Phytomedicine in Joint Disorders
Abstract
:1. Introduction
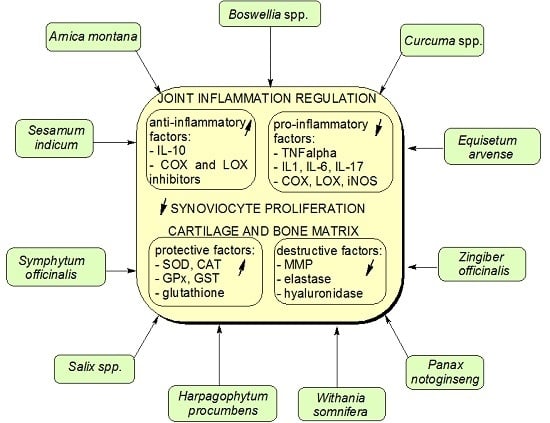
2. Anti-Arthritic Medicinal Plants
2.1. Arnica montana, family (fam.) Asteraceae
2.2. Boswellia spp., fam. Burseraceae
2.3. Curcuma spp., fam. Zingiberaceae
2.4. Equisetum arvense, fam. Equisetaceae
2.5. Harpagophytum procumbens, fam. Pedaliaceae
2.6. Panax notoginseng, fam. Araliaceae
2.7. Salix spp., fam. Salicaceae
2.8. Sesamum indicum, fam. Pedaliaceae
2.9. Symphytum officinalis, fam. Boraginaceae
2.10. Zingiber officinalis, fam. Zingiberaceae
2.11. Whitania somnifera, fam. Solanaceae
3. Concluding Remarks
Author Contributions
Conflicts of Interest
References
- Hsu, D.-Z.; Chu, P.-Y.; Jou, I.-M. Daily sesame oil supplement attenuates joint pain by inhibiting muscular oxidative stress in osteoarthritis rat model. J. Nutr. Biochem. 2016, 29, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Kim, S.-K.; Shin, I.-H.; Kim, H.-G.; Choe, J.-Y. Effects of AIF on Knee Osteoarthritis Patients: Double-blind, Randomized Placebo-controlled Study. Korean J. Physiol. Pharmacol. 2009, 13, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Peat, G.; McCarney, R.; Croft, P. Knee pain and osteoarthritis in older adults: A review of community burden and current use of primary health care. Ann. Rheum. Dis. 2001, 60, 91–97. [Google Scholar] [CrossRef] [PubMed]
- McWilliams, D.F.; Leeb, B.F.; Muthuri, S.G.; Doherty, M.; Zhang, W. Occupational risk factors for osteoarthritis of the knee: A meta-analysis. Osteoarthr. Cartil. 2011, 19, 829–839. [Google Scholar] [CrossRef]
- Smith, R.L.; Carter, D.R.; Schurman, D.J. Pressure and shear differentially alter human articular chondrocyte metabolism: A review. Clin. Orthop. Relat. Res. 2004, 427, S89–S95. [Google Scholar]
- Murphy, N.J.; Eyles, J.P.; Hunter, D.J. Hip Osteoarthritis: Etiopathogenesis and Implications for Management. Adv. Ther. 2016, 33, 1921–1946. [Google Scholar] [CrossRef]
- Dougados, M. Monitoring osteoarthritis progression and therapy. Osteoarthr. Cartil. 2004, 12 (Suppl. A), S55–S60. [Google Scholar] [CrossRef] [PubMed]
- Lequesne, M.G.; Mery, C.; Samson, M.; Gerard, P. Indexes of severity for osteoarthritis of the hip and knee. Validation—Value in comparison with other assessment tests. Scand. J. Rheumatol. Suppl. 1987, 65, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.J.; Bland, J.M.; Davidson, P.M.; Newton, P.J.; Oxberry, S.G.; Abernethy, A.P.; Currow, D.C. The relationship between two performance scales: New York Heart Association Classification and Karnofsky Performance Status Scale. J. Pain Symptom Manag. 2014, 47, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.L.H.; Or, T.C.T.; Ho, M.H.K.; Lau, A.S.Y. Scientific Basis of Botanical Medicine as Alternative Remedies for Rheumatoid Arthritis. Clin. Rev. Allergy Immunol. 2013, 44, 284–300. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Landewe, R.; Breedveld, F.C.; Buch, M.; Burmester, G.; Dougados, M.; Emery, P.; Gaujoux-Viala, C.; Gossec, L.; Nam, J.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann. Rheum. Dis. 2014, 73, 492–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochberg, M.C.; Altman, R.D.; April, K.T.; Benkhalti, M.; Guyatt, G.; McGowan, J.; Towheed, T.; Welch, V.; Wells, G.; Tugwell, P. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012, 64, 465–474. [Google Scholar] [CrossRef]
- Hyrich, K.L.; Watson, K.D.; Silman, A.J.; Symmons, D.P.M. Predictors of response to anti-TNF-alpha therapy among patients with rheumatoid arthritis: Results from the British Society for Rheumatology Biologics Register. Rheumatology 2006, 45, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.M.; Hyrich, K.L.; Lunt, M.; Watson, K.D.; Symmons, D.P.M.; Ashcroft, D.M. Effectiveness of rituximab in patients with rheumatoid arthritis: Observational study from the British Society for Rheumatology Biologics Register. J. Rheumatol. 2012, 39, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, G.; Dinarello, C.A. Treating rheumatological diseases and co-morbidities with interleukin-1 blocking therapies. Rheumatology 2015, 54, 2134–2144. [Google Scholar] [CrossRef] [PubMed]
- Nishina, N.; Kaneko, Y.; Kameda, H.; Kuwana, M.; Takeuchi, T. Reduction of plasma IL-6 but not TNF-alpha by methotrexate in patients with early rheumatoid arthritis: A potential biomarker for radiographic progression. Clin. Rheumatol. 2013, 32, 1661–1666. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Tawab, M.; Werz, O.; Schubert-Zsilavecz, M. Boswellia serrata: An overall assessment of in vitro, preclinical, pharmacokinetic and clinical data. Clin. Pharmacokinet. 2011, 50, 349–369. [Google Scholar] [CrossRef] [PubMed]
