Therapeutic Effects of Olive and Its Derivatives on Osteoarthritis: From Bench to Bedside
Abstract
:1. Introduction
2. Effects of Olive and Its Derivatives in Animal Models of OA
3. Effects of Olive and Its Derivatives in Human Studies
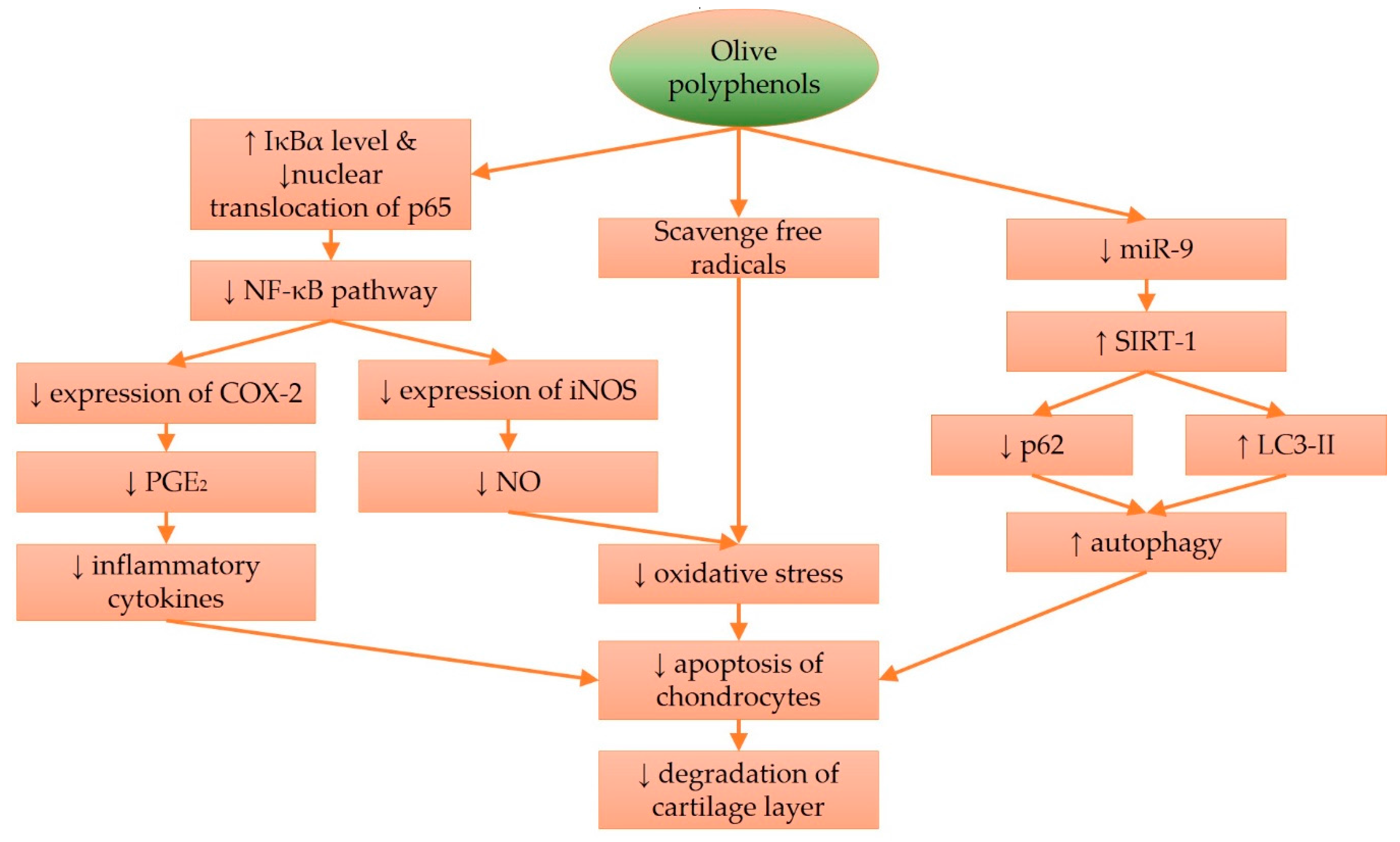
4. Molecular Effects of Olive and Its Derivatives in Cartilage Protection
5. Perspectives on the Use of Olive and Its Derivatives in Combating OA
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kraus, V.B.; Blanco, F.J.; Englund, M.; Karsdal, M.A.; Lohmander, L.S. Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use. Osteoarthr. Cartil. 2015, 23, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Osteoarthritis (OA). Available online: http://www.cdc.gov/arthritis/basics/osteoarthritis.htm (accessed on 18 June 2016).
