The Anticancer Properties of Herba Epimedii and Its Main Bioactive Componentsicariin and Icariside II
Abstract
:1. Introduction
2. Anticancer Effects of Herba Epimedii in Vitro and in Vivo
3. Anticancer Effects of Icariin and Icariside II in Vitro and in Vivo
4. Icariin and Icariside II as Adjuvant Therapy
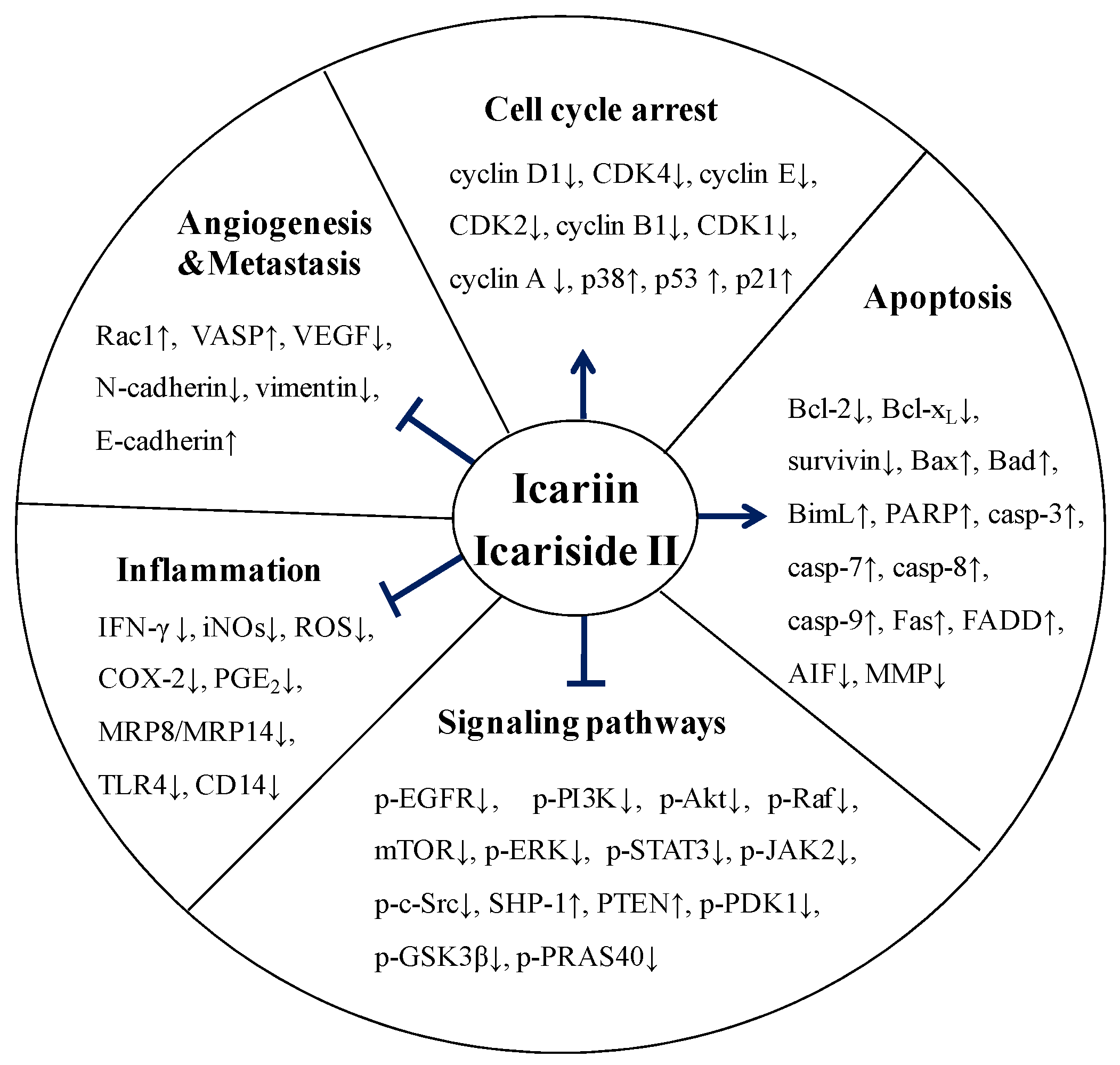
5. Molecular Mechanisms of Anticancer Activity of Icariin and Icariside II
5.1. Effect on Cell Cycle Regulation
5.2. Effect on Apoptosis
5.3. Effect on Angiogenesis and Metastasis
5.4. Effect on Multiple Signaling Pathways
5.5. Anti-Inflammatory Activity
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.G.; Quinn, M.L.; Fabricant, D.S.; Farnsworth, N.R. Plants used against cancer-an extension of Jonathan Hartwell. J. Ethnopharmacol. 2000, 73, 347–377. [Google Scholar] [CrossRef]
- Amin, A.R.; Kucuk, O.; Khuri, F.R.; Shin, D.M. Perspectives for cancer prevention with natural compounds. J. Clin. Oncol. 2009, 27, 2712–2725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, L.X.; Ouyang, L.; Cheng, Y.; Liu, B. Plant natural compounds: Targeting pathways of autophagy as anti-cancer therapeutic agents. Cell Prolif. 2012, 45, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Patil, D.; Rajamohanan, P.R.; Ahmad, A. Isolation, purification and characterization of vinblastine and vincristine from endophytic fungus Fusarium oxysporum isolated from Catharanthus roseus. PLoS ONE 2013, 8, e71805. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, L.; Somfai, S.; Gal, F.; Kellner, B. Comparative studies concerning the tumour inhibition and the toxicology of vinblastine and vincristine. Neoplasma 1970, 17, 345–347. [Google Scholar] [PubMed]
- Cipriani, D. Clinical experience with vinblastine and vincristine. Cancro 1968, 21, 185–189. [Google Scholar] [PubMed]
- Editorial Committee of China Pharmacopoeia. The Chinese Pharmacopoeia, Part I, 2015 ed.; China Chemical Industry Press: Beijing, China, 2015; p. 486. [Google Scholar]
- Ma, H.; He, X.; Yang, Y.; Li, M.; Hao, D.; Jia, Z. The genus Epimedium: An ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2011, 134, 519–541. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Wu, C.F.; Lai, W.P.; Yang, X.J.; Cheung, P.Y.; Yao, X.S.; Leung, P.C.; Wong, M.S. The osteoprotective effect of Herba epimedii (HEP) extract in vivo and in vitro. Evid. Based Complement. Altern. Med. 2005, 2, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.; Guo, Y.; Ma, R.; Fu, M.; Niu, J.; Gao, S.; Zhang, D. Herba Epimedii: An Ancient Chinese Herbal Medicine in the Prevention and Treatment of Osteoporosis. Curr. Pharm. Des. 2016, 22, 328–349. [Google Scholar] [CrossRef] [PubMed]
- Niu, R. Action of the drug Herba Epimedii on testosterone of the mouse plasma and its accessory sexual organ before and after processing. China J. Chin. Mater. Med. 1989, 14, 530–574. [Google Scholar]
- Yu, L.; Li, H.; Huang, G.; Bai, Y.; Dong, Y. Clinical observations on treatment of 120 cases of coronary heart disease with herba epimedii. J. Tradit. Chin. Med. 1992, 12, 30–34. [Google Scholar] [PubMed]
- Zhao, Y.L.; Song, H.R.; Fei, J.X.; Liang, Y.; Zhang, B.H.; Liu, Q.P.; Wang, J.; Hu, P. The effects of Chinese yam-epimedium mixture on respiratory function and quality of life in patients with chronic obstructive pulmonary disease. J. Tradit. Chin. Med. 2012, 32, 203–207. [Google Scholar] [CrossRef]
- Lin, C.C.; Ng, L.T.; Hsu, F.F.; Shieh, D.E.; Chiang, L.C. Cytotoxic effects of Coptis chinensis and Epimedium sagittatum extracts and their major constituents (berberine, coptisine and icariin) on hepatoma and leukaemia cell growth. Clin. Exp. Pharmacol. Physiol. 2004, 31, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Maryam, A.; Qazi, J.I.; Ma, T. Targeting Apoptosis and Multiple Signaling Pathways with Icariside II in Cancer Cells. Int. J. Biol. Sci. 2015, 11, 1100–1112. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liang, M.; Zhang, X.; Zhang, C.; Shen, Z.; Zhang, W. Simultaneous determination of nine flavonoids and qualitative evaluation of Herba Epimedii by high performance liquid chromatography with ultraviolet detection. J. Sep. Sci. 2007, 30, 3207–3213. [Google Scholar] [CrossRef] [PubMed]