- McAlindon, T.E.; Bannuru, R.R.; Sullivan, M.C.; Arden, N.K.; Berenbaum, F.; Bierma-Zeinstra, S.M.; Hawker, G.A.; Henrotin, Y.; Hunter, D.J.; Kawaguchi, H.; et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr. Cartil. 2014, 22, 363–388. [Google Scholar] [CrossRef] [PubMed]
- Matucci, A.; Cammelli, D.; Cantini, F.; Goletti, D.; Marino, V.; Milano, G.M.; Scarpa, R.; Tocci, G.; Maggi, E.; Vultaggio, A. Influence of anti-TNF immunogenicity on safety in rheumatic disease: A narrative review. Expert Opin. Drug Saf. 2016, 15, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Cabral, V.P.; Andrade, C.A.; Passos, S.R.; Martins, M.F.; Hökerberg, Y.H. Severe infection in patients with rheumatoid arthritis taking anakinra, rituximab, or abatacept: A systematic review of observational studies. Rev. Bras. Reumatol. 2016, 56, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Mameli, A.; Barcellona, D.; Marongiu, F. Fatal Cytopenia Induced by Low-Dose Methotrexate in Elderly With Rheumatoid Arthritis. Identification of Risk Factors. Am. J. Ther. 2017, 24, e106–e107. [Google Scholar] [CrossRef]
- Umar, S.; Umar, K.; Sarwar, A.H.M.G.; Khan, A.; Ahmad, N.; Ahmad, S.; Katiyar, C.K.; Husain, S.A.; Khan, H.A. Boswellia serrata extract attenuates inflammatory mediators and oxidative stress in collagen induced arthritis. Phytomedicine 2014, 21, 847–856. [Google Scholar] [CrossRef]
- Akhtar, N.; Miller, M.J.; Haqqi, T.M. Effect of a Herbal-Leucine mix on the IL-1β-induced cartilage degradation and inflammatory gene expression in human chondrocytes. BMC Complement. Altern. Med. 2011, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Arif, M.; Nirala, R.K.; Gupta, R.; Thakur, S.C. Cumulative therapeutic effects of phytochemicals in Arnica montana flower extract alleviated collagen-induced arthritis: Inhibition of both pro-inflammatory mediators and oxidative stress. J. Sci. Food Agric. 2016, 96, 1500–1510. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Hernández, E.; César Casasola-Vargas, J.; Lino-Pérez, L.; Burgos-Vargas, R.; Vázquez-Mellado, J. Frecuencia de uso de medicinas complementarias y alternativas en sujetos que acuden por primera vez al servicio de reumatología. Análisis de 800 casos. Reumatol. Clín. 2006, 2, 183–189. [Google Scholar] [CrossRef]
- Knuesel, O.; Weber, M.; Suter, A. Arnica montana gel in osteoarthritis of the knee: An open, multicenter clinical trial. Adv. Ther. 2002, 19, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Widrig, R.; Suter, A.; Saller, R.; Melzer, J. Choosing between NSAID and Arnica for topical treatment of hand osteoarthritis in a randomised, double-blind study. Rheumatol. Int. 2007, 27, 585–591. [Google Scholar] [CrossRef]
- Ross, S.M. Osteoarthritis. Holist. Nurs. Pract. 2008, 22, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Cameron, M.; Chrubasik, S. Topical herbal therapies for treating osteoarthritis. CDSR 2013, 2013, CD010538. [Google Scholar] [CrossRef]
- Ammon, H.P.T. Boswellic acids (components of frankincense) as the active principle in treatment of chronic inflammatory diseases. Wien. Med. Wochenschr. 2002, 152, 373–378. (In German) [Google Scholar] [CrossRef] [PubMed]
- Sengupta, K.; Krishnaraju, A.V.; Vishal, A.A.; Mishra, A.; Trimurtulu, G.; Sarma, K.V.S.; Raychaudhuri, S.K.; Raychaudhuri, S.P. Comparative efficacy and tolerability of 5-Loxin and AflapinAgainst osteoarthritis of the knee: A double blind, randomized, placebo controlled clinical study. Int. J. Med. Sci. 2010, 7, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Blain, E.J.; Ali, A.Y.; Duance, V.C. Boswellia frereana (frankincense) suppresses cytokine-induced matrix metalloproteinase expression and production of pro-inflammatory molecules in articular cartilage. Phyther. Res. 2009, 24, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Sumantran, V.N.; Joshi, A.K.; Boddul, S.; Koppikar, S.J.; Warude, D.; Patwardhan, B.; Chopra, A.; Chandwaskar, R.; Wagh, U.V. Antiarthritic Activity of a Standardized, Multiherbal, Ayurvedic Formulation containing Boswellia serrata: In Vitro Studies on Knee Cartilage from Osteoarthritis Patients. Phyther. Res. 2011, 25, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.; Chaskar, S.; Athavale, N.; Chitre, D. Inhibition of LPS-Induced TNF-α and NO Production in Mouse Macrophage and Inflammatory Response in Rat Animal Models by a Novel Ayurvedic Formulation, BV-9238. Phyther. Res. 2014, 28, 1479–1485. [Google Scholar] [CrossRef]
- Cameron, M.; Chrubasik, S. Oral herbal therapies for treating osteoarthritis. CDSR 2014, 2014, CD002947. [Google Scholar] [CrossRef]
- Vishal, A.A.; Mishra, A.; Raychaudhuri, S.P. A double blind, randomized, placebo controlled clinical study evaluates the early efficacy of aflapin in subjects with osteoarthritis of knee. Int. J. Med. Sci. 2011, 8, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Belcaro, G.; Dugall, M.; Luzzi, R.; Ledda, A.; Pellegrini, L.; Cesarone, M.R.; Hosoi, M.; Errichi, M.; Francis, S.; Cornelli, U. FlexiQule (Boswellia extract) in the supplementary management of osteoarthritis: A supplement registry. Minerva Med. 2014, 105, 9–16. [Google Scholar]
