caffeine on 24 h energy expenditure and fat oxidation in men. Br. J. Nutr. 2005, 94, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Dulloo, A.G.; Duret, C.; Rohrer, D.; Girardier, L.; Mensi, N.; Fathi, M.; Chantre, P.; Vandermander, J. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am. J. Clin. Nutr. 1999, 70, 1040–1045. [Google Scholar] [PubMed]
- Tjønna, A.E.; Lee, S.J.; Rognmo, Ø.; Stølen, T.O.; Bye, A.; Haram, P.M.; Loennechen, J.P.; Al-Share, Q.Y.; Skogvoll, E.; Slørdahl, S.A. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: A pilot study. Circulation 2008, 118, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.D.; Close, G.L.; MacLaren, D.P.; Gregson, W.; Drust, B.; Morton, J.P. High-intensity interval running is perceived to be more enjoyable than moderate-intensity continuous exercise: Implications for exercise adherence. J. Sports Sci. 2011, 29, 547–453. [Google Scholar] [CrossRef] [PubMed]
- Trapp, E.G.; Chisholm, D.J.; Boutcher, S.H. Metabolic response of trained and untrained women during high-intensity intermittent cycle exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R2370–R2375. [Google Scholar] [CrossRef] [PubMed]
- Heydari, M.; Freund, J.; Boutcher, S.H. The effect of high-intensity intermittent exercise on body composition of overweight young males. J. Obes. 2012. [Google Scholar] [CrossRef] [PubMed]
- Dunn, S.L.; Siu, W.; Freund, J.; Boutcher, S.H. The effect of a lifestyle intervention on metabolic health in young women. Diabetes Metab. Syn. Obes. 2014, 7, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Gahreman, D.; Wang, R.; Boutcher, Y.N.; Boutcher, S.H. Green tea, intermittent sprinting exercise, and fat oxidation. Nutrients 2015, 7, 5646–5663. [Google Scholar] [CrossRef] [PubMed]
- Gahreman, D.; Boutcher, Y.N.; Bustamante, S.; Boutcher, S.H. The combined effect of green tea and acute interval sprinting exercise on fat oxidation of trained and untrained males. J. Exerc. Nutr. Biochem. 2016, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- The GT Capsule. Available online: http://www.gnc.com (accessed on 17 August 2016).
- Borg, G. Psychological basis of perceived exertion. Med. Sci. Sports. Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Talanian, J.L.; Galloway, S.D.; Heigenhauser, G.J.; Bonen, A.; Spriet, L.L. Two weeks of high-intensity aerobic interval training increases the capacity for fat oxidation during exercise in women. J. Appl. Physiol. 2007, 102, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Heydari, M.; Boutcher, Y.N.; Boutcher, S.H. High-intensity intermittent exercise and cardiovascular and autonomic function. Clin. Auton. Res. 2013, 23, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Heydari, M.; Boutcher, Y.N.; Boutcher, S.H. The effects of high-intensity intermittent exercise training on cardiovascular response to mental and physical challenge. Int. J. Psychophysiol. 