- Pei, L.K.; Guo, B.L. A review on research of raw material and cut crude drug of Herba epimedii in last ten years. China J. Chin. Mater. Med. 2007, 32, 466–471. [Google Scholar]
- Xie, X.; Pei, F.; Wang, H.; Tan, Z.; Yang, Z.; Kang, P. Icariin: A promising osteoinductive compound for repairing bone defect and osteonecrosis. J. Biomater. Appl. 2015, 30, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Liu, B.; Sun, J.; Lv, Y.; Luo, Q.; Liu, F.; Dong, J. Regulation of Th17/Treg function contributes to the attenuation of chronic airway inflammation by icariin in ovalbumin-induced murine asthma model. Immunobiology 2015, 220, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.R.; Xu, X.Z.; Wang, Y.H.; Chen, J.W.; Xu, S.W.; Gu, L.Q.; Liu, P.Q. Icariin derivative inhibits inflammation through suppression of p38 mitogen-activated protein kinase and nuclear factor-kappaB pathways. Biol. Pharm. Bull. 2010, 33, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Chen, Y.; Wang, Y.; Liu, J. Roles of the antioxidant properties of icariin and its phosphorylated derivative in the protection against duck virus hepatitis. BMC Vet. Res. 2014, 10. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Choi, Y.J.; Sung, S.H.; Shin, D.I.; Kim, J.W.; Kim, Y.C. Antihepatotoxic activity of icariin, a major constituent of Epimedium koreanum. Planta Med. 1995, 61, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Xu, C.; Wu, X.; Liu, F.; Du, Y.; Sun, J.; Tao, J.; Dong, J. Icariin exerts an antidepressant effect in an unpredictable chronic mild stress model of depression in rats and is associated with the regulation of hippocampal neuroinflammation. Neuroscience 2015, 294, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, H.; Xu, C.; Yang, G.; Tao, J.; Huang, J.; Wu, J.; Duan, X.; Cao, Y.; Dong, J. Neuroprotective effects of icariin on corticosterone-induced apoptosis in primary cultured rat hippocampal neurons. Brain Res. 2011, 1375, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Pei, H.; Lan, C. Icariin Exerts Protective Effect Against Myocardial Ischemia/Reperfusion Injury in Rats. Cell Biochem. Biophys. 2015, 73, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Zhai, M.; He, L.; Ju, X.; Shao, L.; Li, G.; Zhang, Y.; Liu, Y.; Zhao, H. Icariin Acts as a Potential Agent for Preventing Cardiac Ischemia/Reperfusion Injury. Cell Biochem. Biophys. 2015, 72, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, K.; Yan, M.; Zhang, Y.; Wang, Y.; Ren, L. Effects and mechanisms of icariin on atherosclerosis. Int. J. Clin. Exp. Med. 2015, 8, 3585–3589. [Google Scholar] [PubMed]
- Xia, Q.; Xu, D.; Huang, Z.; Liu, J.; Wang, X.; Wang, X.; Liu, S. Preparation of icariside II from icariin by enzymatic hydrolysis method. Fitoterapia 2010, 81, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Gu, F.; Zhang, Y.; Liu, T.; Guo, P.; Huang, Y. Icariside II promotes osteogenic differentiation of bone marrow stromal cells in beagle canine. Int. J. Clin. Exp. Pathol. 2015, 8, 4367–4377. [Google Scholar] [PubMed]
- Yin, C.; Deng, Y.; Gao, J.; Li, X.; Liu, Y.; Gong, Q. Icariside II, a novel phosphodiesterase-5 inhibitor, attenuates streptozotocin-induced cognitive deficits in rats. Neuroscience 2016, 328, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Xiong, D.; Yin, C.; Liu, B.; Shi, J.; Gong, Q. Icariside II protects against cerebral ischemia-reperfusion injury in rats via nuclear factor-kappaB inhibition and peroxisome proliferator-activated receptor up-regulation. Neurochem. Int. 2016, 96, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tang, L.; Li, Q. Antiproliferative activities of alcohol extracts of Herba Epimedii and Cortex Fraxini on breast cancer cell proliferation: In vitro study. China Pharm. 2007, 18, 1124–1127. [Google Scholar]