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Salmon, J.H.; Rat, A.C.; Sellam, J.; Michel, M.; Eschard, J.P.; Guillemin, F.; Jolly, D.; Fautrel, B. Economic impact of lower-limb osteoarthritis worldwide: A systematic review of cost-of-illness studies. Osteoarthr. Cartil. 2016, 24, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Szychlinska, M.A.; Mobasheri, A. Age-related degeneration of articular cartilage in the pathogenesis of osteoarthritis: Molecular markers of senescent chondrocytes. Histol. Histopathol. 2015, 30, 1–12. [Google Scholar] [PubMed]
- Mobasheri, A.; Matta, C.; Zakany, R.; Musumeci, G. Chondrosenescence: Definition, hallmarks and potential role in the pathogenesis of osteoarthritis. Maturitas 2015, 80, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.T.; Meng, H.; Ma, L.F.; Zhang, L.; Yu, H.M.; Wang, Z.Z.; Guo, A. Role of autophagy in the progression of osteoarthritis: The autophagy inhibitor, 3-methyladenine, aggravates the severity of experimental osteoarthritis. Int. J. Mol. Med. 2017, 39, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Castrogiovanni, P.; Trovato, F.M.; Weinberg, A.M.; Al-Wasiyah, M.K.; Alqahtani, M.H.; Mobasheri, A. Biomarkers of chondrocyte apoptosis and autophagy in osteoarthritis. Int. J. Mol. Sci. 2015, 16, 20560–20575. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Aiello, F.C.; Szychlinska, M.A.; Di Rosa, M.; Castrogiovanni, P.; Mobasheri, A. Osteoarthritis in the XXist century: Risk factors and behaviours that influence disease onset and progression. Int. J. Mol. Sci. 2015, 16, 6093–6112. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Castrogiovanni, P.; Loreto, C.; Castorina, S.; Pichler, K.; Weinberg, A.M. Post-traumatic caspase-3 expression in the adjacent areas of growth plate injury site: A morphological study. Int. J. Mol. Sci. 2013, 14, 15767–15784. [Google Scholar] [CrossRef] [PubMed]
- Nakki, A.; Rodriguez-Fontenla, C.; Gonzalez, A.; Harilainen, A.; Leino-Arjas, P.; Heliovaara, M.; Eriksson, J.G.; Tallroth, K.; Videman, T.; Kaprio, J.; et al. Association study of MMP8 gene in osteoarthritis. Connect. Tissue Res. 2016, 57, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Pozgan, U.; Caglic, D.; Rozman, B.; Nagase, H.; Turk, V.; Turk, B. Expression and activity profiling of selected cysteine cathepsins and matrix metalloproteinases in synovial fluids from patients with rheumatoid arthritis and osteoarthritis. Biol. Chem. 2010, 391, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Scanzello, C.R.; Umoh, E.; Pessler, F.; Diaz-Torne, C.; Miles, T.; Dicarlo, E.; Potter, H.G.; Mandl, L.; Marx, R.; Rodeo, S.; et al. Local cytokine profiles in knee osteoarthritis: Elevated synovial fluid interleukin-15 differentiates early from end-stage disease. Osteoarthr. Cartil. 2009, 17, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Janusz, M.J.; Little, C.B.; King, L.E.; Hookfin, E.B.; Brown, K.K.; Heitmeyer, S.A.; Caterson, B.; Poole, A.R.; Taiwo, Y.O. Detection of aggrecanase- and MMP-generated catabolic neoepitopes in the rat iodoacetate model of cartilage degeneration. Osteoarthr. Cartil. 2004, 12, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, S.; Skwara, A.; Bloch, M.; Dankbar, B. Differential induction and regulation of matrix metalloproteinases in osteoarthritic tissue and fluid synovial fibroblasts. Osteoarthr. Cartil. 2004, 12, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, H.; Kohsaka, H.; Liu, M.F.; Higashiyama, H.; Hirata, Y.; Kanno, K.; Saito, I.; Miyasaka, N. Nitric oxide production and inducible nitric oxide synthase expression in inflammatory arthritides. J. Clin. Investig. 1995, 96, 2357–2363. [Google Scholar] [CrossRef] [PubMed]
- Hiran, T.S.; Moulton, P.J.; Hancock, J.T. Detection of superoxide and NADPH oxidase in porcine articular chondrocytes. Free Radic. Biol. Med. 1997, 23, 736–743. [Google Scholar] [CrossRef]
- Rathakrishnan, C.; Tiku, K.; Raghavan, A.; Tiku, M.L. Release of oxygen radicals by articular chondrocytes: A study of luminol-dependent chemiluminescence and hydrogen peroxide secretion. J. Bone Miner. Res. 1992, 7, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.M.; Gilbert, S.J.; Caterson, B.; Sandell, L.J.; Archer, C.W. Oxidative stress induces expression of osteoarthritis markers procollagen IIA and 3B3(-) in adult bovine articular cartilage. Osteoarthr. Cartil. 2008, 16, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Ostalowska, A.; Birkner, E.; Wiecha, M.; Kasperczyk, S.; Kasperczyk, A.; Kapolka, D.; Zon-Giebel, A. Lipid peroxidation and antioxidant enzymes in synovial fluid of patients with primary and secondary osteoarthritis of the knee joint. Osteoarthr. Cartil. 2006, 14, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Tiku, M.L.; Liesch, J.B.; Robertson, F.M. Production of hydrogen peroxide by rabbit articular chondrocytes. Enhancement by cytokines. J. Immunol. 1990, 1990, 690–696. [Google Scholar]
- Scott, J.L.; Gabrielides, C.; Davidson, R.K.; Swingler, T.E.; Clark, I.M.; Wallis, G.A.; Boot-Handford, R.P.; Kirkwood, T.B.; Taylor, R.W.; Young, D.A. Superoxide dismutase downregulation in osteoarthritis progression and end-stage disease. Ann. Rheum. Dis. 2010, 69, 1502–1510. [Google Scholar] [CrossRef] [PubMed]
