Neuroprotective Effects of Aged Garlic Extract on Cognitive Dysfunction and Neuroinflammation Induced by ?-Amyloid in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Materials and Plant Extract Preparation
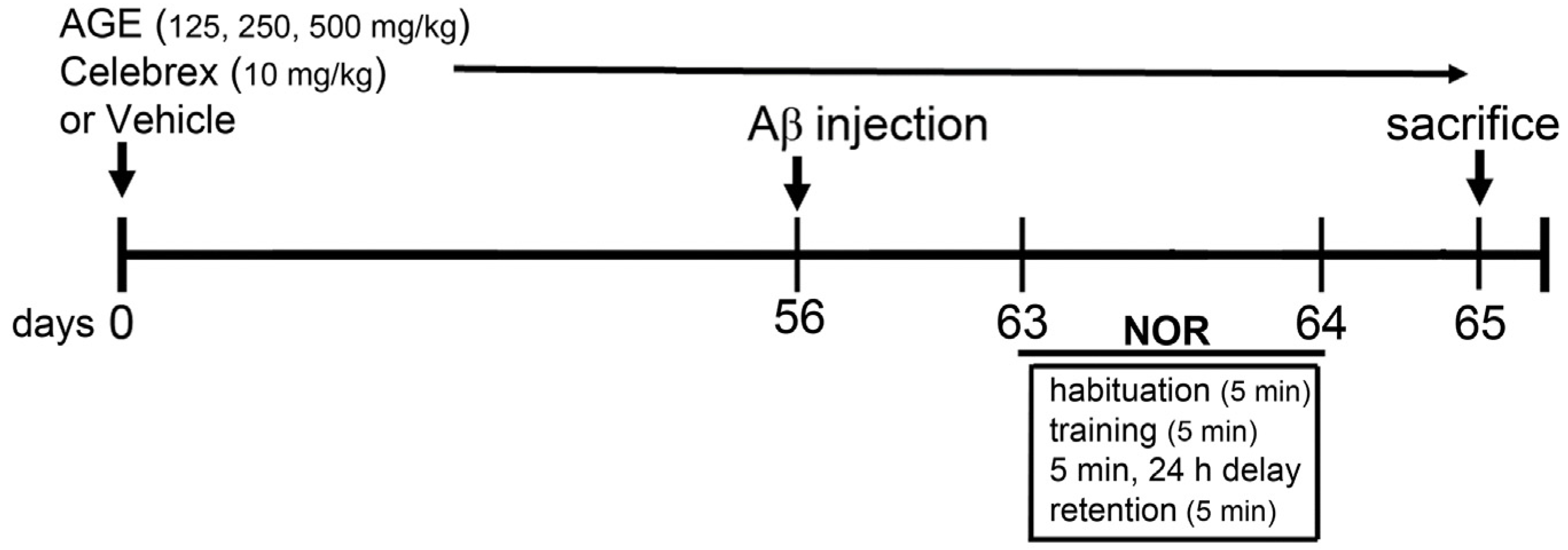
2.3. Aβ (1-42) Injection and Drug Treatments
2.4. Novel Object Recognition (NOR)
2.5. Tissue Processing
2.6. Immunohistochemistry
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
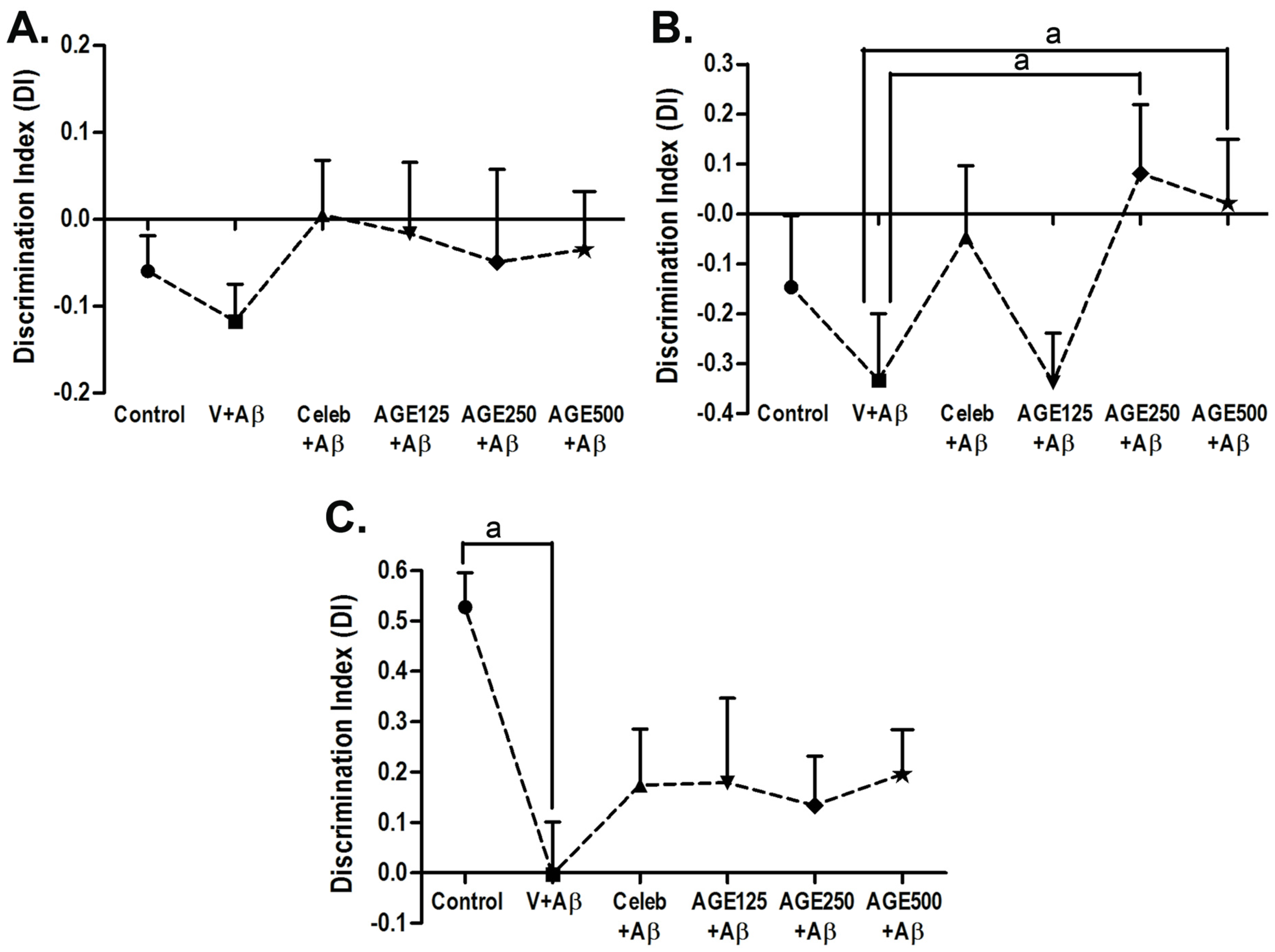
3.1. Effect of AGE On Recognition Memory
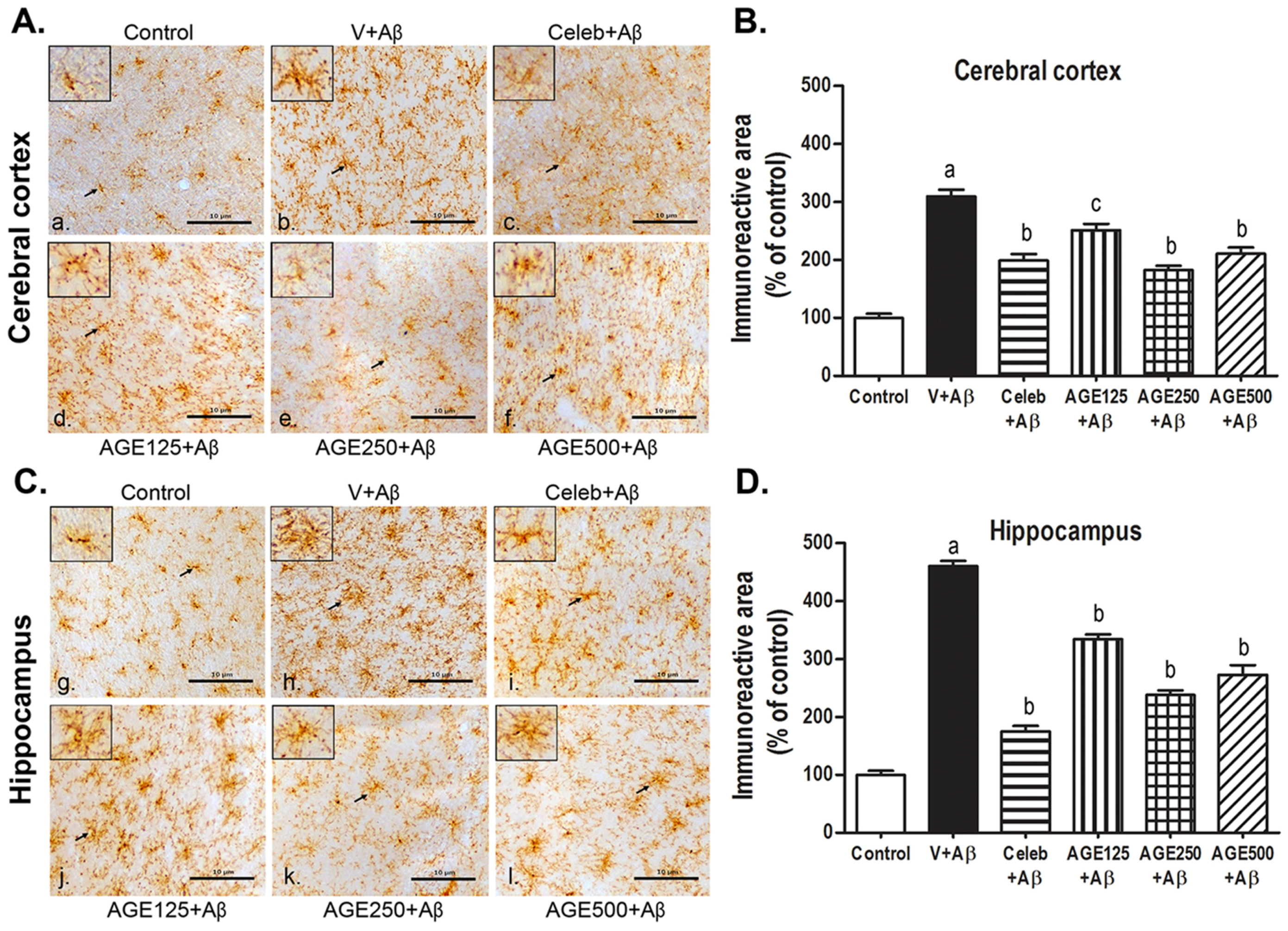
3.2. Effect of AGE on Microglia Activation in the Cerebral Cortex and Hippocampus
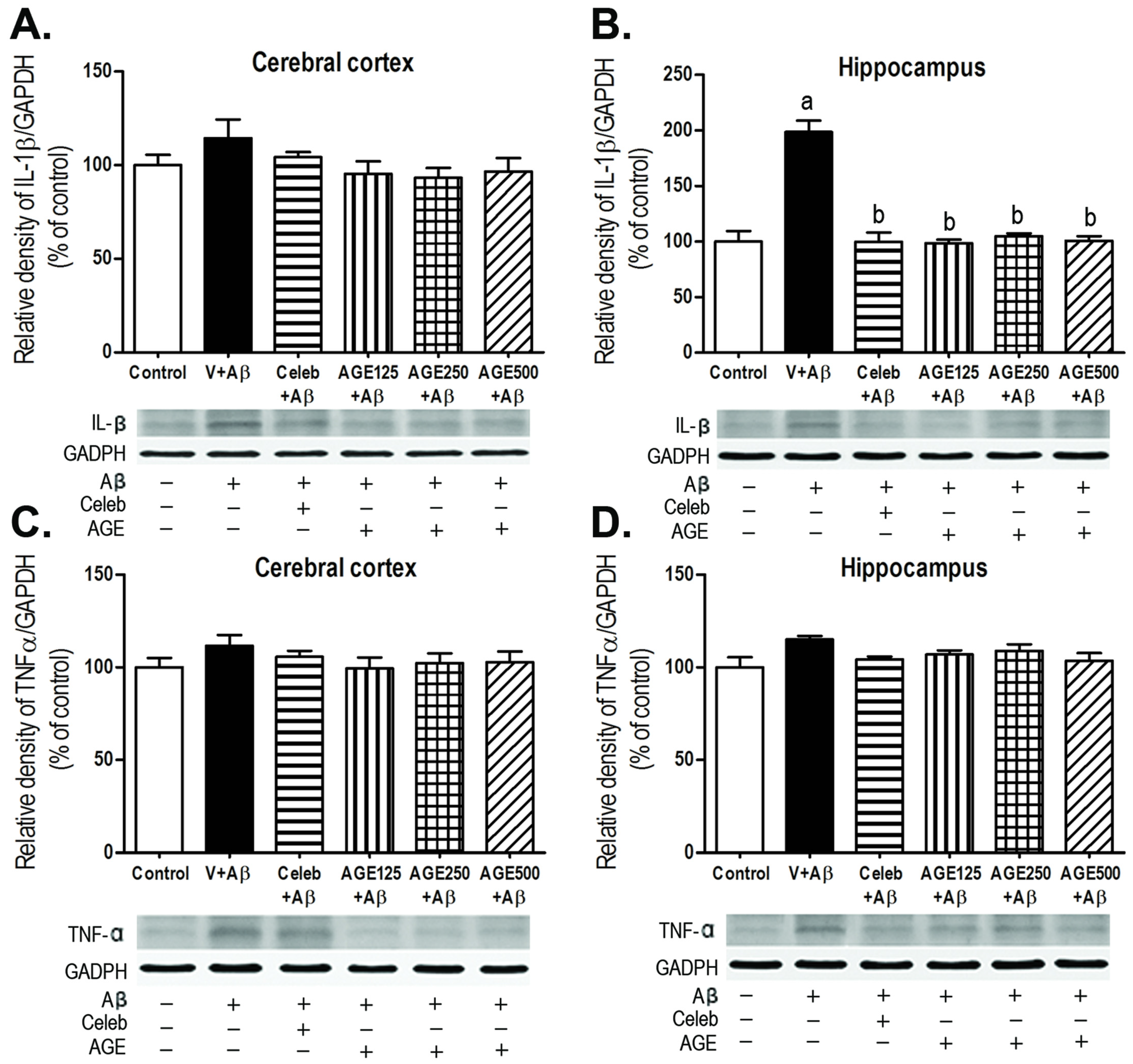
3.3. Effect of AGE on IL-1β and TNFα Expression in the Cerebral Cortex and Hippocampus
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Aβ | β-amyloid |
| AD | Alzheimer’s disease |
| AGE | Aged garlic extract |
| ANOVA | analysis of variance |
| AP | anterior-posterior |
| BSA | bovine serum albumin |
| BW | body weight |
| Celeb | Celebrex |
| CD11b | anti-integrin αM |
| COX-2 | cyclooygenase-2 |
| CRD-HHP | Center for Research and Development of Herbal Health Products |
| DI | discrimination index |
| DPX | Depex mounting medium |
| F | familiar object |
| G | Gram |
| GAPDH | anti-glyceraldehyde 3 phosphate dehydrogenase |
| H | Hour |
| H2O2 | hydrogen peroxide |
| ICV | Intracerebroventricular |
| IL-1β | interleukin-1β |
| Kg | Kilogram |
| M | Molar |
| Mg | Milligram |
| Min | Minute |
| MO | Missouri |
| μL | Microliter |
| µm | Micrometer |
| ML | medial-lateral |
| N | novel object |
| NLRP3 | NOD-, LRR- and pyrin domain-containing 3 |
| NOR | novel object recognition |
| NSAIDs | non-steroidal anti-inflammatory drugs |
| PBS | phosphate buffer solution |
| ROS | reactive oxygen species |
| SAC | S-allyl cysteine |
| S.E.M. | standard error of mean |
| SI | superior-inferior |
| TBS-T | tris-buffered saline containing 0.1% tween 20 |
| TLR | Toll-like receptor |
| TNFα | tumor necrosis factor-α |
| USA | United States of America |
| V | Vehicle |
References
- Blennow, K.; de Leon, M.J.; Zetterberg, H. Alzheimer’s disease. Lancet 2006, 368, 387–403. [Google Scholar] [CrossRef]