- Yao, X.; Jia, L.Q.; Tan, H.Y.; Gao, F.Y.; Cui, J.; Li, H. Effects of Epimedium on rat tumor growth and bone destruction of breast cancer rats with bone metastasis. Beijing J. Trad. Chin. Med. 2008, 27, 882–884. [Google Scholar]
- Wang, Z.M.; Song, N.; Ren, Y.L. Anti-proliferative and cytoskeleton-disruptive effects of icariin on HepG2 cells. Mol. Med. Rep. 2015, 12, 6815–6820. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dong, P.; Wang, J.; Zhang, J.; Gu, J.; Wu, X.; Wu, W.; Fei, X.; Zhang, Z.; Wang, Y.; et al. Icariin, a natural flavonol glycoside, induces apoptosis in human hepatoma SMMC-7721 cells via a ROS/JNK-dependent mitochondrial pathway. Cancer Lett. 2010, 298, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhu, D.; Lou, Y. A novel anticancer agent, icaritin, induced cell growth inhibition, G1 arrest and mitochondrial transmembrane potential drop in human prostate carcinoma PC-3 cells. Eur. J. Pharmacol. 2007, 564, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Lee, H.J.; Ahn, K.S.; Kim, S.H.; Nam, D.; Kim, D.K.; Choi, D.Y.; Ahn, K.S.; Lu, J.; Kim, S.H. Cyclooxygenase-2/prostaglandin E2 pathway mediates icariside II induced apoptosis in human PC-3 prostate cancer cells. Cancer Lett. 2009, 280, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Yang, Y.; Liu, Y.; Jiang, S.; Di, S.; Hu, W.; Ma, Z.; Li, T.; Zhu, Y.; Xin, Z.; et al. Icariin displays anticancer activity against human esophageal cancer cells via regulating endoplasmic reticulum stress-mediated apoptotic signaling. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, K.; Zhao, F. Icariin regulates the proliferation and apoptosis of human ovarian cancer cells through microRNA-21 by targeting PTEN, RECK and Bcl-2. Oncol. Rep. 2015, 33, 2829–2836. [Google Scholar] [CrossRef] [PubMed]
- Di, S.; Fan, C.; Yang, Y.; Jiang, S.; Liang, M.; Wu, G.; Wang, B.; Xin, Z.; Hu, W.; Zhu, Y.; et al. Activation of endoplasmic reticulum stress is involved in the activity of icariin against human lung adenocarcinoma cells. Apoptosis 2015, 20, 1229–1241. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Feng, L.; Zhong, R.; Xia, Z.; Zhang, L.; Cui, L.; Yan, H.; Jia, X.; Zhang, Z. Icariside II inhibits the EMT of NSCLC cells in inflammatory microenvironment via down-regulation of Akt/NF-kappaB signaling pathway. Mol. Carcinog. 2016. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, J.; Hu, S.; Liu, J. Icariin Induced B16 Melanoma Tumor Cells Apoptosis, Suppressed Tumor Growth and Metastasis. Iran. J. Public Health 2014, 43, 847–848. [Google Scholar] [PubMed]
- Wu, J.; Song, T.; Liu, S.; Li, X.; Li, G.; Xu, J. Icariside II inhibits cell proliferation and induces cell cycle arrest through the ROS-p38-p53 signaling pathway in A375 human melanoma cells. Mol. Med. Rep. 2015, 11, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xu, J.; Eksioglu, E.A.; Chen, X.; Zhou, J.; Fortenbery, N.; Wei, S.; Dong, J. Icariside II induces apoptosis of melanoma cells through the downregulation of survival pathways. Nutr. Cancer 2013, 65, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Guan, M.; Wong, P.F.; Yu, H.; Dong, J.; Xu, J. Icariside II potentiates paclitaxel-induced apoptosis in human melanoma A375 cells by inhibiting TLR4 signaling pathway. Food Chem. Toxicol. 2012, 50, 3019–3024. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Hao, J.; Pu, J.; Zhao, L.; Lu, Z.; Hu, J.; Yu, Q.; Wang, Y.; Xie, Y.; Li, G. Icariin induces apoptosis in mouse MLTC-10 Leydig tumor cells through activation of the mitochondrial pathway and down-regulation of the expression of piwil4. Int. J. Oncol. 2011, 39, 973–980. [Google Scholar] [PubMed]