- Regan, E.; Flannelly, J.; Bowler, R.; Tran, K.; Nicks, M.; Carbone, B.D.; Glueck, D.; Heijnen, H.; Mason, R.; Crapo, J. Extracellular superoxide dismutase and oxidant damage in osteoarthritis. Arthritis Rheumatol. 2005, 52, 3479–3491. [Google Scholar] [CrossRef] [PubMed]
- Aigner, T.; Fundel, K.; Saas, J.; Gebhard, P.M.; Haag, J.; Weiss, T.; Zien, A.; Obermayr, F.; Zimmer, R.; Bartnik, E. Large-scale gene expression profiling reveals major pathogenetic pathways of cartilage degeneration in osteoarthritis. Arthritis Rheumatol. 2006, 54, 3533–3544. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Romero, C.; Calamia, V.; Mateos, J.; Carreira, V.; Martinez-Gomariz, M.; Fernandez, M.; Blanco, F.J. Mitochondrial dysfunction of osteoarthritic human articular chondrocytes analyzed by proteomics. Mol. Cell. Proteom. 2009, 8, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Altay, M.A.; Erturk, C.; Bilge, A.; Yapti, M.; Levent, A.; Aksoy, N. Evaluation of prolidase activity and oxidative status in patients with knee osteoarthritis: Relationships with radiographic severity and clinical parameters. Rheumatol. Int. 2015, 35, 1725–1731. [Google Scholar] [CrossRef] [PubMed]
- Maneiro, E.; Lopez-Armada, M.J.; de Andres, M.C.; Carames, B.; Martin, M.A.; Bonilla, A.; Del Hoyo, P.; Galdo, F.; Arenas, J.; Blanco, F.J. Effect of nitric oxide on mitochondrial respiratory activity of human articular chondrocytes. Ann. Rheum. Dis. 2005, 64, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.; Jung, A.; Murphy, A.; Andreyev, A.; Dykens, J.; Terkeltaub, R. Mitochondrial oxidative phosphorylation is a downstream regulator of nitric oxide effects on chondrocyte matrix synthesis and mineralization. Arthritis Rheumatol. 2000, 43, 1560–1570. [Google Scholar] [CrossRef]
- Rachek, L.I.; Grishko, V.I.; Ledoux, S.P.; Wilson, G.L. Role of nitric oxide-induced mtDNA damage in mitochondrial dysfunction and apoptosis. Free Radic. Biol. Med. 2006, 40, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.E.; Bruckner, P.; Pujol, J.P.L. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthr. Cartil. 2003, 11, 747–755. [Google Scholar] [CrossRef]
- Musumeci, G.; Loreto, C.; Carnazza, M.L.; Martinez, G. Characterization of apoptosis in articular cartilage derived from the knee joints of patients with osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Satareh, M.; Ochs, R.L.; Lotz, M. Fas/fas ligand expression and induction of apoptosis in chondrocytes. Arthritis Rheumatol. 1997, 40, 1749–1755. [Google Scholar] [CrossRef]
- Aigner, T.; Hemmel, M.; Neureiter, D.; Gebhard, P.M.; Zeiler, G.; Kirchner, T.; McKenna, L. Apoptotic cell death is not a widespread phenomenon in normal aging and osteoarthritic human articular knee cartilage. Arthritis Rheumatol. 2001, 44, 1304–1312. [Google Scholar] [CrossRef]
- Doerks, T.; Copley, R.R.; Schultz, J.; Ponting, C.P.; Bork, P. Systematic identification of novel protein domain families associated with nuclear functions. Genome Res. 2002, 12, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Castrogiovanni, P.; Trovato, F.M.; Loreto, C.; Nsir, H.; Szychlinska, M.A.; Musumeci, G. Nutraceutical supplements in the management and prevention of osteoarthritis. Int. J. Mol. Sci. 2016, 17, 2042. [Google Scholar] [CrossRef] [PubMed]
- Bohensky, J.; Leshinsky, S.; Srinivas, V.; Shapiro, I.M. Chondrocyte autophagy is stimulated by HIF-1 dependent AMPK activation and mtor suppression. Pediatr. Nephrol. 2010, 25, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Terkeltaub, R.; Yang, B.; Lotz, M.; Liu-Bryan, R. Chondrocyte AMP-activated protein kinase activity suppresses matrix degradation responses to proinflammatory cytokines interleukin-1β and tumor necrosis factor α. Arthritis Rheumatol. 2011, 63, 1928–1937. [Google Scholar] [CrossRef] [PubMed]
- Thoms, B.L.; Dudek, K.A.; Lafont, J.E.; Murphy, C.L. Hypoxia promotes the production and inhibits the destruction of human articular cartilage. Arthritis Rheumatol. 2013, 65, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Gabay, O.; Sanchez, C.; Dvir-Ginzberg, M.; Gagarina, V.; Zaal, K.J.; Song, Y.; He, X.H.; McBurney, M.W. Sirtuin 1 enzymatic activity is required for cartilage homeostasis in vivo in a mouse model. Arthritis Rheumatol. 2013, 65, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, T.; Sasaki, H.; Takayama, K.; Ishida, K.; Matsumoto, T.; Kubo, S.; Matsuzaki, T.; Nishida, K.; Kurosaka, M.; Kuroda, R. The overexpression of SIRT1 inhibited osteoarthritic gene expression changes induced by interleukin-1β in human chondrocytes. J. Orthop. Res. 2013, 31, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, T.; Matsushita, T.; Takayama, K.; Matsumoto, T.; Nishida, K.; Kuroda, R.; Kurosaka, M. Disruption of Sirt1 in chondrocytes causes accelerated progression of osteoarthritis under mechanical stress and during ageing in mice. Ann. Rheum. Dis. 2014, 73, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Petursson, F.; Husa, M.; June, R.; Lotz, M.; Terkeltaub, R.; Liu-Bryan, R. Linked decreases in liver kinase B1 and AMP-activated protein kinase activity modulate matrix catabolic responses to biomechanical injury in chondrocytes. Arthritis Res. Ther. 2013, 15, R77. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Csaki, C.; Nebrich, S.; Mobasheri, A. Resveratrol suppresses interleukin-1β-induced inflammatory signaling and apoptosis in human articular chondrocytes: Potential for use as a novel nutraceutical for the treatment of osteoarthritis. Biochem. Pharmacol. 2008, 76, 1426–1439. [Google Scholar] [CrossRef] [PubMed]