2013, 87, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Trilk, J.L.; Singhak, A.; Bigelman, K.A.; Cureton, K.J. Effect of sprint interval training on circulatory function during exercise in sedentary, overweight/obese women. Eur. J. Appl. Physiol. 2011, 111, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Milanovic, Z.; Sporis, G.; Weston, M. Effectiveness of high-intensity interval training (HIT) and continuous endurance training for VO2max improvements: A systematic review and meta-analysis of controlled trials. Sports Med. 2015, 45, 1469–1481. [Google Scholar] [CrossRef] [PubMed]
- Blair, S.N. Physical inactivity: The biggest public health problem of the 21st century. Br. J. Sports Med. 2009, 43, 1–2. [Google Scholar]
- Kuk, J.L.; Katzmarzyk, P.T.; Nichman, M.Z.; Church, T.S.; Blair, S.N.; Ross, R. Visceral fat is an independent predictor of all-cause mortality in men. Obesity 2006, 14, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Dagnone, D.; Jones, P.J.; Smith, H.; Paddags, A.; Hudson, R.; Janssen, I. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men: A randomized, controlled trial. Ann. Int. Med. 2000, 133, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Okauchi, Y.; Nishzawa, H.; Funahashi, T.; Ogawa, T.; Noguchi, M.; Ryo, M.; Kihara, S.; Iwahashi, H.; Yamagata, K.; Nakamura, T.; et al. Reduction of visceral fat is associated with decrease in the number of metabolic risk factors in Japanese men. Diabetes Care 2007, 30, 2392–2394. [Google Scholar] [CrossRef] [PubMed]
- Boudou, P.; Sobngwi, E.; Mauvais-Jarvis, F.; Vexiau, P.; Gautier, J.F. Absence of exercise-induced variations in adiponectin levels despite decreased abdominal adiposity and improved insulin sensitivity in type 2 diabetic men. Eur. J. Endocrinol. 2003, 149, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Stiegler, P.; Cunliffe, A. The role of diet and exercise for the maintenance of fat-free mass and resting metabolic rate during weight loss. Sports Med. 2006, 36, 239–262. [Google Scholar] [CrossRef] [PubMed]
- Shimotoyodome, A.; Haramizu, S.; Inaba, M.; Murase, T.; Tokimitsu, I. Exercise and green tea extract stimulate fat oxidation and prevent obesity in mice. Med. Sci. Sports Exerc. 2005, 37, 1884–1892. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, S.; Raederstorff, D.; Wang, Y.; Teixeira, S.R.; Elste, V.; Weber, P. TEAVIGO (epigallocatechin gallate) supplementation prevents obesity in rodents by reducing adipose tissue mass. Ann. Nutr. Metab. 