- Wang, Y.; Dong, H.; Zhu, M.; Ou, Y.; Zhang, J.; Luo, H.; Luo, R.; Wu, J.; Mao, M.; Liu, X.; et al. Icariin exterts negative effects on human gastric cancer cell invasion and migration by vasodilator-stimulated phosphoprotein via Rac1 pathway. Eur. J. Pharmacol. 2010, 635, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sun, X.H.; Fan, W.J.; Jiang, X.M.; Li, A.W. Icariin induces S-phase arrest and apoptosis in medulloblastoma cells. Cell Mol. Biol. 2016, 62, 123–129. [Google Scholar] [PubMed]
- Zhang, C.; Yang, L.; Geng, Y.D.; An, F.L.; Xia, Y.Z.; Guo, C.; Luo, J.G.; Zhang, L.Y.; Guo, Q.L.; Kong, L.Y. Icariside II, a natural mTOR inhibitor, disrupts aberrant energy homeostasis via suppressing mTORC1–4E-BP1 axis in sarcoma cells. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.D.; Zhang, C.; Shi, Y.M.; Xia, Y.Z.; Guo, C.; Yang, L.; Kong, L.Y. Icariside II-induced mitochondrion and lysosome mediated apoptosis is counterbalanced by an autophagic salvage response in hepatoblastoma. Cancer Lett. 2015, 366, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.D.; Yang, L.; Zhang, C.; Kong, L.Y. Blockade of epidermal growth factor receptor/mammalian target of rapamycin pathway by Icariside II results in reduced cell proliferation of osteosarcoma cells. Food Chem. Toxicol. 2014, 73, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Eun, J.S.; Kim, D.K.; Li, R.H.; Shin, T.Y.; Park, H.; Cho, N.P.; Soh, Y. Icariside II from Epimedium koreanum inhibits hypoxia-inducible factor-1alpha in human osteosarcoma cells. Eur. J. Pharmacol. 2008, 579, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zuo, F.; Du, J.; Wong, P.F.; Qin, H.; Xu, J. Icariside II induces apoptosis via inhibition of the EGFR pathways in A431 human epidermoid carcinoma cells. Mol. Med. Rep. 2013, 8, 597–602. [Google Scholar] [PubMed]
- Kang, S.H.; Jeong, S.J.; Kim, S.H.; Kim, J.H.; Jung, J.H.; Koh, W.; Kim, J.H.; Kim, D.K.; Chen, C.Y.; Kim, S.H. Icariside II induces apoptosis in U937 acute myeloid leukemia cells: Role of inactivation of STAT3-related signaling. PLoS ONE 2012, 7, e28706. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chen, X.; Guo, B.; Huang, W.; Shen, T.; Sun, X.; Xiao, P.; Zhou, Q. Induction of apoptosis by Icariside II through extrinsic and intrinsic signaling pathways in human breast cancer MCF7 cells. Biosci. Biotechnol. Biochem. 2012, 76, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Ahn, K.S.; Jeong, S.J.; Kwon, T.R.; Jung, J.H.; Yun, S.M.; Han, I.; Lee, S.G.; Kim, D.K.; Kang, M.; et al. Janus activated kinase 2/signal transducer and activator of transcription 3 pathway mediates icariside II-induced apoptosis in U266 multiple myeloma cells. Eur. J. Pharmacol. 2011, 654, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wu, J.; Chen, X.; Fortenbery, N.; Eksioglu, E.; Kodumudi, K.N.; Pk, E.B.; Dong, J.; Djeu, J.Y.; Wei, S. Icariin and its derivative, ICT, exert anti-inflammatory, anti-tumor effects, and modulate myeloid derived suppressive cells (MDSCs) functions. Int. Immunopharmacol. 2011, 11, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.X.; Fichtner, I.; Becker, M.; Lemm, M.; Wang, X.M. Anti-proliferative efficacy of icariin on HepG2 hepatoma and its possible mechanism of action. Am. J. Chin. Med. 2009, 37, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Y.; Guo, H.; Guo, M. Synergistic Anti-Cancer Effects of Icariin and Temozolomide in Glioblastoma. Cell Biochem. Biophys. 2015, 71, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, H.; Dai, L.; Song, T.; Li, P.; Liu, Y.; Wang, L. Arsenic Trioxide and Icariin Show Synergistic Anti-leukemic Activity. Cell Biochem. Biophys. 2015, 73, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, M.; Wang, L.; Ji, S.; Zhang, J.; Zhang, C. Icariin synergizes with arsenic trioxide to suppress human hepatocellular carcinoma. Cell Biochem. Biophys. 