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L.; et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging 2000, 21, 383–421. [Google Scholar] [CrossRef]
- Mattson, M.P.; Maudsley, S.; Martin, B. A neural signaling triumvirate that influences ageing and age-related disease: Insulin/IGF-1, bdnf and serotonin. Ageing Res. Rev. 2004, 3, 445–464. [Google Scholar] [CrossRef] [PubMed]
- McGeer, E.G.; McGeer, P.L. The importance of inflammatory mechanisms in Alzheimer disease. Exp. Gerontol. 1998, 33, 371–378. [Google Scholar] [CrossRef]
- Hickman, S.E.; Allison, E.K.; El Khoury, J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J. Neurosci. 2008, 28, 8354–8360. [Google Scholar] [CrossRef] [PubMed]
- Johnston, H.; Boutin, H.; Allan, S.M. Assessing the contribution of inflammation in models of Alzheimer’s disease. Biochem. Soc. Trans. 2011, 39, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.; Lue, L.F. Microglial chemotaxis, activation, and phagocytosis of amyloid beta-peptide as linked phenomena in Alzheimer’s disease. Neurochem. Int. 2001, 39, 333–340. [Google Scholar] [CrossRef]
- Gonzalez-Scarano, F.; Baltuch, G. Microglia as mediators of inflammatory and degenerative diseases. Annu. Rev. Neurosci. 1999, 22, 219–240. [Google Scholar] [CrossRef] [PubMed]
- Eikelenboom, P.; Zhan, S.S.; van Gool, W.A.; Allsop, D. Inflammatory mechanisms in Alzheimer’s disease. Trends Pharmacol. Sci. 1994, 15, 447–450. [Google Scholar] [CrossRef]
- Amagase, H.; Petesch, B.L.; Matsuura, H.; Kasuga, S.; Itakura, Y. Intake of garlic and its bioactive components. J. Nutr. 2001, 131, 955S–962S. [Google Scholar] [PubMed]
- Chauhan, N.B. Multiplicity of garlic health effects and Alzheimer’s disease. J. Nutr. Health Aging 2005, 9, 421–432. [Google Scholar] [PubMed]
- Borek, C. The health benefits of aged garlic extract. Townsend Lett. Dr. Patients 2004, 8–9, 112–116. [Google Scholar]
- Borek, C. Garlic reduces dementia and heart-disease risk. J. Nutr. 2006, 136 (Suppl. 3), 810S–812S. [Google Scholar] [PubMed]
- Brown, C.; Gwebu, E.T. Effect of aged garlic extract on caspase-3 activity in PC12 cells. J. Ala. Acad. Sci. 2003, 74, 132–133. [Google Scholar]