- Belcaro, G.; Feragalli, B.; Cornelli, U.; Dugall, M. Hand “stress” arthritis in young subjects: Effects of Flexiqule (pharma-standard Boswellia extract). A preliminary case report. Minerva Gastroenterol. Dietol. 2015, in press. [Google Scholar]
- Kizhakkedath, R. Clinical evaluation of a formulation containing Curcuma longa and Boswellia serrata extracts in the management of knee osteoarthritis. Mol. Med. Rep. 2013, 8, 1542–1548. [Google Scholar] [CrossRef] [PubMed]
- Sander, O.; Herborn, G.; Rau, R. Is H15 (resin extract of Boswellia serrata, “incense”) a useful supplement to established drug therapy of chronic polyarthritis? Results of a double-blind pilot study. Z. Rheumatol. 1998, 57, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, C.; Wu, Y.; Ai, Y.; Lee, D.Y.-W.; Dai, R. Comparative pharmacokinetic study of two boswellic acids in normal and arthritic rat plasma after oral administration of Boswellia serrata extract or Huo Luo Xiao Ling Dan by LC-MS. Biomed. Chromatogr. 2014, 28, 1402–1408. [Google Scholar] [CrossRef] [PubMed]
- Hamidpour, R.; Hamidpour, S.; Hamidpour, M.; Shahlari, M. Frankincense (Rǔ Xiāng; Boswellia Species): From the Selection of Traditional Applications to the Novel Phytotherapy for the Prevention and Treatment of Serious Diseases. J. Tradit. Complement. Med. 2013, 3, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.L.; Bani, S.; Singh, G.B. Anti-arthritic activity of boswellic acids in bovine serum albumin (BSA)-induced arthritis. Int. J. Immunopharmacol. 1989, 11, 647–652. [Google Scholar] [CrossRef]
- Singh, S.; Khajuria, A.; Taneja, S.C.; Johri, R.K.; Singh, J.; Qazi, G.N. Boswellic acids: A leukotriene inhibitor also effective through topical application in inflammatory disorders. Phytomedicine 2008, 15, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Ammon, H.P.T. Modulation of the immune system by Boswellia serrata extracts and boswellic acids. Phytomedicine 2010, 17, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Khajuria, A.; Gupta, A.; Suden, P.; Singh, S.; Malik, F.; Singh, J.; Gupta, B.D.; Suri, K.A.; Srinivas, V.K.; Ella, K.; et al. Immunomodulatory activity of biopolymeric fraction BOS 2000 from Boswellia serrata. Phyther. Res. 2008, 22, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, K.; Kolla, J.N.; Krishnaraju, A.V.; Yalamanchili, N.; Rao, C.V.; Golakoti, T.; Raychaudhuri, S.; Raychaudhuri, S.P. Cellular and molecular mechanisms of anti-inflammatory effect of Aflapin: A novel Boswellia serrata extract. Mol. Cell. Biochem. 2011, 354, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Dash, B. Caraka Samhita, 2006th ed.; Chowkhamba Sanskrit Series Office: Varanasi, India, 2006. [Google Scholar]
- Funk, J.L.; Frye, J.B.; Oyarzo, J.N.; Kuscuoglu, N.; Wilson, J.; McCaffrey, G.; Stafford, G.; Chen, G.; Lantz, R.C.; Jolad, S.D.; et al. Efficacy and mechanism of action of turmeric supplements in the treatment of experimental arthritis. Arthritis Rheum. 2006, 54, 3452–3464. [Google Scholar] [CrossRef] [PubMed]
- Taty Anna, K.; Elvy Suhana, M.R.; Das, S.; Faizah, O.; Hamzaini, A.H. Anti-inflammatory effect of Curcuma longa (turmeric) on collagen-induced arthritis: An anatomico-radiological study. Clin. Ter. 2011, 162, 201–207. [Google Scholar] [PubMed]
- Kamarudin, T.A.; Othman, F.; Mohd Ramli, E.S.; Md Isa, N.; Das, S. Protective effect of curcumin on experimentally induced arthritic rats: Detailed histopathological study of the joints and white blood cell count. EXCLI J. 2012, 11, 226–236. [Google Scholar] [PubMed]
- Chang, H.-I.; Su, Y.-H.; Lin, Y.-J.; Chen, P.-J.; Shi, C.-S.; Chen, C.-N.; Yeh, C.-C. Evaluation of the protective effects of curcuminoid (curcumin and bisdemethoxycurcumin)-loaded liposomes against bone turnover in a cell-based model of osteoarthritis. Drug Des. Dev. Ther. 2015, 9, 2285–2300. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, G.; El-Menshawy, O. Protective effects of ginger-turmeric rhizomes mixture on joint inflammation, atherogenesis, kidney dysfunction and other complications in a rat model of human rheumatoid arthritis. Int. J. Rheum. Dis. 2013, 16, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Kuptniratsaikul, V.; Dajpratham, P.; Taechaarpornkul, W.; Buntragulpoontawee, M.; Lukkanapichonchut, P.; Chootip, C.; Saengsuwan, J.; Tantayakom, K.; Laongpech, S. Efficacy and safety of Curcuma domestica extracts compared with ibuprofen in patients with knee osteoarthritis: A multicenter study. Clin. Interv. Aging 2014, 9, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Daily, J.W.; Yang, M.; Park, S. Efficacy of Turmeric Extracts and Curcumin for Alleviating the Symptoms of Joint Arthritis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Med. Food 2016, 19, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Banerjee, S.; Sil, P.C. The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: A recent update. Food Chem. Toxicol. 