2014, 68, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, L.; Xia, Y.; Guo, C.; Kong, L. Icariin enhances cytotoxicity of doxorubicin in human multidrug-resistant osteosarcoma cells by inhibition of ABCB1 and down-regulation of the PI3K/Akt pathway. Biol. Pharm. Bull. 2015, 38, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.B.; Li, X.X.; Zheng, H.T.; Li, D.W.; Cai, G.X.; Peng, J.J.; Gu, W.L.; Guan, Z.Q.; Xu, Y.; Cai, S.J. Icariin-mediated inhibition of NF-kappaB activity enhances the in vitro and in vivo antitumour effect of 5-fluorouracil in colorectal cancer. Cell Biochem. Biophys. 2014, 69, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.C.; Liu, J.L.; Ding, Y.B.; Xia, J.G.; Chen, G.Y. Icariin potentiates the antitumor activity of gemcitabine in gallbladder cancer by suppressing NF-kappaB. Acta Pharmacol. Sin. 2013, 34, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.G.; Alvero, A.B.; Chen, R.; Silasi, D.A.; Abrahams, V.M.; Chan, S.; Visintin, I.; Rutherford, T.; Mor, G. TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Res. 2006, 66, 3859–3868. [Google Scholar] [CrossRef] [PubMed]
- Nurse, P.; Masui, Y.; Hartwell, L. Understanding the cell cycle. Nat. Med. 1998, 4, 1103–1106. [Google Scholar] [CrossRef] [PubMed]
- Sa, G.; Das, T. Anti cancer effects of curcumin: Cycle of life and death. Cell Div. 2008, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Elledge, S.J. Cell cycle checkpoints: Preventing an identity crisis. Science 1996, 274, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Leonard, C.J.; Canman, C.E.; Kastan, M.B. The role of p53 in cell-cycle control and apoptosis: Implications for cancer. Important Adv. Oncol. 1995, 121, 33–42. [Google Scholar]
- Kastan, M.B.; Canman, C.E.; Leonard, C.J. P53, cell cycle control and apoptosis: Implications for cancer. Cancer Metastasis Rev. 1995, 14, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Bulavin, D.V.; Saito, S.; Hollander, M.C.; Sakaguchi, K.; Anderson, C.W.; Appella, E.; Fornace, A.J., Jr. Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J. 1999, 18, 6845–6854. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, K.; Van Bockstaele, D.R.; Berneman, Z.N. The cell cycle: A review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003, 36, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; Peters, G. Genetic alterations of cyclins, cyclin-dependent kinases, and Cdk inhibitors in human cancer. Adv. Cancer Res. 1996, 68, 67–108. [Google Scholar] [PubMed]
- Noble, M.; Barrett, P.; Endicott, J.; Johnson, L.; McDonnell, J.; Robertson, G.; Zawaira, A. Exploiting structural principles to design cyclin-dependent kinase inhibitors. Biochim. Biophys. Acta 2005, 1754, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Cotter, T.G. Apoptosis and cancer: The genesis of a research field. Nat. Rev. Cancer 2009, 9, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, A.; Dixit, V.M. Death receptors: Signaling and modulation. Science 1998, 281, 1305–1308. [Google Scholar] [CrossRef] [PubMed]
- Kischkel, F.C.; Hellbardt, S.; Behrmann, I.; Germer, M.; Pawlita, M.; Krammer, P.H.; Peter, M.E. Cytotoxicity-Dependent Apo-1 (Fas/Cd95)-Associated Proteins Form a Death-Inducing Signaling Complex (Disc) with the Receptor. EMBO. J. 1995, 14, 5579–5588. [Google Scholar] [PubMed]
- Saelens, X.; Festjens, N.; Vande Walle, L.; van Gurp, M.; van Loo, G.; Vandenabeele, P. Toxic proteins released from mitochondria in cell death. Oncogene 2004, 23, 2861–2874. [Google Scholar] [CrossRef] [PubMed]
- Chinnaiyan, A.M. The apoptosome: Heart and soul of the cell death machine. Neoplasia. 1999, 1, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Cory, S.; Adams, J.M. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer. 2002, 2, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Schuler, M.; Green, D.R. Mechanisms of p53-dependent apoptosis. Biochem. Soc. Trans. 2001, 29, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Igney, F.H.; Krammer, P.H. Death and anti-death: Tumour resistance to apoptosis. Nat. Rev. Cancer 2002, 2, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B. Nuclear factor-kappaB: The enemy within. Cancer Cell 2004, 6, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, Q. Therapeutic targets of multiple angiogenic factors for the treatment of cancer and metastasis. Adv. Cancer Res. 2007, 97, 203–224. [Google Scholar] [PubMed]