- Griffin, W.S.; Sheng, J.G.; Royston, M.C.; Gentleman, S.M.; McKenzie, J.E.; Graham, D.I.; Roberts, G.W.; Mrak, R.E. Glial-neuronal interactions in Alzheimer’s disease: The potential role of a ‘cytokine cycle’ in disease progression. Brain Pathol. 1998, 8, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.; McNeil, B.; Taylor, C.; Holl, G.; Ruff, D.; Gwebu, E. Effect of aged garlic extract on human recombinant caspace-3 activity. J. Ala. Acad. Sci. 2003, 74, 121–122. [Google Scholar]
- Mbyirukira, G.; Gwebu, E.T. Aged garlic extract protects serum deprived PC12 cells from apoptosis. J. Ala. Acad. Sci. 2003, 74, 127–128. [Google Scholar]
- Numagami, Y.; Sato, S.; Ohnishi, S.T. Attenuation of rat ischemic brain damage by aged garlic extracts: A possible protecting mechanism as antioxidants. Neurochem. Int. 1996, 29, 135–143. [Google Scholar] [CrossRef]
- Kasuga, S.; Uda, N.; Kyo, E.; Ushijima, M.; Morihara, N.; Itakura, Y. Pharmacologic activities of aged garlic extract in comparison with other garlic preparations. J. Nutr. 2001, 131, 1080S–1084S. [Google Scholar] [PubMed]
- Nishiyama, N.; Moriguchi, T.; Saito, H. Beneficial effects of aged garlic extract on learning and memory impairment in the senescence-accelerated mouse. Exp. Gerontol. 1997, 32, 149–160. [Google Scholar] [CrossRef]
- Moriguchi, T.; Saito, H.; Nishiyama, N. Anti-ageing effect of aged garlic extract in the inbred brain atrophy mouse model. Clin. Exp. Pharmacol. Physiol. 1997, 24, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hu, J.; Jiang, L.; Xu, S.; Zheng, B.; Wang, C.; Zhang, J.; Wei, X.; Chang, L.; Wang, Q. Brilliant blue g improves cognition in an animal model of Alzheimer’s disease and inhibits amyloid-beta-induced loss of filopodia and dendrite spines in hippocampal neurons. Neuroscience 2014, 279, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.B.; Yang, X.M.; Li, T.J.; Cheng, Y.F.; Zhang, H.T.; Xu, J.P. Memory deficits and neurochemical changes induced by C-reactive protein in rats: Implication in Alzheimer’s disease. Psychopharmacology 2009, 204, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, R.; Kitazawa, M.; Passos, G.F.; Baglietto-Vargas, D.; Cheng, D.; Cribbs, D.H.; LaFerla, F.M. Aspirin-triggered lipoxin A4 stimulates alternative activation of microglia and reduces Alzheimer disease-like pathology in mice. Am. J. Pathol. 2013, 182, 1780–1789. [Google Scholar] [CrossRef] [PubMed]