2015, 83, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Nonose, N.; Pereira, J.A.; Machado, P.R.M.; Rodrigues, M.R.; Sato, D.T.; Martinez, C.A.R. Oral administration of curcumin (Curcuma longa) can attenuate the neutrophil inflammatory response in zymosan-induced arthritis in rats. Acta Cir. Bras. 2014, 29, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Alishiri, G.H.; Parvin, S.; Sahebkar, A. Mitigation of Systemic Oxidative Stress by Curcuminoids in Osteoarthritis: Results of a Randomized Controlled Trial. J. Diet. Suppl. 2016, 13, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-J.; Dai, L.; Zhao, L.-X.; Zhu, X.; Cao, S.; Gao, Y.-J. Intrathecal curcumin attenuates pain hypersensitivity and decreases spinal neuroinflammation in rat model of monoarthritis. Sci. Rep. 2015, 5, 10278. [Google Scholar] [CrossRef]
- Zou, S.; Wang, C.; Cui, Z.; Guo, P.; Meng, Q.; Shi, X.; Gao, Y.; Yang, G.; Han, Z. β-Elemene induces apoptosis of human rheumatoid arthritis fibroblast-like synoviocytes via reactive oxygen species-dependent activation of p38 mitogen-activated protein kinase. Pharmacol. Rep. 2016, 68, 7–11. [Google Scholar] [CrossRef]
- Gründemann, C.; Lengen, K.; Sauer, B.; Garcia-Käufer, M.; Zehl, M.; Huber, R. Equisetum arvense (common horsetail) modulates the function of inflammatory immunocompetent cells. BMC Complement. Altern. Med. 2014, 14, 283. [Google Scholar] [CrossRef]
- Farinon, M.; Lora, P.S.; Francescato, L.N.; Bassani, V.L.; Henriques, A.T.; Xavier, R.M.; de Oliveira, P.G. Effect of Aqueous Extract of Giant Horsetail (Equisetum giganteum L.) in Antigen-Induced Arthritis. Open Rheumatol. J. 2013, 7, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Qu, Q.; Li, M.; Miao, S.; Li, X.; Cai, W. Horsetail mixture on rheumatoid arthritis and its regulation on TNF-α and IL-10. Pak. J. Pharm. Sci. 2014, 27, 2019–2023. [Google Scholar] [PubMed]
- Zgrajka, W.; Turska, M.; Rajtar, G.; Majdan, M.; Parada-Turska, J. Kynurenic acid content in anti-rheumatic herbs. Ann. Agric. Environ. Med. 2013, 20, 800–802. [Google Scholar] [PubMed]
- Varga, G.; Erces, D.; Fazekas, B.; Fulop, M.; Kovacs, T.; Kaszaki, J.; Fulop, F.; Vecsei, L.; Boros, M. N-Methyl-d-aspartate receptor antagonism decreases motility and inflammatory activation in the early phase of acute experimental colitis in the rat. Neurogastroenterol. Motil. 2010, 22, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Parada-Turska, J.; Rzeski, W.; Zgrajka, W.; Majdan, M.; Kandefer-Szerszen, M.; Turski, W. Kynurenic acid, an endogenous constituent of rheumatoid arthritis synovial fluid, inhibits proliferation of synoviocytes in vitro. Rheumatol. Int. 2006, 26, 422–426. [Google Scholar] [CrossRef]
- Parada-Turska, J.; Zgrajka, W.; Majdan, M. Kynurenic acid in synovial fluid and serum of patients with rheumatoid arthritis, spondyloarthropathy, and osteoarthritis. J. Rheumatol. 2013, 40, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, M.; Busse, W.R. The Complete German Commission E Monographs; American Botanical Council: Austin, TX, USA, 1998. [Google Scholar]
- Fiebich, B.; Heinrich, M.; Hiller, K.; Kammerer, N. Inhibition of TNF-α synthesis in LPS-stimulated primary human monocytes by Harpagophytum extract SteiHap 69. Phytomedicine 2001, 8, 28–30. [Google Scholar] [CrossRef]
- Lanhers, M.; Fleurentin, J.; Mortier, F.; Al, E. Antiinflammatory and analgesic effects of an aqueous extract of Harpagophytum procumbens. Planta Med. 1992, 58, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Haqqi, T.M. Current nutraceuticals in the management of osteoarthritis: A review. Ther. Adv. Musculoskelet. Dis. 2012, 4, 181–207. [Google Scholar] [CrossRef] [PubMed]
- Wegener, T.; Lupke, N. Treatment of patients with arthrosis of hip or knee with an aqueous extract of devil’s claw (Harpagophytum procumbens DC.). Phytother. Res. 2003, 17, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Chantre, P.; Cappelaere, A.; Leblan, D.; Al, E. Efficacy and tolerance of Harpagophytum procumbens versus diacerhein in treatment of osteoarthritis. Phytomedicine 2000, 7, 177–183. [Google Scholar] [CrossRef]
- Chrubasik, S.; Thanner, J.; Kunzel, O.; Al, E. Comparison of outcome measures during treatment with the proprietary Harpagophytum extract Doloteffin in patients with pain in the lower back, knee, or hip. Phytomedicine 2002, 9, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Harpagophytum procumbens (devil’s claw). Monograph. Altern. Med. Rev. 2008, 13, 248–252.