2005, 49, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, T.; Nomura, S.; Someya, Y.; Akimoto, S.; Tachiyashiki, K.; Imaizumi, K. Effect of endurance training supplemented with green tea extract on substrate metabolism during exercise in humans. Scand. J. Med. Sci. Sports 2011, 21, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.D.; Roberts, M.G.; Tarpey, M.D.; Weekes, J.C.; Thomas, C.H. The effect of a decaffeinated green tea extract formula on fat oxidation, body composition and exercise performance. J. Int. Soc. Sports Nutr. 2015, 145, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Murase, T.; Haramizu, S.; Shimotodome, A.; Nagasawa, A.; Tokimitsu, I. Green tea extract improves endurance capacity and increases muscle lipid oxidation in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R708–R715. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Meng, X.; Yang, C.S. Enzymology of methylation of tea catechins and inhibition of catechol-O-methyltransferase by (–)-epigallocatechin gallate. Drug Metab. Dispos. 2003, 31, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Kao, Y.H.; Hiipakka, R.A. Green tea: Biochemical and biological basis for health benefits. Vitam. Horm. 2001, 62, 91–94. [Google Scholar]


| Variable | GT + ISE | ISE | GT | C | ||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Weight | 88.48 ± 5.57 | 86.79 ± 5.44 * | 88.08 ± 3.84 | 86.43 ± 3.75 * | 85.83 ± 1.95 | 85.72 ± 2 | 91.19 ± 4.03 | 91.87 ± 4.17 |
| BMI | 29.04 ± 1.28 | 28.48 ± 1.26 * | 28.67 ± 0.78 | 28.13 ± 0.77 * | 26.95 ± 0.29 | 26.92 ± 0.32 | 29.51 ± 1.31 | 29.71 ± 1.3 |
| Waist circumference | 96.17 ± 3.4 | 92.88 ± 3.38 * | 94.25 ± 2.17 | 89.88 ± 2.33 * | 92.42 ± 1.15 | 92.46 ± 1.59 | 95.08 ± 2.75 | 96.07 ± 2.88 |
| (mL/kg/min) | 34.23 ± 2.11 | 40.38 ± 2.21 * | 34.08 ± 1.35 | 39.32 ± 0.94 * | 39.77 ± 2.26 | 40.39 ± 1.68 | 27.68 ± 1.34 | 28.19 ± 1.40 |
| Cholesterol (mmol/L) | 4.61 ± 0.36 | 4.23 ± 0.3 * | 4.2 ± 0.28 | 3.92 ± 0.26 | 4.61 ± 0.3 | 4.57 ± 0.26 | 4.38 ± 0.27 | 4.38 ± 0.26 |
| HDL (mmol/L) | 1.06 ± 0.12 | 1.02 ± 0.09 | 1.34 ± 0.12 | 1.35 ± 0.11 | 1.15 ± 0.09 | 1.17 ± 0.09 | 0.98 ± 0.09 | 0.97 ± 0.1 |
| Triglycerides (mmol/L) | 1.42 ± 0.23 | 1.21 ± 0.15 | 1.0 ± 0.11 | 0.79 ± 0.11 * | 0.98 ± 0.17 | 0.99 ± 0.19 | 1.39 ± 0.19 | 1.38 ± 0.23 |
| LDL (mmol/L) | 2.9 ± 0.3 | 2.87 ± 0.35 | 2.42 ± 0.19 | 2.25 ± 0.18 | 3.03 ± 0.22 | 2.95 ± 0.23 | 2.76 ± 0.25 | 2.74 ± 0.24 |
| TC/HDL (mmol/L) | 4.73 ± 0.53 | 4.46 ± 0.45 | 3.29 ± 0.23 | 3 ± 0.16 | 4.15 ± 0.35 | 4 ± 0.41 | 4.76 ± 0.39 | 4.73 ± 0.36 |
| Glucose (mmol/L) | 5.06 ± 0.08 | 4.96 ± 0.13 | 4.74 ± 0.21 | 4.81 ± 0.15 | 4.8 ± 0.13 | 4.84 ± 0.13 | 4.71 ± 0.15 | 4.73 ± 0.12 |