- Hu, Y.; Liu, K.; Yan, M.; Zhang, Y.; Wang, Y.; Ren, L. Icariin inhibits oxidized low-density lipoprotein-induced proliferation of vascular smooth muscle cells by suppressing activation of extracellular signal-regulated kinase 1/2 and expression of proliferating cell nuclear antigen. Mol. Med. Rep. 2016, 13, 2899–2903. [Google Scholar] [PubMed]
- Ekstrand, A.J.; Sugawa, N.; James, C.D.; Collins, V.P. Amplified and rearranged epidermal growth factor receptor genes in human glioblastomas reveal deletions of sequences encoding portions of the N- and/or C-terminal tails. Proc. Natl. Acad. Sci. USA 1992, 89, 4309–4313. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Lo, H.W. Landscape of EGFR signaling network in human cancers: Biology and therapeutic response in relation to receptor subcellular locations. Cancer Lett. 2012, 318, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Mora, L.B.; Buettner, R.; Seigne, J.; Diaz, J.; Ahmad, N.; Garcia, R.; Bowman, T.; Falcone, R.; Fairclough, R.; Cantor, A.; et al. Constitutive activation of Stat3 in human prostate tumors and cell lines: Direct inhibition of Stat3 signaling induces apoptosis of prostate cancer cells. Cancer Res. 2002, 62, 6659–6666. [Google Scholar] [PubMed]
- Dolled-Filhart, M.; Camp, R.L.; Kowalski, D.P.; Smith, B.L.; Rimm, D.L. Tissue microarray analysis of signal transducers and activators of transcription 3 (Stat3) and phospho-Stat3 (Tyr705) in node-negative breast cancer shows nuclear localization is associated with a better prognosis. Clin. Cancer Res. 2003, 9, 594–600. [Google Scholar] [PubMed]
- Hsiao, J.R.; Jin, Y.T.; Tsai, S.T.; Shiau, A.L.; Wu, C.L.; Su, W.C. Constitutive activation of STAT3 and STAT5 is present in the majority of nasopharyngeal carcinoma and correlates with better prognosis. Brit. J. Cancer 2003, 89, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Santarpia, L.; Lippman, S.M.; El-Naggar, A.K. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert. Opin. Ther. Targets 2012, 16, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.M.; Pruitt, K.; McFall, A.; Shaub, A.; Der, C.J. Understanding Ras: ‘It ain’t over ‘til it’s over’. Trends Cell Biol. 2000, 10, 147–154. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef] [PubMed]
- Guertin, D.A.; Sabatini, D.M. Defining the role of mTOR in cancer. Cancer Cell 2007, 12, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Shacter, E.; Weitzman, S.A. Chronic inflammation and cancer. Oncology 2002, 16, 217–229. [Google Scholar] [PubMed]
- Bunt, S.K.; Sinha, P.; Clements, V.K.; Leips, J.; Ostrand-Rosenberg, S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J. Immunol. 2006, 176, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.; Davydova, J.; Brown, E.; Han, J.; Yamamoto, M.; Vickers, S.M. Delivery of interferon alpha using a novel Cox2-controlled adenovirus for pancreatic cancer therapy. Surgery 2012, 152, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Ristimaki, A.; Honkanen, N.; Jankala, H.; Sipponen, P.; Harkonen, M. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res. 1997, 57, 1276–1280. [Google Scholar] [PubMed]
- Basler, J.W.; Piazza, G.A. Nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 selective inhibitors for prostate cancer chemoprevention. J. Urol. 2004, 171, S59–S63. [Google Scholar] [CrossRef] [PubMed]



| Cancer Types | Components | Cell Lines | Concentrations | Effects and Molecular Targets | Reference | |
|---|---|---|---|---|---|---|
| Con. Range | IC50 | |||||
| Hepatocellular carcinoma | Icariin | HepG2 | 10 μM | NA | G0/G1↑, S↓, Bcl-2↓ | [37] |