- Passos, G.F.; Medeiros, R.; Cheng, D.; Vasilevko, V.; Laferla, F.M.; Cribbs, D.H. The bradykinin B1 receptor regulates abeta deposition and neuroinflammation in Tg-SwDI mice. Am. J. Pathol. 2013, 182, 1740–1749. [Google Scholar] [CrossRef] [PubMed]
- Town, T.; Laouar, Y.; Pittenger, C.; Mori, T.; Szekely, C.A.; Tan, J.; Duman, R.S.; Flavell, R.A. Blocking TGF-beta-Smad2/3 innate immune signaling mitigates Alzheimer-like pathology. Nat. Med. 2008, 14, 681–687. [Google Scholar] [PubMed]
- Mustafa, S.; Walker, A.; Bennett, G.; Wigmore, P.M. 5-fluorouracil chemotherapy affects spatial working memory and newborn neurons in the adult rat hippocampus. Eur. J. Neurosci. 2008, 28, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Deschaux, O.; Bizot, J.C.; Goyffon, M. Apamin improves learning in an object recognition task in rats. Neurosci. Lett. 1997, 222, 159–162. [Google Scholar] [CrossRef]
- Ennaceur, A.; Meliani, K. A new one-trial test for neurobiological studies of memory in rats. III. Spatial vs. Non-spatial working memory. Behav. Brain Res. 1992, 51, 83–92. [Google Scholar] [CrossRef]
- Puma, C.; Bizot, J.C. Intraseptal infusions of a low dose of AP5, a NMDA receptor antagonist, improves memory in an object recognition task in rats. Neurosci. Lett. 1998, 248, 183–186. [Google Scholar] [CrossRef]
- Murray, E.A.; Richmond, B.J. Role of perirhinal cortex in object perception, memory, and associations. Curr. Opin. Neurobiol. 2001, 11, 188–193. [Google Scholar] [CrossRef]
- Reger, M.L.; Hovda, D.A.; Giza, C.C. Ontogeny of rat recognition memory measured by the novel object recognition task. Dev. Psychobiol. 2009, 51, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Takata, K.; Kitamura, Y.; Tsuchiya, D.; Kawasaki, T.; Taniguchi, T.; Shimohama, S. Heat shock protein-90-induced microglial clearance of exogenous amyloid-beta1-42 in rat hippocampus in vivo. Neurosci. Lett. 2003, 344, 87–90. [Google Scholar] [CrossRef]