- Huang, T.H.-W.; Tran, V.H.; Duke, R.K.; Tan, S.; Chrubasik, S.; Roufogalis, B.D.; Duke, C.C. Harpagoside suppresses lipopolysaccharide-induced iNOS and COX-2 expression through inhibition of NF-κB activation. J. Ethnopharmacol. 2006, 104, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Kaszkin, M.; Beck, K.; Koch, E.; Al, E. Downregulation of iNOS expression in rat mesangial cells by special extracts of Harpagophytum procumbens derives from harpagoside-dependent and independent effects. Phytomedicine 2004, 11, 585–595. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, L. Essentials of Chinese Medicine; Liu, Z., Liu, L., Eds.; Springer: London, UK, 2009. [Google Scholar]
- Chang, S.-H.; Choi, Y.; Park, J.-A.; Jung, D.-S.; Shin, J.; Yang, J.-H.; Ko, S.-Y.; Kim, S.-W.; Kim, J.-K. Anti-inflammatory effects of BT-201, an n-butanol extract of Panax notoginseng, observed in vitro and in a collagen-induced arthritis model. Clin. Nutr. 2007, 26, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-H.; Sung, H.-C.; Choi, Y.; Ko, S.-Y.; Lee, B.-E.; Baek, D.-H.; Kim, S.-W.; Kim, J.-K. Suppressive effect of AIF, a water extract from three herbs, on collagen-induced arthritis in mice. Int. Immunopharmacol. 2005, 5, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Z.; Sun, J.; Ma, J.; Zhang, S.; Liu, J.; Zhu, J. Effect of Panax Notoginseng Saponins on autograft tendon healing in bone tunnel: Interface histological characteristics. Zhongguo Gu Shang 2011, 24, 132–136. [Google Scholar] [PubMed]
- Zhang, J.; Wang, J.; Wang, H. Clinical study on effect of total Panax notoginseng saponins on immune related inner environment imbalance in rheumatoid arthritis patients. Zhongguo Zhong Xi Yi Jie He Za Zhi/Chin. J. Integr. Tradit. West. Med. 2007, 27, 589–592. [Google Scholar]
- Appelboom, T. Arthropathy in art and the history of pain management—Through the centuries to cyclooxygenase-2 inhibitors. Rheumatology 2002, 41 (Suppl. 1), 28–34. [Google Scholar] [CrossRef] [PubMed]
- Vlachojannis, J.E.; Cameron, M.; Chrubasik, S. A systematic review on the effectiveness of willow bark for musculoskeletal pain. Phyther. Res. 2009, 23, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Mackowiak, P.A. Brief History of Antipyretic Therapy. Clin. Infect. Dis. 2000, 31, S154–S156. [Google Scholar] [CrossRef]
- Vane, J.R. The fight against rheumatism: From willow bark to COX-1 sparing drugs. J. Physiol. Pharmacol. 2000, 51, 573–586. [Google Scholar] [PubMed]
- Bonaterra, G.A.; Heinrich, E.U.; Kelber, O.; Weiser, D.; Metz, J.; Kinscherf, R. Anti-inflammatory effects of the willow bark extract STW 33-I (Proaktiv®) in LPS-activated human monocytes and differentiated macrophages. Phytomedicine 2010, 17, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Khayyal, M.; El-Ghazaly, M.; Abdallah, D.; Okpanyi, S.; Kelber, O.; Weiser, D. Mechanisms Involved in the Anti-inflammatory Effect of a Standardized Willow Bark Extract. Arzneimittelforschung 2011, 55, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sahu, D.; Das, H.R.; Sharma, D. Amelioration of collagen-induced arthritis by Salix nigra bark extract via suppression of pro-inflammatory cytokines and oxidative stress. Food Chem. Toxicol. 2011, 49, 3395–3406. [Google Scholar] [CrossRef] [PubMed]
- Schmid, B.; Lüdtke, R.; Selbmann, H.K.; Kötter, I.; Tschirdewahn, B.; Schaffner, W.; Heide, L. Efficacy and tolerability of a standardized willow bark extract in patients with osteoarthritis: Randomized placebo-controlled, double blind clinical trial. Phytother. Res. 2001, 15, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Biegert, C.; Wagner, I.; Lüdtke, R.; Kötter, I.; Lohmüller, C.; Günaydin, I.; Taxis, K.; Heide, L. Efficacy and safety of willow bark extract in the treatment of osteoarthritis and rheumatoid arthritis: Results of 2 randomized double-blind controlled trials. J. Rheumatol. 2004, 31, 2121–2130. [Google Scholar]