| SMMC-7721 | 5–20 μM | around 10 μM | cleaved caspase-3/9↑, mitochondria cytochrome c↓, cytosol cytochrome c↑, cleaved PARP1↑, XIAP↓, MMP↓, Bcl-2↓, Bax↑, p-JNK↑, ROS↑ | [38] | ||
| Prostate carcinoma | Icariin | PC-3 | 30 μM | NA | Cyclin D1↓, CDK4↓ | [39] |
| Icariside II | PC-3 | 0–40 μM | around 20 μM | MMP↓, cleaved caspase-3/8/9↑, cleaved PARP↑, COX-2↓, iNOS↓, VEGF↓, PGE2↓ | [40] | |
| Esophageal cancer | Icariin | EC109 | 20–80 μM | 106.13 μM (12 h) | cleaved caspase-9↑, ROS↑, NADPH oxidase activity↑, GSH↓, GRP78↑, ATF4↑, CHOP↑, p-PERK↑, p-eIF2α↑, Bcl2↓, PUMA↑ | [41] |
| 73.65 μM (24 h) | ||||||
| 38.59 μM (36 h) | ||||||
| TE1 | 20–80 μM | 115.29 μM (12 h) | ||||
| 76.77 μM (24 h) | ||||||
| 42.21 μM (36 h) | ||||||
| Ovarian cancer | Icariin | A2780 | 13–50 μM | NA | caspase-3 activity↑, miR-21↓ PTEN↑ RECK↑ Bcl-2↓ | [42] |
| Lung cancer | Icariin | A549 | 25–100 μM | 118.25 μM (12 h) | ROS↑, caspase 3 activity↑, GSH↓, ERS-related molecules↑(p-PERK, ATF6, GRP78, p-eIF2a, and CHOP), Bcl-2↓, PUMA↑ | [43] |
| 86.21 μM (24 h) | ||||||
| 56.8 μM (36 h) | ||||||
| Icariside II | A549 | 0–20 µM | NA | vimentin↓, N-cadherin↓, NF-κB↓, p-IκBα↓, p65/IκB↑, p-Akt↓ p-GSK-3β↓ | [44] | |
| H1299 | 0–20 µM | NA | ||||
| Melanoma | Icariin | B16 | 20–200 μg/mL | 84.3μg/mL (72 h) | procaspase-9↓ cleaved caspase-9↑ | [45] |
| Icariside II | A375 | 0–100 μM | 10.6 μM | G0/G1 phase↑, S↓, G2/M arrest↑, cyclin E↓, CDK2↓, cyclin B1↓, P‑CDK1↓, ROS↑, p-p38↑, p-p53↑, p21↑, cleaved caspase-3↑, survivin↓, p-STAT3↓, p-ERK↓, cleaved PARP↑ | [46,47,48] | |
| SK-MEL-5 | 0–100 μM | 11.1 μM | ||||
| Leydig cell tumor | Icariin | MLTC-1 | 12.5–100 μg/mL | 50 μg/mL (48 h) | S↓, Bcl-2↓, Bax↑, cytochrome c↑, cleaved caspase-3/9↑, piwil4↓ | [49] |
| Gastric adenocarcinoma | Icariin | BGC-823 | 20–200 μg/mL | 128 μg/mL | Rac1↓, VASP↓ | [50] |
| Medulloblastoma | Icariin | Daoy | NA | NA | Cyclin A↓, CDK2↓, Cyclin B1↓, cleaved caspase-3↑, cleaved caspase-9↑, PARP↑, Bcl-2↓ | [51] |
| D341 | NA | NA | ||||
| Sarcoma | Icariside II | U2OS | 0–30 µM | NA | 4E-BP1↑, mTORC1↓, p-S6K(Thr389)↓, p-S6(Ser235/236)↓, p-4E-BP1 (Ser65)↓ | [52] |
| SW1353 | 0–20 µM | NA | ||||
| S180 | 0–20 µM | NA | ||||
| Hepatoblastoma | Icariside II | HepG2 | 0–30 μM | NA | △ψm↓, ROS↑, Bax/Bcl-2↑, cleaved-Bid↑, LAMP1↑, LMP↑, cleaved caspase-8/9/7/3/PARP↑, LC3B-II↑, SQSTM1↑ | [53] |
| Osteosarcoma | Icariside II | MG-63 | 10–35 μM | NA | p-EGFR↓, p-PI3K↓, p-Akt↓, p-PDK1↓, p-Raf↓, p-mTOR↓, p-PDK1↓, p-PRAS40↓, p-GSK3β↓, p-ERK↓ | [54] |
| Saos-2 | 10–35 μM | NA | ||||
| HOS | 0–10 μM | NA | HIF-1α↓, VEGF↓, uPAR↓, ADM↓, MMP2↓, Glut4↓, MCT4↓, aldolase A↓, enolase 1↓ | [55] | ||
| Epidermoid carcinoma | Icariside II | A431 cell line | 0–100 μM | NA | cleaved caspase 9↑, cleaved PARP↑, caspase 9↓, PARP↓, p-STAT3↓, p-ERK↓, p-AKT↑, p-EGFR↑↓ | [56] |
| Acute myeloid leukemia | Icariside II | U937 | 0–50 μM | NA | cleaved PARP↑, procaspase 3↓, Bcl-2↓, Bcl-XL↓, survivin↓, COX-2↓, p-STAT3↓, p-JAK2↓, p-Src↓ | [57] |
| Breast cancer | Icariside II | MCF-7 | 0–100 μM | 72.73 μM (24 h) | MMP↓, cleaved caspase-3/7/8/9↑, cleaved PARP↑, △ψm↓, cytosol cyto c↑, cytosol AIF↑, mitochondrial cyto c↓, mitochondrial AIF↓, Fas↑, FADD↑, Bcl-xL↑, Bax↑, BimL↑ | [58] |
| 57.98 μM (48 h) | ||||||
| 50.95 μM (72 h) | ||||||
| 37.75 μM (96 h) | ||||||
| MDA-MB-231 | 0–100 μM | 97.14 μM (24 h) | ||||
| 62.75 μM (48 h) | ||||||
| 42.40 μM (72 h) | ||||||
| 38.65 μM (96 h) | ||||||
| Multiple myeloma | Icariside II | U266 | 0-100 μM | NA | p-STAT3↓, p-JAK2↓, p-c-Src↓, SHP-1↑, PTEN↑, cyclin D1↓, Bcl-2↓, Bcl-xL↓, survivin↓ VEGF↓, COX-2↓, cleaved caspase-3↑, p-PARP↑ | [59] |
| Components | Tumor Models | Transplantation | Treatment | Results | Reference |
|---|---|---|---|---|---|
| Icariin | Esophageal cancer EC109 | Subcutaneous injection | Given by i.p. 60 and 120 mg/kg every day for 20 days | Significantly inhibit tumor growth | [41] |