- Meda, L.; Cassatella, M.A.; Szendrei, G.I.; Otvos, L., Jr.; Baron, P.; Villalba, M.; Ferrari, D.; Rossi, F. Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature 1995, 374, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Valencia, A.; Moran, J. Reactive oxygen species induce different cell death mechanisms in cultured neurons. Free Radic. Biol. Med. 2004, 36, 1112–1125. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Kim, M.J.; Jin Heo, H.; Kim, J.K.; Jin Jun, W.; Kim, H.K.; Kim, E.K.; Ok Kim, M.; Yon Cho, H.; Hwang, H.J.; et al. Ameliorative effect of 1,2-benzenedicarboxylic acid dinonyl ester against amyloid beta peptide-induced neurotoxicity. Amyloid 2009, 16, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Jana, A.; Yatish, K.; Freidt, M.B.; Fung, Y.K.; Martinson, J.A.; Pahan, K. Reactive oxygen species up-regulate CD11b in microglia via nitric oxide: Implications for neurodegenerative diseases. Free Radic. Biol. Med. 2008, 45, 686–699. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.B. Effect of aged garlic extract on APP processing and tau phosphorylation in Alzheimer’s transgenic model Tg2576. J. Ethnopharmacol. 2006, 108, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Hoozemans, J.J.; Rozemuller, A.J.; van Haastert, E.S.; Eikelenboom, P.; van Gool, W.A. Neuroinflammation in Alzheimer’s disease wanes with age. J. Neuroinflamm. 2011, 8, 171. [Google Scholar] [CrossRef] [PubMed]
- Latta, C.H.; Brothers, H.M.; Wilcock, D.M. Neuroinflammation in Alzheimer’s disease; a source of heterogeneity and target for personalized therapy. Neuroscience 2015, 302, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Griffin, W.S.; Stanley, L.C.; Ling, C.; White, L.; MacLeod, V.; Perrot, L.J.; White, C.L., 3rd; Araoz, C. Brain interleukin 1 and S-100 immunoreactivity are elevated in down syndrome and Alzheimer disease. Proc. Natl. Acad. Sci. USA 1989, 86, 7611–7615. [Google Scholar] [CrossRef] [PubMed]