- Beer, A.-M.; Wegener, T. Willow bark extract (Salicis cortex) for gonarthrosis and coxarthrosis—Results of a cohort study with a control group. Phytomedicine 2008, 15, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Uehleke, B.; Müller, J.; Stange, R.; Kelber, O.; Melzer, J. Willow bark extract STW 33-I in the long-term treatment of outpatients with rheumatic pain mainly osteoarthritis or back pain. Phytomedicine 2013, 20, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Nahrstedt, A.; Schmidt, M.; Jäggi, R.; Metz, J.; Khayyal, M.T. Willow bark extract: The contribution of polyphenols to the overall effect. Wien. Med. Wochenschr. 2007, 157, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Drummond, E.M.; Harbourne, N.; Marete, E.; Martyn, D.; Jacquier, J.; O’Riordan, D.; Gibney, E.R. Inhibition of Proinflammatory Biomarkers in THP1 Macrophages by Polyphenols Derived From Chamomile, Meadowsweet and Willow bark. Phyther. Res. 2013, 27, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Shara, M.; Stohs, S.J. Efficacy and Safety of White Willow Bark (Salix alba) Extracts. Phyther. Res. 2015, 29, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.-Z.; Chen, S.-J.; Chu, P.-Y.; Liu, M.-Y. Therapeutic effects of sesame oil on monosodium urate crystal-induced acute inflammatory response in rats. Springerplus 2013, 2, 659. [Google Scholar] [CrossRef] [PubMed]
- Sotnikova, R.; Ponist, S.; Navarova, J.; Mihalova, D.; Tomekova, V.; Strosova, M.; Bauerova, K. Effects of sesame oil in the model of adjuvant arthritis. Neuro Endocrinol. Lett. 2009, 30 (Suppl. 1), 22–24. [Google Scholar]
- Yadav, N.V.; Sadashivaiah; Ramaiyan, B.; Acharya, P.; Belur, L.; Talahalli, R.R. Sesame Oil and Rice Bran Oil Ameliorates Adjuvant-Induced Arthritis in Rats: Distinguishing the Role of Minor Components and Fatty Acids. Lipids 2016, 51, 1385–1395. [Google Scholar] [CrossRef] [PubMed]
- Eftekhar Sadat, B.; Khadem Haghighian, M.; Alipoor, B.; Malek Mahdavi, A.; Asghari Jafarabadi, M.; Moghaddam, A. Effects of sesame seed supplementation on clinical signs and symptoms in patients with knee osteoarthritis. Int. J. Rheum. Dis. 2013, 16, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Khadem Haghighian, M.; Alipoor, B.; Malek Mahdavi, A.; Eftekhar Sadat, B.; Asghari Jafarabadi, M.; Moghaddam, A. Effects of sesame seed supplementation on inflammatory factors and oxidative stress biomarkers in patients with knee osteoarthritis. Acta Med. Iran. 2015, 53, 207–213. [Google Scholar] [PubMed]
- Yashaswini, P.S.; Rao, A.G.A.; Singh, S.A. Inhibition of lipoxygenase by sesamol corroborates its potential anti-inflammatory activity. Int. J. Biol. Macromol. 2017, 94, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Hemshekhar, M.; Thushara, R.M.; Jnaneshwari, S.; Devaraja, S.; Kemparaju, K.; Girish, K.S. Attenuation of adjuvant-induced arthritis by dietary sesamol via modulation of inflammatory mediators, extracellular matrix degrading enzymes and antioxidant status. Eur. J. Nutr. 2013, 52, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Khansai, M.; Boonmaleerat, K.; Pothacharoen, P.; Phitak, T.; Kongtawelert, P. Ex vivo model exhibits protective effects of sesamin against destruction of cartilage induced with a combination of tumor necrosis factor-alpha and oncostatin M. BMC Complement. Altern. Med. 2016, 16. [Google Scholar] [CrossRef] [PubMed]
- Cavero, R.Y.; Calvo, M.I. Medicinal plants used for musculoskeletal disorders in Navarra and their pharmacological validation. J. Ethnopharmacol. 2015, 168, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Dell’Agli, M.; Badea, M.; Dima, L.; Colombo, E.; Sangiovanni, E.; Restani, P.; Bosisio, E. Plant food supplements with anti-inflammatory properties: A systematic review (II). Crit. Rev. Food Sci. Nutr. 2013, 53, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Gilca, M.; Gaman, L.; Lixandru, D.; Stoian, I. Estimating the yin-yang nature of Western herbs: A potential tool based on antioxidation-oxidation theory. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Hiermann, A.; Writzel, M. Antiphlogistic glycopeptide from the roots of Symphytum officinale. Pharm. Pharmacol. Lett. 1998, 8, 154–157. [Google Scholar]
- Mascolo, N.; Autore, G.; Capasso, F.; Menghini, A.; Fasulo, M.P. Biological screening of Italian medicinal plants for anti-inflammatory activity. Phyther. Res. 1987, 1, 28–31. [Google Scholar] [CrossRef]
- Laslett, L.L.; Quinn, S.J.; Darian-Smith, E.; Kwok, M.; Fedorova, T.; Körner, H.; Steels, E.; March, L.; Jones, G. Treatment with 4Jointz reduces knee pain over 12 weeks of treatment in patients with clinical knee osteoarthritis: A randomised controlled trial. Osteoarthr. Cartil. 2012, 20, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Grube, B.; Grünwald, J.; Krug, L.; Staiger, C. Efficacy of a comfrey root (Symphyti offic. radix) extract ointment in the treatment of patients with painful osteoarthritis of the knee: Results of a double-blind, randomised, bicenter, placebo-controlled trial. Phytomedicine 2007, 14, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Gracza, L.; Koch, H.; Loffler, E. Biochemical-pharmacologic studies of medicinal plants. 1. Isolation of rosmarinic acid from Symphytum officinale L. and its anti-inflammatory activity in an in vitro model. Arch. Pharm. 1985, 318, 1090–1095. [Google Scholar] [CrossRef]