- Dave, M.; Attur, M.; Palmer, G.; Al-Mussawir, H.E.; Kennish, L.; Patel, J.; Abramson, S.B. The antioxidant resveratrol protects against chondrocyte apoptosis via effects on mitochondrial polarization and ATP production. Arthritis Rheumatol. 2008, 58, 2786–2797. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Wang, J.G.; Xiao, D.M.; Fan, M.; Wang, D.P.; Xiong, J.Y.; Chen, Y.; Ding, Y.; Liu, S.L. Resveratrol inhibits interleukin 1β-mediated inducible nitric oxide synthase expression in articular chondrocytes by activating SIRT1 and thereby suppressing nuclear factor-κB activity. Eur. J. Pharmacol. 2012, 674, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, J.S.; Chen, J.W.; Li, F.; Tian, J. Effect of resveratrol on cartilage protection and apoptosis inhibition in experimental osteoarthritis of rabbit. Rheumatol. Int. 2012, 32, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, A.G.; Bentley, G. Chondrocyte multiplication in osteoarthritic articular cartilage. J. Bone Jt. Surg. 1973, 55, 588–594. [Google Scholar]
- Carames, B.; Taniguchi, N.; Otsuki, S.; Blanco, F.J.; Lotz, M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheumatol. 2010, 62, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Dvir-Ginzberg, M.; Gagarina, V.; Lee, E.J.; Booth, R.; Gabay, O.; Hall, D.J. Tumor necrosis factor α-mediated cleavage and inactivation of SirT1 in human osteoarthritic chondrocytes. Arthritis Rheumatol. 2011, 63, 2363–2373. [Google Scholar] [CrossRef] [PubMed]
- Dvir-Ginzberg, M.; Gagarina, V.; Lee, E.J.; Hall, D.J. Regulation of cartilage-specific gene expression in human chondrocytes by SirT1 and nicotinamide phosphoribosyltransferase. J. Biol. Chem. 2008, 283, 36300–36310. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Petursson, F.; Viollet, B.; Lotz, M.; Terkeltaub, R.; Liu-Bryan, R. Peroxisome proliferator-activated receptor gamma coactivator 1α and FoxO3a mediate chondroprotection by AMP-activated protein kinase. Arthritis Rheumatol. 2014, 66, 3073–3082. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S.; Kakutani, K.; Yurube, T.; Maeno, K.; Takada, T.; Zhang, Z.; Kurakawa, T.; Terashima, Y.; Ito, M.; Ueha, T.; et al. Recombinant human SIRT1 protects against nutrient deprivation-induced mitochondrial apoptosis through autophagy induction in human intervertebral disc nucleus pulposus cells. Arthritis Res. Ther. 2015, 17, 253. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Kawakami, Y.; Kobayashi, M.; Greco, N.; Cummins, J.H.; Matsushita, T.; Kuroda, R.; Kurosaka, M.; Fu, F.H.; Huard, J. Local intra-articular injection of rapamycin delays articular cartilage degeneration in a murine model of osteoarthritis. Arthritis Res. Ther. 2014, 16, 482. [Google Scholar] [CrossRef] [PubMed]
- Caramés, B.; Kiosses, W.B.; Akasaki, Y.; Brinson, D.C.; Eap, W.; Koziol, J.; Lotz, M.K. Glucosamine activates autophagy in vitro and in vivo. Arthritis Rheumatol. 2013, 65, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Bruyere, O.; Altman, R.D.; Reginster, J.Y. Efficacy and safety of glucosamine sulfate in the management of osteoarthritis: Evidence from real-life setting trials and surveys. Semin. Arthritis Rheum. 2016, 45, S12–S17. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Woo, J.H.; Choi, S.J.; Ji, J.D.; Song, G.G. Effect of glucosamine or chondroitin sulfate on the osteoarthritis progression: A meta-analysis. Rheumatol. Int. 2010, 30, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Sawitzke, A.D.; Shi, H.; Finco, M.F.; Dunlop, D.D.; Bingham, C.O., 3rd; Harris, C.L.; Singer, N.G.; Bradley, J.D.; Silver, D.; Jackson, C.G.; et al. The effect of glucosamine and/or chondroitin sulfate on the progression of knee osteoarthritis: A report from the glucosamine/chondroitin arthritis intervention trial. Arthritis Rheumatol. 2008, 58, 3183–3191. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Beaumont, G.; Ivorra, J.A.; Del Carmen Trabado, M.; Blanco, F.J.; Benito, P.; Martin-Mola, E.; Paulino, J.; Marenco, J.L.; Porto, A.; Laffon, A.; et al. Glucosamine sulfate in the treatment of knee osteoarthritis symptoms: A randomized, double-blind, placebo-controlled study using acetaminophen as a side comparator. Arthritis Rheumatol. 2007, 56, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Reginster, J.Y.; Deroisy, R.; Rovati, L.C.; Lee, R.L.; Lejeune, E.; Bruyere, O.; Giacovelli, G.; Henrotin, Y.; Dacre, J.E.; Gossett, C. Long-term effects of glucosamine sulphate on osteoarthritis progression: A randomised, placebo-controlled clinical trial. Lancet 2001, 357, 251–256. [Google Scholar] [CrossRef]