- Griffin, W.S.; Sheng, J.G.; Roberts, G.W.; Mrak, R.E. Interleukin-1 expression in different plaque types in Alzheimer’s disease: Significance in plaque evolution. J. Neuropathol. Exp. Neurol. 1995, 54, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.G.; Mrak, R.E.; Griffin, W.S. Glial-neuronal interactions in alzheimer disease: Progressive association of IL-1alpha+ microglia and S100beta+ astrocytes with neurofibrillary tangle stages. J. Neuropathol. Exp. Neurol. 1997, 56, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Grilli, M.; Goffi, F.; Memo, M.; Spano, P. Interleukin-1beta and glutamate activate the NF-kappaB/Rel binding site from the regulatory region of the amyloid precursor protein gene in primary neuronal cultures. J. Biol. Chem. 1996, 271, 15002–15007. [Google Scholar] [PubMed]
- Yang, Y.; Quitschke, W.W.; Brewer, G.J. Upregulation of amyloid precursor protein gene promoter in rat primary hippocampal neurons by phorbol ester, IL-1 and retinoic acid, but not by reactive oxygen species. Brain Res. Mol. Brain Res. 1998, 60, 40–49. [Google Scholar] [CrossRef]
- Rogers, J.T.; Leiter, L.M.; McPhee, J.; Cahill, C.M.; Zhan, S.S.; Potter, H.; Nilsson, L.N. Translation of the alzheimer amyloid precursor protein mRNA is up-regulated by interleukin-1 through 5′-untranslated region sequences. J. Biol. Chem. 1999, 274, 6421–6431. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.M.; Chiozzi, P.; Ferrari, D.; Colaianna, M.; Idzko, M.; Falzoni, S.; Fellin, R.; Trabace, L.; Di Virgilio, F. Activation of microglia by amyloid β requires P2X7 receptor expression. J. Immunol. 2009, 182, 4378–4385. [Google Scholar] [CrossRef] [PubMed]
- Fenger, C.; Drojdahl, N.; Wirenfeldt, M.; Sylvest, L.; Jorgensen, O.S.; Meldgaard, M.; Lambertsen, K.L.; Finsen, B. Tumor necrosis factor and its p55 and p75 receptors are not required for axonal lesion-induced microgliosis in mouse fascia dentata. Glia 2006, 54, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Borre, Y.; Lemstra, S.; Westphal, K.G.; Morgan, M.E.; Olivier, B.; Oosting, R.S. Celecoxib delays cognitive decline in an animal model of neurodegeneration. Behav. Brain Res. 2012, 234, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.R.; Stuart, L.M.; Wilkinson, K.; van Gils, J.M.; Deng, J.; Halle, A.; Rayner, K.J.; Boyer, L.; Zhong, R.; Frazier, W.A. CD36 ligands promote sterile inflammation through assembly of a toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 2010, 11, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Doens, D.; Fernández, P.L. Microglia receptors and their implications in the response to amyloid β for Alzheimer’s disease pathogenesis. J. Neuroinflamm. 