- Gracza, L. Prüfung der membranabdichtenden Wirkung eines Phytopharmakons und dessen Wirkstoffe. Z. Phytother. 1987, 8, 78–81. [Google Scholar]
- Huang, S.-H.; Lee, C.-H.; Wang, H.-M.; Chang, Y.-W.; Lin, C.-Y.; Chen, C.-Y.; Chen, Y.-H. 6-Dehydrogingerdione restrains lipopolysaccharide-induced inflammatory responses in RAW 264.7 macrophages. J. Agric. Food Chem. 2014, 62, 9171–9179. [Google Scholar] [CrossRef] [PubMed]
- Van Breemen, R.B.; Tao, Y.; Li, W. Cyclooxygenase-2 inhibitors in ginger (Zingiber officinale). Fitoterapia 2011, 82, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Grzanna, R.; Lindmark, L.; Frondoza, C.G. Ginger—An herbal medicinal product with broad anti-inflammatory actions. J. Med. Food 2005, 8, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Ippoushi, K.; Takeuchi, A. Repeated oral administration of a squeezed ginger (Zingiber officinale) extract augmented the serum corticosterone level and had anti-inflammatory properties. Biosci. Biotechnol. Biochem. 2010, 74, 2248–2252. [Google Scholar] [CrossRef] [PubMed]
- Naderi, Z.; Mozaffari-Khosravi, H.; Dehghan, A.; Nadjarzadeh, A.; Huseini, H.F. Effect of ginger powder supplementation on nitric oxide and C-reactive protein in elderly knee osteoarthritis patients: A 12-week double-blind randomized placebo-controlled clinical trial. J. Tradit. Complement. Med. 2016, 6, 199–203. [Google Scholar] [CrossRef]
- Altman, R.D.; Marcussen, K.C. Effects of a ginger extract on knee pain in patients with osteoarthritis. Arthritis Rheum. 2001, 44, 2531–2538. [Google Scholar] [CrossRef]
- Alipour, Z.; Asadizaker, M.; Fayazi, S.; Yegane, N.; Kochak, M.; Haghighi Zadeh, M.H. The Effect of Ginger on Pain and Satisfaction of Patients with Knee Osteoarthritis. Jundishapur J. Chronic Dis. Care 2016, 6, e34798. [Google Scholar] [CrossRef]
- Haghighi, M.; Khalvat, A.; Toliat, T.; Jallaei, S. Comparing the effects of ginger extract and ibuprofen in patients with osteoporosis. Arch. Iran. Med. 2005, 8, 267–271. [Google Scholar]
- Drozdov, V.N.; Kim, V.A.; Tkachenko, E.V.; Varvanina, G.G. Influence of a specific ginger combination on gastropathy conditions in patients with osteoarthritis of the knee or hip. J. Altern. Complement. Med. 2012, 18, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Bliddal, H.; Rosetzsky, A.; Schlichting, P.; Weidner, M.S.; Andersen, L.A.; Ibfelt, H.H.; Christensen, K.; Jensen, O.N.; Barslev, J. A randomized, placebo-controlled, cross-over study of ginger extracts and ibuprofen in osteoarthritis. Osteoarthr. Cartil. 2000, 8, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Park, S.H.; Lee, M.; Kim, H.-J.; Ryu, S.Y.; Kim, N.D.; Hwang, B.Y.; Hong, J.T.; Han, S.-B.; Kim, Y. 1-Dehydro-[10]-gingerdione from ginger inhibits IKKβ activity for NF-κB activation and suppresses NF-κB-regulated expression of inflammatory genes. Br. J. Pharmacol. 2012, 167, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Bhalla, M.; de Jager, P.; Gilca, M. An overview on Ashwagandha: A Rasayana (Rejuvenator) of Ayurveda. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Aggarwal, A.; Maurya, R.; Naik, S. Withania somnifera inhibits NF-κB and AP-1 transcription factors in human peripheral blood and synovial fluid mononuclear cells. Phytother. Res. 2007, 21, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Sehgal, P.K.; Mandal, A.B.; Sayeed, S. Protective effect of Withania somnifera and Cardiospermum halicacabum extracts against collagenolytic degradation of collagen. Appl. Biochem. Biotechnol. 2011, 165, 1075–1091. [Google Scholar] [CrossRef] [PubMed]
- Rasool, M.; Varalakshmi, P. Protective effect of Withania somnifera root powder in relation to lipid peroxidation, antioxidant status, glycoproteins and bone collagen on adjuvant-induced arthritis in rats. Fundam. Clin. Pharmacol. 2007, 21, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Ramakanth, G.S.H.; Uday Kumar, C.; Kishan, P.V.; Usharani, P. A randomized, double blind placebo controlled study of efficacy and tolerability of Withaina somnifera extracts in knee joint pain. J. Ayurveda Integr. Med. 2016, 7, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Sabina, E.P.; Chandal, S.; Rasool, M.K. Inhibition of monosodium urate crystal-induced inflammation by withaferin A. J. Pharm. Pharm. Sci. 2008, 11, 46–55. [Google Scholar] [PubMed]