- El-Arman, M.M.; El-Fayoumi, G.; El-Shal, E.; El-Boghdady, I.; El-Ghaweet, A. Aggrecan and cartilage oligomeric matrix protein in serum and synovial fluid of patients with knee osteoarthritis. HSS J. 2010, 6, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Pratta, M.A.; Su, J.L.; Leesnitzer, M.A.; Struglics, A.; Larsson, S.; Lohmander, L.S.; Kumar, S. Development and characterization of a highly specific and sensitive sandwich elisa for detection of aggrecanase-generated aggrecan fragments. Osteoarthr. Cartil. 2006, 14, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Struglics, A.; Larsson, S.; Pratta, M.A.; Kumar, S.; Lark, M.W.; Lohmander, L.S. Human osteoarthritis synovial fluid and joint cartilage contain both aggrecanase- and matrix metalloproteinase-generated aggrecan fragments. Osteoarthr. Cartil. 2006, 14, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, A.; Beauvais, P.J.; Saed-Nejad, F. Differential levels of synovial fluid aggrecan aggregate components in experimental osteoarthritis and joint disuse. J Orthop. Res. 1994, 12, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Tiku, M.L.; Gupta, S.; Deshmukh, D.R. Aggrecan degradation in chondrocytes is mediated by reactive oxygen species and protected by antioxidants. Free Radic. Res. 1999, 30, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, R.; Loreto, C.; Barbato, E.; Caltabiano, R.; Lombardo, C.; Musumeci, G.; Lo Muzio, L. MMP-13 (collagenase 3) localization in human temporomandibular joint discs with internal derangement. Acta Histochem. 2008, 110, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Lipari, L.; Gerbino, A. Expression of gelatinases (MMP-2, MMP-9) in human articular cartilage. Int. J. Immunopathol. Pharmacol. 2013, 26, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, Z.; Xue, R.; Singh, G.K.; Shi, K.; Lv, Y.; Yang, L. Combined effects of TNF-α, IL-1β, and HIF-1α on MMP-2 production in ACL fibroblasts under mechanical stretch: An in vitro study. J. Orthop. Res. 2011, 29, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, R.; Rusu, M.C.; Loreto, F.; Loreto, C.; Musumeci, G. Immunolocalization and expression of lubricin in the bilaminar zone of the human temporomandibular joint disc. Acta Histochem. 2012, 114, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Castrogiovanni, P.; Trovato, F.M.; Imbesi, R.; Giunta, S.; Szychlinska, M.A.; Loreto, C.; Castorina, S.; Mobasheri, A. Physical activity ameliorates cartilage degeneration in a rat model of aging: A study on lubricin expression. Scand. J. Med. Sci. Sports 2015, 25, e222–e230. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Carnazza, M.L.; Loreto, C.; Leonardi, R.; Loreto, C. β-defensin-4 (HBD-4) is expressed in chondrocytes derived from normal and osteoarthritic cartilage encapsulated in PEGDA scaffold. Acta Histochem. 2012, 114, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Carnazza, M.L.; Leonardi, R.; Loreto, C. Expression of β-defensin-4 in “an in vivo and ex vivo model” of human osteoarthritic knee meniscus. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Tagliafierro, L.; Officioso, A.; Sorbo, S.; Basile, A.; Manna, C. The protective role of olive oil hydroxytyrosol against oxidative alterations induced by mercury in human erythrocytes. Food Chem. Toxicol. 2015, 82, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wang, X.; Hou, C.; Yang, L.; Li, H.; Guo, J.; Huo, C.; Wang, M.; Miao, Y.; Liu, J.; et al. Oleuropein improves mitochondrial function to attenuate oxidative stress by activating the Nrf2 pathway in the hypothalamic paraventricular nucleus of spontaneously hypertensive rats. Neuropharmacology 2017, 113, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Maalej, A.; Mahmoudi, A.; Bouallagui, Z.; Fki, I.; Marrekchi, R.; Sayadi, S. Olive phenolic compounds attenuate deltamethrin-induced liver and kidney toxicity through regulating oxidative stress, inflammation and apoptosis. Food Chem. Toxicol. 2017, 106, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Kalaiselvan, I.; Dicson, S.M.; Kasi, P.D. Olive oil and its phenolic constituent tyrosol attenuates dioxin-induced toxicity in peripheral blood mononuclear cells via an antioxidant-dependent mechanism. Nat. Prod. Res. 2015, 29, 2129–2132. [Google Scholar] [CrossRef] [PubMed]
- Cabrerizo, S.; De La Cruz, J.P.; Lopez-Villodres, J.A.; Munoz-Marin, J.; Guerrero, A.; Reyes, J.J.; Labajos, M.T.; Gonzalez-Correa, J.A. Role of the inhibition of oxidative stress and inflammatory mediators in the neuroprotective effects of hydroxytyrosol in rat brain slices subjected to hypoxia reoxygenation. J. Nutr. Biochem. 2013, 24, 2152–2157. [Google Scholar] [CrossRef] [PubMed]