2014, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.-K.; Ji, K.; Min, K.; Joe, E.-H. Brain inflammation and microglia: Facts and misconceptions. Exp. Neurobiol. 2013, 22, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Kosuge, Y.; Sakikubo, T.; Horie, K.; Ishikawa, N.; Obokata, N.; Yokoyama, E.; Yamashina, K.; Yamamoto, M.; Saito, H. Protective effect of S-allyl-l-cysteine, a garlic compound, on amyloid β-protein-induced cell death in nerve growth factor-differentiated PC12 cells. Neurosci. Res. 2003, 46, 119–125. [Google Scholar] [CrossRef]
- Jeong, Y.Y.; Ryu, J.H.; Shin, J.-H.; Kang, M.J.; Kang, J.R.; Han, J.; Kang, D. Comparison of anti-oxidant and anti-inflammatory effects between fresh and aged black garlic extracts. Molecules 2016, 21, 430. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-H.; Ryu, J.H.; Kang, M.J.; Hwang, C.R.; Han, J.; Kang, D. Short-term heating reduces the anti-inflammatory effects of fresh raw garlic extracts on the LPS-induced production of NO and pro-inflammatory cytokines by downregulating allicin activity in raw 264.7 macrophages. Food Chem. Toxicol. 2013, 58, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Qi, J.; Feng, F.; Wang, M.D.; Bao, G.; Wang, T.; Xiang, M.; Xie, W.F. Neuroprotective effect of allicin against traumatic brain injury via Akt/endothelial nitric oxide synthase pathway-mediated anti-inflammatory and anti-oxidative activities. Neurochem. Int. 2014, 68, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Saenthaweesuk, S. Effect of Substituting Garlic Herbal Powder for Antibiotic Growth Promoters in Ration on Growth Performance, Carcass Characteristic, Meat Quality, Blood Chemistry, Plasma Lipids and Antioxidising Response of Broilers. Ph.D. Thesis, Khon-Kaen University, Khon-Kaen, Thailand, 2006. [Google Scholar]