- Grover, A.; Shandilya, A.; Punetha, A.; Bisaria, V.S.; Sundar, D. Inhibition of the NEMO/IKKβ association complex formation, a novel mechanism associated with the NF-κB activation suppression by Withania somnifera’s key metabolite withaferin A. BMC Genom. 2010, 11 (Suppl. 4), S25. [Google Scholar] [CrossRef] [PubMed]
- Heyninck, K.; Lahtela-Kakkonen, M.; van der Veken, P.; Haegeman, G.; Vanden Berghe, W. Withaferin A inhibits NF-κB activation by targeting cysteine 179 in IKKβ. Biochem. Pharmacol. 2014, 91, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Bee, T.A.; Liew, A.; Hons, P. Dietary Supplements Used in Osteoarthritis. Proc. Singap. Healthc. 2010, 19, 237–247. [Google Scholar] [CrossRef]
- Posadzki, P.; Watson, L.; Ernst, E. Contamination and adulteration of herbal medicinal products (HMPs): An overview of systematic reviews. Eur. J. Clin. Pharmacol. 2013, 69, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Ernst, E. Risks of herbal medicinal products. Pharmacoepidemiol. Drug Saf. 2004, 13, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Posadzki, P.; Watson, L.; Ernst, E. Herb-drug interactions: An overview of systematic reviews. Br. J. Clin. Pharmacol. 2013, 75, 603–618. [Google Scholar] [CrossRef] [PubMed]
- Bhattaram, V.A.; Graefe, U.; Kohlert, C.; Veit, M.; Derendorf, H. Pharmacokinetics and bioavailability of herbal medicinal products. Phytomedicine 2002, 9 (Suppl. 3), 1–33. [Google Scholar] [CrossRef] [PubMed]
| Plant | Active Phytochemicals | Mechanism of Action | References |
|---|---|---|---|
| Arnica montana | phenols, flavonoids | (−) NO, TNF-α, IL-1β, IL-6, IL-12, anti-type II collagen antibodies, (+) antioxidants (AM) | [24] |
| Boswelia spp. | boswelic acids | (−) PGE1-S, cathepsin G, LOX-5, MMP-9, MMP-13, COX-2, NO, PGE1, TNF-α, IL-1, IL-2, IL-4, IL-6, IFN-γ (in vitro, AM) | [17,30,31,41] |
| (−) leukocyte infiltration in knee (AM) | [43] | ||
| Curcuma spp. | curcuminoids | (+) SOD, GSH, (−) MDA (HS) | [58] |
| (−) neutrophil infiltrate in knee, (AM), (−) IL-1β, TNFα, MCP-1, and MIP-1α (in vitro, AM) | [57,59] | ||
| β-elemene | (+) p38 MAPK (in vitro) | [60] | |
| Equisetum arvense | kynurenic acid | (−) synoviocyte proliferation (in vitro) | [64,66] |
| Harpagophytum procumbens | iridoid glycosides | (−) iNOS and COX-2 (in vitro) | [76] |
| Panax notoginseng | saponins | (−) TNF-alpha, IL-1, iNOS, MMP-13 (AM) | [79,80] |
| Salix spp. | salicin, polyphenols, flavonoids | (−) TNFα, COX-2, IL-1, IL-6 (in vitro) | [87,95] |
| Sesamum indicum | sesamin, sesamol, sesamolin | (−) thiobarbituric acid reactive substances, LOX (in vitro), TNF-α, IL-1β, IL-6, hyaluronidase, MMP-13, MMP-3, MMP-9, exoglycosidases, cathepsin D, phosphatases, COX-2, PGE2, ROS, H2O2, MDA (AM), IL-6 (HS) | [1,97,98,101,102,103] |
| (+) GSH, GPx (AM) | |||
| Symphitum officinalis | rosmarinic acids, glycopeptides, amino acids | (−) PG (in vitro) | [108,112,113] |
| Zingiber officinalis | gingerdione derivatives, 10-gingerol, 8,10-shogaol | (−) COX-1, COX-2, LOX, iNOS, TNF-α, IL-1β, IL-6, MCP-1, κB kinase β (in vitro, AM), NO (HS) | [114,115,117,118,124] |
| (+) cortisone (AM) | |||
| Whitania somnifera | whitaferin A | (−)TNF-alpha, IL-1β, IL-12, collagenase (in vitro), NF kB (docking studies) | [126,127,131,132] |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragos, D.; Gilca, M.; Gaman, L.; Vlad, A.; Iosif, L.; Stoian, I.; Lupescu, O. Phytomedicine in Joint Disorders. Nutrients 2017, 9, 70. https://doi.org/10.3390/nu9010070
Dragos D, Gilca M, Gaman L, Vlad A, Iosif L, Stoian I, Lupescu O. Phytomedicine in Joint Disorders. Nutrients. 2017; 9(1):70. https://doi.org/10.3390/nu9010070
Chicago/Turabian StyleDragos, Dorin, Marilena Gilca, Laura Gaman, Adelina Vlad, Liviu Iosif, Irina Stoian, and Olivera Lupescu. 2017. "Phytomedicine in Joint Disorders" Nutrients 9, no. 1: 70. https://doi.org/10.3390/nu9010070
APA StyleDragos, D., Gilca, M., Gaman, L., Vlad, A., Iosif, L., Stoian, I., & Lupescu, O. (2017). Phytomedicine in Joint Disorders. Nutrients, 9(1), 70. https://doi.org/10.3390/nu9010070