| Variable | GT + ISE | ISE | GT | C | ||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Total body fat mass (kg) | 28.47 ± 3.6 | 26.19 ± 3.5 * | 29.63 ± 2.3 | 27.68 ± 2.3 * | 21.48 ± 1.3 | 20.93 ± 1.7 | 33.06 ± 3.2 | 33.04 ± 3.4 |
| Abdominal fat mass (kg) | 2.31 ± 0.3 | 2.07 ± 0.2 * | 2.33 ± 0.2 | 2.2 ± 0.2 * | 1.66 ± 0.1 | 1.61 ± 0.2 | 2.56 ± 0.3 | 2.57 ± 0.3 |
| Arm lean mass (kg) | 6.98 ± 0.4 | 7.17 ± 0.4 | 6.96 ± 0.4 | 6.91 ± 0.4 | 8.33 ± 0.4 | 8.37 ± 0.4 | 6.80 ± 0.4 | 6.86 ± 0.4 |
| Trunk lean mass (kg) | 26.35 ± 1.1 | 26.55 ± 1.2 | 25.63 ± 1.0 | 25.920.9 | 28.11 ± 1.0 | 28.16 ± 1.0 | 25.63 ± 0.1 | 25.69 ± 1.0 |
| Leg lean mass (kg) | 20.3 ± 1.0 | 20.52 ± 0.9 | 18.97 ± 0.9 | 19.28 ± 0.8 | 21.44 ± 0.7 | 21.35 ± 0.7 | 19.47 ± 0.1 | 19.4 ± 0.6 |
| Total lean mass (kg) | 58.13 ± 2.4 | 58.7 ± 2.4 * | 55.76 ± 2.3 | 56.32 ± 2.0 * | 62.32 ± 1.9 | 62.31 ± 2.0 | 55.72 ± 1.7 | 55.67 ± 1.6 |
| Variable | GT + ISE | ISE | GT | C | ||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Total energy intake (kJ·day-1) | 8655 ± 528 | 8934 ± 485 | 7703 ± 260 | 7753 ± 244 | 8235 ± 412 | 8572 ± 436 | 8568 ± 342 | 8641 ± 343 |
| % carbohydrates | 43.42 ± 2.5 | 41.31 ± 2.1 | 43.11 ± 2.1 | 43.33 ± 2.0 | 49.16 ± 1.4 | 45.99 ± 1.6 | 46.18 ± 1.8 | 46.78 ± 2.0 |
| % protein | 18.27 ± 0.8 | 18.52 ± 0.7 | 19.45 ± 1.3 | 18.93 ± 1.3 | 19.61 ± 1.2 | 20.31 ± 1.1 | 18.18 ± 1.1 | 18.33 ± 1.5 |
| % fat | 35.22 ± 2.5 | 35.88 ± 2.1 | 37.28 ± 2.0 | 37.58 ± 1.8 | 31.01 ± 1.4 | 33.22 ± 1.5 | 35.66 ± 1.7 | 34.89 ± 1.4 |
| % saturated fat | 12.46 ± 1.1 | 12.57 ± 1.1 | 13.71 ± 0.9 | 13.11 ± 0.9 | 11.21 ± 0.7 | 11.02 ± 0.7 | 12.40 ± 0.7 | 11.29 ± 0.6 |
| % mono-unsaturated fat | 13.28 ± 0.97 | 13.76 ± 0.78 | 14.01 ± 0.82 | 14.27 ± 0.85 | 11.40 ± 0.51 | 12.96 ± 0.68 | 13.58 ± 0.68 | 13.84 ± 0.70 |
| % poly- unsaturated fat | 6.46 ± 0.60 | 6.87 ± 0.65 | 6.23 ± 0.59 | 6.91 ± 0.77 | 5.11 ± 0.65 | 6.16 ± 0.76 | 6.26 ± 0.61 | 6.50 ± 0.7 |
| Cholesterol (mg) | 356.24 ± 40 | 402.66 ± 36 | 337.68 ± 21 | 312.87 ± 25 | 406.98 ± 63 | 450.28 ± 43 | 304.83 ± 41 | 381.80 ± 56 |
| Fibre (g) | 21.75 ± 3.0 | 25.58 ± 2.8 | 16.78 ± 2.1 | 19.44 ± 2.3 | 19.43 ± 2.6 | 19.07 ± 3.1 | 20.28 ± 2.4 | 21.39 ± 2.8 |
| Sodium (mg) | 3039 ± 490 | 3269 ± 449 | 2379 ± 399 | 2375 ± 398 | 3121 ± 480 | 3323 ± 472 | 2389 ± 345 | 2546 ± 334 |
| Group | Variable | Rest | Submaximal Exercise (W) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 60 W | 90 W | 120 W | 150 W | 180 W | 210 W | 240 W | ||||
| Exercise | RER | Pre | 0.85 ± 0.01 | 0.88 ± 0.03 | 0.90 ± 0.03 | 0.92 ± 0.03 | 0.99 ± 0.03 | 1.07 ± 0.03 | 1.14 ± 0.04 | 1.21 ±0.04 |
| Post | 0.86 ± 0.01 | 0.85 ± 0.02 | 0.85 ± 0.02 | 0.87 ± 0.03 | 0.91 ± 0.03 | 0.98 ± 0.03 | 1.04 ± 0.03 | 1.10 ± 0.04 * | ||
| HR | Pre | 63 ± 3 | 98 ± 3.1 | 104 ± 3.2 | 115 ± 3.4 | 127 ± 4.2 | 139 ± 4.9 | 153 ± 4.8 | 166 ± 4.1 | |