- Scoditti, E.; Nestola, A.; Massaro, M.; Calabriso, N.; Storelli, C.; De Caterina, R.; Carluccio, M.A. Hydroxytyrosol suppresses MMP-9 and COX-2 activity and expression in activated human monocytes via PKCα and PKCβ 1 inhibition. Atherosclerosis 2014, 232, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Liu, L.; Pan, H.; Ma, Y.; Wang, D.; Kang, K.; Wang, J.; Sun, B.; Sun, X.; Jiang, H. Protective effects of hydroxytyrosol on liver ischemia/reperfusion injury in mice. Mol. Nutr. Food Res. 2013, 57, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Maria Trovato, F.; Imbesi, R.; Castrogiovanni, P. Effects of dietary extra-virgin olive oil on oxidative stress resulting from exhaustive exercise in rat skeletal muscle: A morphological study. Acta Histochem. 2014, 116, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Takashima, T.; Sakata, Y.; Iwakiri, R.; Shiraishi, R.; Oda, Y.; Inoue, N.; Nakayama, A.; Toda, S.; Fujimoto, K. Feeding with olive oil attenuates inflammation in dextran sulfate sodium-induced colitis in rat. J. Nutr. Biochem. 2014, 25, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Sepodes, B.; Rocha, J.; Direito, R.; Fernandes, A.; Brites, D.; Freitas, M.; Fernandes, E.; Bronze, M.R.; Figueira, M.E. Protective effects of hydroxytyrosol-supplemented refined olive oil in animal models of acute inflammation and rheumatoid arthritis. J. Nutr. Biochem. 2015, 26, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Fidalgo, S.; Villegas, I.; Aparicio-Soto, M.; Cardeno, A.; Rosillo, M.A.; Gonzalez-Benjumea, A.; Marset, A.; Lopez, O.; Maya, I.; Fernandez-Bolanos, J.G.; et al. Effects of dietary virgin olive oil polyphenols: Hydroxytyrosyl acetate and 3,4-dihydroxyphenylglycol on DSS-induced acute colitis in mice. J. Nutr. Biochem. 2015, 26, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Pirozzi, C.; Lama, A.; Simeoli, R.; Paciello, O.; Pagano, T.B.; Mollica, M.P.; Di Guida, F.; Russo, R.; Magliocca, S.; Canani, R.B.; et al. Hydroxytyrosol prevents metabolic impairment reducing hepatic inflammation and restoring duodenal integrity in a rat model of NAFLD. J. Nutr. Biochem. 2016, 30, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Lamy, S.; Ben Saad, A.; Zgheib, A.; Annabi, B. Olive oil compounds inhibit the paracrine regulation of TNF-α-induced endothelial cell migration through reduced glioblastoma cell cyclooxygenase-2 expression. J. Nutr. Biochem. 2016, 27, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Zheng, A.; Li, H.; Xu, J.; Cao, K.; Li, H.; Pu, W.; Yang, Z.; Peng, Y.; Long, J.; Liu, J.; et al. Hydroxytyrosol improves mitochondrial function and reduces oxidative stress in the brain of db/db mice: Role of AMP-activated protein kinase activation. Br. J. Nutr. 2015, 113, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Kikusato, M.; Muroi, H.; Uwabe, Y.; Furukawa, K.; Toyomizu, M. Oleuropein induces mitochondrial biogenesis and decreases reactive oxygen species generation in cultured avian muscle cells, possibly via an up-regulation of peroxisome proliferator-activated receptor gamma coactivator-1α. Anim. Sci. J. 2016, 87, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Stiuso, P.; Bagarolo, M.L.; Ilisso, C.P.; Vanacore, D.; Martino, E.; Caraglia, M.; Porcelli, M.; Cacciapuoti, G. Protective effect of tyrosol and S-adenosylmethionine against ethanol-induced oxidative stress of Hepg2 cells involves sirtuin 1, p53 and Erk1/2 signaling. Int. J. Mol. Sci. 2016, 17, 622. [Google Scholar] [CrossRef] [PubMed]
- Samuel, S.M.; Thirunavukkarasu, M. Akt/FOXO3a/SIRT1-mediated cardioprotection by n-tyrosol against ischemic stress in rat in vivo model of myocardial infarction: Switching gears toward survival and longevity. J. Agric. Food Chem. 2008, 56, 9692–9698. [Google Scholar] [CrossRef] [PubMed]
- Lauretti, E.; Iuliano, L.; Praticò, D. Extra-virgin olive oil ameliorates cognition and neuropathology of the 3xTg mice: Role of autophagy. Ann. Clin. Transl. Neurol. 2017, 4, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, R.; Pignatelli, P.; Nocella, C.; Loffredo, L.; Pastori, D.; Vicario, T.; Petruccioli, A.; Bartimoccia, S.; Violi, F. Extra virgin olive oil blunt post-prandial oxidative stress via NOX2 down-regulation. Atherosclerosis 2014, 235, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Camargo, A.; Rangel-Zuniga, O.A.; Haro, C.; Meza-Miranda, E.R.; Pena-Orihuela, P.; Meneses, M.E.; Marin, C.; Yubero-Serrano, E.M.; Perez-Martinez, P.; Delgado-Lista, J.; et al. Olive oil phenolic compounds decrease the postprandial inflammatory response by reducing postprandial plasma lipopolysaccharide levels. Food Chem. 2014, 162, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Bogani, P.; Galli, C.; Villa, M.; Visioli, F. Postprandial anti-inflammatory and antioxidant effects of extra virgin olive oil. Atherosclerosis 2007, 190, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.A.; Cheon, E.J. Animal model of osteoarthritis. J. Rheum. Dis. 2012, 19, 239–247. [Google Scholar] [CrossRef]
- Gong, D.Z.; Geng, C.Y.; Jiang, L.P.; Wang, L.H.; Yoshimura, H.; Zhong, L.F. Repair effect of olive leaf extract on experimental cartilaginous injuries in rabbits. Chin. J. Pharmacol. Toxicol. 2013, 27, 200–204. [Google Scholar]
- Del Monaco, G.; Officioso, A.; D’Angelo, S.; La Cara, F.; Ionata, E.; Marcolongo, L.; Squillaci, G.; Maurelli, L.; Morana, A. Characterization of extra virgin olive oils produced with typical Italian varieties by their phenolic profile. Food Chem. 2015, 184, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Montano, A.; Hernandez, M.; Garrido, I.; Llerena, J.L.; Espinosa, F. Fatty acid and phenolic compound concentrations in eight different monovarietal virgin olive oils from Extremadura and the relationship with oxidative stability. Int. J. Mol. Sci. 2016, 17, E1960. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Trovato, F.M.; Pichler, K.; Weinberg, A.M.; Loreto, C.; Castrogiovanni, P. Extra-virgin olive oil diet and mild physical activity prevent cartilage degeneration in an osteoarthritis model: An in vivo and in vitro study on lubricin expression. J. Nutr. Biochem. 2013, 24, 2064–2075. [Google Scholar] [CrossRef] [PubMed]