- Gu, X.; Wu, H.; Fu, P. Allicin attenuates inflammation and suppresses HLA-B27 protein expression in ankylosing spondylitis mice. BioMed Res. Int. 2013, 2013, 171573. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Guo, H.; Cha, Y.; Ban, Y.H.; Seo da, W.; Choi, Y.; Kim, T.S.; Lee, S.P.; Kim, J.C.; Choi, E.K.; et al. Cereboost, an american ginseng extract, improves cognitive function via up-regulation of choline acetyltransferase expression and neuroprotection. Regul. Toxicol. Pharmacol. 2016, 78, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.P.; Chu, T.; Yang, F.; Beech, W.; Frautschy, S.A.; Cole, G.M. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 2001, 21, 8370–8377. [Google Scholar] [PubMed]
- Rezai-Zadeh, K.; Shytle, D.; Sun, N.; Mori, T.; Hou, H.; Jeanniton, D.; Ehrhart, J.; Townsend, K.; Zeng, J.; Morgan, D. Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J. Neurosci. 2005, 25, 8807–8814. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nillert, N.; Pannangrong, W.; Welbat, J.U.; Chaijaroonkhanarak, W.; Sripanidkulchai, K.; Sripanidkulchai, B. Neuroprotective Effects of Aged Garlic Extract on Cognitive Dysfunction and Neuroinflammation Induced by ?-Amyloid in Rats. Nutrients 2017, 9, 24. https://doi.org/10.3390/nu9010024
Nillert N, Pannangrong W, Welbat JU, Chaijaroonkhanarak W, Sripanidkulchai K, Sripanidkulchai B. Neuroprotective Effects of Aged Garlic Extract on Cognitive Dysfunction and Neuroinflammation Induced by ?-Amyloid in Rats. Nutrients. 2017; 9(1):24. https://doi.org/10.3390/nu9010024
Chicago/Turabian StyleNillert, Nutchareeporn, Wanassanun Pannangrong, Jariya Umka Welbat, Wunnee Chaijaroonkhanarak, Kittisak Sripanidkulchai, and Bungorn Sripanidkulchai. 2017. "Neuroprotective Effects of Aged Garlic Extract on Cognitive Dysfunction and Neuroinflammation Induced by ?-Amyloid in Rats" Nutrients 9, no. 1: 24. https://doi.org/10.3390/nu9010024
APA StyleNillert, N., Pannangrong, W., Welbat, J. U., Chaijaroonkhanarak, W., Sripanidkulchai, K., & Sripanidkulchai, B. (2017). Neuroprotective Effects of Aged Garlic Extract on Cognitive Dysfunction and Neuroinflammation Induced by ?-Amyloid in Rats. Nutrients, 9(1), 24. https://doi.org/10.3390/nu9010024