| Icariin | Lung adenocarcinoma A549 | Subcutaneous injection | Given by i.p. 100 or 150 mg/kg (5 days/week) for 4 weeks | Significantly inhibit tumor growth | [43] |
| Icariin | Melanoma B16 | Subcutaneous injection into the right flank | Given by p.o. 65 mg/kg every day for 20 days | Apparently inhibit tumor growth | [45] |
| Icariin | Mammary carcinoma 4 T1-Neu | Subcutaneous inoculation tumor bearing mice | Given by i.p. 100 mg/kg three times a week starting on day 7 until day 28 | 61% reduction of tumor growth | [60] |
| Icariin | Hepatoma SMMC-7721 | Subcutaneous injectioninto the armpit | Given by i.p. 15, 30, and 60 mg/kg every day for 20 days | 38.7%, 54.7%, and 69.9% inhibition in tumor volume, respectively | [38] |
| Icariin | Hepatoma HepG2 | Subcutaneous injection | Given by i.g. 80 mg/kg for 35 days | 55.6% inhibition in tumor weight; 47.2% inhibition in tumor volume | [61] |
| Icariside II | Sarcoma S180 | Subcutaneous injection into the right armpit | Given by i.v. 10, 20, 30 mg/kg everyday for 9 days | 33.0%, 51.3%, and 62.6% reduction in tumor weight, respectively | [52] |
| Icariside II | Lung cancer A549 | Subcutaneous injection into the flank area | Given by i.v. 30 and 60 mg/kg once every 3 days for 24 consecutive days | Strongly suppress tumor volume | [44] |
| Icariside II | Liver carcinoma H22 | Inoculation | Given by i.v.10, 20, 30 mg/kg everyday for 9 days | Inhibit tumor growth | [53] |
| Icariside II | Sarcoma S180 | Subcutaneous injection into the right flanks | Given by i.p. 10, 20 and 30 mg/kg everyday for 10 days | Inhibit tumor proliferation | [54] |
| Icariside II | Melanoma B16 | Subcutaneous injection into the right flank | Given by i.p. 50 mg/kg and 100 mg/kg 3 times for a week | 41% and 49% decrease in tumor volume | [47] |
| Component | Chemotherapeutic Drugs | Cancer Types | Cell Lines | Tumor Models | Molecular Targets | Reference |
|---|---|---|---|---|---|---|
| Icariin | Temozolomide | Glioblastomamultiforme | U87MG | NF-κB↓ | [62] | |
| Icariin | Arsenic Trioxide | Acute promyelocytic leukemia | HL-60 | Xenograft murine model (HepG2) | ROS↑ | [63,64] |
| Hepatocellular carcinoma | NB4 | |||||
| SMMC-7721 | ROS↑ NF-κB↓cyclin D1↓ Bcl-2↓Bcl-xL↓ COX-2↓survivin↓ VEGF↓ | |||||
| HepG2 | ||||||
| Icariin | Doxorubicin | Osteosarcoma | MG-63/DOX | MDR1↓ PI3K/Akt pathway↓ | [65] | |
| Icariin | 5-Fluorouracil | Colorectal cancer | HT29 | Xenograft murine model (HCT116) | NF-κB↓ cyclin D1↓ caspase-8↑ caspase-9↑ caspase-3↑Bax↑ PARP↑Bcl-xL↑ | [66] |
| HCT116 | ||||||
| Icariin | Gemcitabine | Gallbladder cancer | GBC-SD | Xenograft murine model (GBC-SD) | NF-κB↓ caspase-3↑ G0/G1 phase arrest↑ Bcl-2↓Bcl-xL↓ | [67] |
| SGC-996 | ||||||
| Icariside II | Paclitaxel | Melanoma | A375 | TLR4–MyD88–ERK↓ caspase-3↑ IL-8 ↓ VEGF↓ | [48] | |
| Icariside II | Bortezomib | Multiple myeloma | U266 | STAT3↓ JAK2↓ c-Src↓ SHP-1↓ PTEN↓ Bcl-2↓Bcl-xL↓survivin↓cyclin D1↓ COX-2↓ VEGF↓ | [59] | |
| Thalidomide | U266 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Wu, J.; Luo, Q.; Mo, S.; Lyu, Y.; Wei, Y.; Dong, J. The Anticancer Properties of Herba Epimedii and Its Main Bioactive Componentsicariin and Icariside II. Nutrients 2016, 8, 563. https://doi.org/10.3390/nu8090563
Chen M, Wu J, Luo Q, Mo S, Lyu Y, Wei Y, Dong J. The Anticancer Properties of Herba Epimedii and Its Main Bioactive Componentsicariin and Icariside II. Nutrients. 2016; 8(9):563. https://doi.org/10.3390/nu8090563
Chicago/Turabian StyleChen, Meixia, Jinfeng Wu, Qingli Luo, Shuming Mo, Yubao Lyu, Ying Wei, and Jingcheng Dong. 2016. "The Anticancer Properties of Herba Epimedii and Its Main Bioactive Componentsicariin and Icariside II" Nutrients 8, no. 9: 563. https://doi.org/10.3390/nu8090563
APA StyleChen, M., Wu, J., Luo, Q., Mo, S., Lyu, Y., Wei, Y., & Dong, J. (2016). The Anticancer Properties of Herba Epimedii and Its Main Bioactive Componentsicariin and Icariside II. Nutrients, 8(9), 563. https://doi.org/10.3390/nu8090563