- Jay, G.D.; Waller, K.A. The biology of lubricin: Near frictionless joint motion. Matrix Biol. 2014, 39, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Mevel, E.; Merceron, C.; Vinatier, C.; Krisa, S.; Richard, T.; Masson, M.; Lesoeur, J.; Hivernaud, V.; Gauthier, O.; Abadie, J.; et al. Olive and grape seed extract prevents post-traumatic osteoarthritis damages and exhibits in vitro anti IL-1β activities before and after oral consumption. Sci. Rep. 2016, 6, 33527. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, M.N.; Sanchez, C.; Membrez Scalfo, F.; Drion, P.; Comblain, F.; Taralla, S.; Donneau, A.F.; Offord, E.A.; Henrotin, Y. Oleuropein or rutin consumption decreases the spontaneous development of osteoarthritis in the Hartley guinea pig. Osteoarthr. Cartil. 2015, 23, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Mobasheri, A.; Trovato, F.M.; Szychlinska, M.A.; Imbesi, R.; Castrogiovanni, P. Post-operative rehabilitation and nutrition in osteoarthritis. F1000Research 2014, 3, 116. [Google Scholar] [CrossRef] [PubMed]
- Bitler, C.M.; Matt, K.; Irving, M.; Hook, G.; Yusen, J.; Eagar, F.; Kirschner, K.; Walker, B.; Crea, R. Olive extract supplement decreases pain and improves daily activities in adults with osteoarthritis and decreases plasma homocysteine in those with rheumatoid arthritis. Nutr. Res. 2007, 27, 470–477. [Google Scholar] [CrossRef]
- Bohlooli, S.; Jastan, M.; Nakhostin-Roohi, B.; Mohammadi, S.; Baghaei, Z. A pilot double-blinded, randomized, clinical trial of topical virgin olive oil versus piroxicam gel in osteoarthritis of the knee. J. Clin. Rheumatol. 2012, 18, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Takeda, R.; Koike, T.; Taniguchi, I.; Tanaka, K. Double-blind placebo-controlled trial of hydroxytyrosol of Olea europaea on pain in gonarthrosis. Phytomedicine 2013, 20, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Gelmini, F.; Ruscica, M.; MacChi, C.; Bianchi, V.; Maffei Facino, R.; Beretta, G.; Magni, P. Unsaponifiable fraction of unripe fruits of Olea europaea: An interesting source of anti-inflammatory constituents. Planta Med. 2016, 82, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Nsir, H.; Szychlinska, M.A.; Cardile, V.; Graziano, A.C.E.; Avola, R.; Esafi, H.; Bendini, A.; Zarouk, M.; Loreto, C.; Rapisarda, V.; et al. Polar and apolar extra virgin olive oil and leaf extracts as a promising anti-inflammatory natural treatment for osteoarthritis. Acta Histochem. 2017, 119, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Iacono, A.; Gomez, R.; Sperry, J.; Conde, J.; Bianco, G.; Meli, R.; Gomez-Reino, J.J.; Smith, A.B., III; Gualillo, O. Effect of oleocanthal and its derivatives on inflammatory response induced by lipopolysaccharide in a murine chondrocyte cell line. Arthritis Rheumatol. 2010, 62, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Scotece, M.; Gómez, R.; Conde, J.; Lopez, V.; Gómez-Reino, J.J.; Lago, F.; Smith, A.B., III; Gualillo, O. Further evidence for the anti-inflammatory activity of oleocanthal: Inhibition of MIP-1α and IL-6 in J774 macrophages and in ATDC5 chondrocytes. Life Sci. 2012, 91, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Facchini, A.; Cetrullo, S.; D‘Adamo, S.; Guidotti, S.; Minguzzi, M.; Facchini, A.; Borzi, R.M.; Flamigni, F. Hydroxytyrosol prevents increase of osteoarthritis markers in human chondrocytes treated with hydrogen peroxide or growth-related oncogene α. PLoS ONE 2014, 9, e109724. [Google Scholar] [CrossRef] [PubMed]
- D‘Adamo, S.; Cetrullo, S.; Guidotti, S.; Borzi, R.M.; Flamigni, F. Hydroxytyrosol modulates the levels of microRNA-9 and its target sirtuin-1 thereby counteracting oxidative stress-induced chondrocyte death. Osteoarthr. Cartil. 2017, 25, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Carames, B.; López de Figueroa, P.; Ribeiro, M.; Calamia, V.; Romero, C.R.; Blanco, F.J. Deficient autophagy induces premature senescence in aging and osteoarthritis. Osteoarthr. Cartil. 2015, 23, A33–A34. [Google Scholar] [CrossRef]
- Li, Y.S.; Zhang, F.J.; Zeng, C.; Luo, W.; Xiao, W.F.; Gao, S.G.; Lei, G.H. Autophagy in osteoarthritis. Jt. Bone Spine 2016, 83, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Cetrullo, S.; D‘Adamo, S.; Guidotti, S.; Borzi, R.M.; Flamigni, F. Hydroxytyrosol prevents chondrocyte death under oxidative stress by inducing autophagy through sirtuin 1-dependent and -independent mechanisms. Biochim. Biophys. Acta 2016, 1860, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Ichimura, Y. Physiological significance of selective degradation of p62 by autophagy. FEBS Lett. 2010, 584, 1374–1378. [Google Scholar] [CrossRef] [PubMed]
- Rosillo, M.A.; Sanchez-Hidalgo, M.; Gonzalez-Benjumea, A.; Fernandez-Bolanos, J.G.; Lubberts, E.; Alarcon-de-la-Lastra, C. Preventive effects of dietary hydroxytyrosol acetate, an extra virgin olive oil polyphenol in murine collagen-induced arthritis. Mol. Nutr. Food Res. 2015, 59, 2537–2546. [Google Scholar] [CrossRef] [PubMed]