| Post | 58 ± 2 | 93 ± 3.5 | 97 ± 3.0 | 108 ± 3.5 | 119 ± 3.9 | 129 ± 4.2 | 141 ± 4.4 | 153 ± 4.4 * | ||
| Exercise/green tea | RER | Pre | 0.85 ± 0.02 | 0.84 ± 0.01 | 0.86 ± 0.02 | 0.92 ± 0.02 | 1.00 ± 0.02 | 1.10 ± 0.02 | 1.15 ± 0.03 | 1.22 ± 0.03 |
| Post | 0.83 ± 0.02 | 0.83 ± 0.01 | 0.82 ± 0.02 | 0.86 ± 0.01 | 0.92 ± 0.02 | 0.99 ± 0.02 | 1.05 ± 0.02 | 1.10 ± 0.04 * | ||
| HR | Pre | 62 ± 2 | 100 ± 3.5 | 109 ± 3.2 | 119 ± 3.0 | 130 ± 3.0 | 143 ± 3.4 | 156 ± 4.1 | 168 ± 4.1 | |
| Post | 58 ± 1 | 94 ± 3.4 | 100 ± 2.8 | 108 ± 2.5 | 119 ± 2.4 | 133 ± 3.3 | 144 ± 3.3 | 157 ± 4.0 * | ||
| Green tea | RER | Pre | 0.85 ± 0.02 | 0.84 ± 0.02 | 0.84 ± 0.02 | 0.88± 0.02 | 0.94± 0.02 | 1.01 ± 0.02 | 1.08 ± 0.03 | 1.15 ± 0.03 |
| Post | 0.83 ± 0.01 | 0.82 ± 0.02 | 0.83 ± 0.02 | 0.85 ± 0.02 | 0.90 ± 0.02 | 0.98 ± 0.03 | 1.06 ± 0.03 | 1.14 ± 0.04 * | ||
| HR | Pre | 62 ± 3 | 93 ± 2.3 | 100 ± 2.5 | 109 ± 3.1 | 120 ± 3.6 | 132 ± 4.3 | 145 ± 4.6 | 157 ± 4.8 | |
| Post | 61 ± 3 | 91 ± 3.2 | 97 ± 3.4 | 107 ± 4.0 | 118 ± 4.6 | 129 ± 5.5 | 142 ± 5.5 | 154 ± 5.5 | ||
| Control | RER | Pre | 0.85 ± 0.02 | 0.85 ± 0.02 | 0.85 ± 0.02 | 0.91 ± 0.01 | 1.01 ± 0.02 | 1.10 ± 0.02 | 1.18 ± 0.02 | 1.24 ± 0.03 |
| Post | 0.87 ± 0.02 | 0.84 ± 0.01 | 0.84 ± 0.02 | 0.91 ± 0.02 | 1.01 ± 0.02 | 1.09 ± 0.02 | 1.16 ± 0.02 | 1.22 ± 0.03 | ||
| HR | Pre | 63 ± 2 | 105 ± 2.5 | 112 ± 3.0 | 123 ± 3.4 | 136 ± 4.0 | 148 ± 4.1 | 161 ± 3.8 | 169 ± 3.4 | |
| Post | 65 ± 2 | 103 ± 2.2 | 112 ± 2.1 | 122 ± 2.7 | 134 ± 3.0 | 146 ± 3.3 | 158 ± 3.2 | 168 ± 2.6 | ||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gahreman, D.; Heydari, M.; Boutcher, Y.; Freund, J.; Boutcher, S. The Effect of Green Tea Ingestion and Interval Sprinting Exercise on the Body Composition of Overweight Males: A Randomized Trial. Nutrients 2016, 8, 510. https://doi.org/10.3390/nu8080510
Gahreman D, Heydari M, Boutcher Y, Freund J, Boutcher S. The Effect of Green Tea Ingestion and Interval Sprinting Exercise on the Body Composition of Overweight Males: A Randomized Trial. Nutrients. 2016; 8(8):510. https://doi.org/10.3390/nu8080510
Chicago/Turabian StyleGahreman, Daniel, Mehrdad Heydari, Yati Boutcher, Judith Freund, and Stephen Boutcher. 2016. "The Effect of Green Tea Ingestion and Interval Sprinting Exercise on the Body Composition of Overweight Males: A Randomized Trial" Nutrients 8, no. 8: 510. https://doi.org/10.3390/nu8080510
APA StyleGahreman, D., Heydari, M., Boutcher, Y., Freund, J., & Boutcher, S. (2016). The Effect of Green Tea Ingestion and Interval Sprinting Exercise on the Body Composition of Overweight Males: A Randomized Trial. Nutrients, 8(8), 510. https://doi.org/10.3390/nu8080510