- St-Laurent-Thibault, C.; Arseneault, M.; Longpre, F.; Ramassamy, C. Tyrosol and hydroxytyrosol, two main components of olive oil, protect N2a cells against amyloid-β-induced toxicity. Involvement of the NF-κB signaling. Curr. Alzheimer Res. 2011, 8, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Miro-Casas, E.; Covas, M.I.; Fito, M.; Farre-Albadalejo, M.; Marrugat, J.; de la Torre, R. Tyrosol and hydroxytyrosol are absorbed from moderate and sustained doses of virgin olive oil in humans. Eur. J. Clin. Nutr. 2003, 57, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Vissers, M.N.; Zock, P.L.; Roodenburg, A.J.; Leenen, R.; Katan, M.B. Olive oil phenols are absorbed in humans. J. Nutr. 2002, 132, 409–417. [Google Scholar] [PubMed]
- Tuck, K.L.; Freeman, M.P.; Hayball, P.J.; Stretch, G.L.; Stupans, I. The in vivo fate of hydroxytyrosol and tyrosol, antioxidant phenolic constituents of olive oil, after intravenous and oral dosing of labeled compounds to rats. J. Nutr. 2001, 131, 1993–1996. [Google Scholar] [PubMed]
- Miro-Casas, E.; Covas, M.I.; Farre, M.; Fito, M.; Ortuno, J.; Weinbrenner, T.; Roset, P.; de la Torre, R. Hydroxytyrosol disposition in humans. Clin. Chem. 2003, 49, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Galli, C.; Bornet, F.; Mattei, A.; Patelli, R.; Galli, G.; Caruso, D. Olive oil phenolics are dose-dependently absorbed in humans. FEBS Lett. 2000, 468, 159–160. [Google Scholar] [CrossRef]
- Parkinson, L.; Cicerale, S. The health benefiting mechanisms of virgin olive oil phenolic compounds. Molecules 2016, 21, 1734. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.F.; Tan, L.S.; Buang, F. Transdermal anti-inflammatory activity of bilayer film containing olive compound hydroxytyrosol: Physical assessment, in vivo dermal safety and efficacy study in Freund‘s adjuvant-induced arthritic rat model. Drug Dev. Ind. Pharm. 2017, 43, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Siddique, M.I.; Katas, H.; Amin, M.; Ng, S.F.; Zulfakar, M.H.; Buang, F.; Jamil, A. Minimization of local and systemic adverse effects of topical glucocorticoids by nanoencapsulation: In vivo safety of hydrocortisone-hydroxytyrosol loaded chitosan nanoparticles. J. Pharm. Sci. 2015, 104, 4276–4286. [Google Scholar] [CrossRef] [PubMed]
- Flaiz, L.; Freire, M.; Cofrades, S.; Mateos, R.; Weiss, J.; Jimenez-Colmenero, F.; Bou, R. Comparison of simple, double and gelled double emulsions as hydroxytyrosol and n-3 fatty acid delivery systems. Food Chem. 2016, 213, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Cofrades, S.; Bou, R. Bioaccessibility of hydroxytyrosol and n-3 fatty acids as affected by the delivery system: Simple, double and gelled double emulsions. J. Food Sci. Technol. 2017, 54, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Junker, S.; Frommer, K.W.; Krumbholz, G.; Tsiklauri, L.; Gerstberger, R.; Rehart, S.; Steinmeyer, J.; Rickert, M.; Wenisch, S.; Schett, G.; et al. Expression of adipokines in osteoarthritis osteophytes and their effect on osteoblasts. Matrix Biol. 2017, 62, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, R.; Inada, H.; Koike, T.; Yamano, T. Effects of leptin to cultured growth plate chondrocytes. Horm. Res. Paediatr. 2003, 60, 91–98. [Google Scholar] [CrossRef]
- Presle, N.; Pottie, P.; Dumond, H.; Guillaume, C.; Lapicque, F.; Pallu, S.; Mainard, D.; Netter, P.; Terlain, B. Differential distribution of adipokines between serum and synovial fluid in patients with osteoarthritis. Contribution of joint tissues to their articular production. Osteoarthr. Cartil. 2006, 14, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Reseland, J.E.; Syversen, U.; Bakke, I.; Qvigstad, G.; Eide, L.G.; Hjertner, Ø.; Gordeladze, J.O.; Drevon, C.A. Leptin is expressed in and secreted from primary cultures of human osteoblasts and promotes bone mineralization. J. Bone Miner. Res. 2001, 16, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Dumond, H.; Presle, N.; Terlain, B.; Mainard, D.; Loeuille, D.; Netter, P.; Pottie, P. Evidence for a key role of leptin in osteoarthritis. Arthritis Rheumatol. 2003, 48, 3118–3129. [Google Scholar] [CrossRef] [PubMed]
- Vuolteenaho, K.; Koskinen, A.; Kukkonen, M.; Nieminen, R.; Paivarinta, U.; Moilanen, T.; Moilanen, E. Leptin enhances synthesis of proinflammatory mediators in human osteoarthritic cartilage—Mediator role of NO in leptin-induced PGE2, IL-6, and IL-8 production. Mediat. Inflamm. 2009, 2009, 345838. [Google Scholar] [CrossRef] [PubMed]
- Simopoulou, T.; Malizos, K.N.; Iliopoulos, D.; Stefanou, N.; Papatheodorou, L.; Ioannou, M.; Tsezou, A. Differential expression of leptin and leptin‘s receptor isoform (Ob-Rb) mRNA between advanced and minimally affected osteoarthritic cartilage; effect on cartilage metabolism. Osteoarthr. Cartil. 2007, 15, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Cnop, M.; Havel, P.J.; Utzschneider, K.M.; Carr, D.B.; Sinha, M.K.; Boyko, E.J.; Retzlaff, B.M.; Knopp, R.H.; Brunzell, J.D.; Kahn, S.E. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: Evidence for independent roles of age and sex. Diabetologia 2003, 46, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.; Chen, L.; Hsieh, M.S.; Chang, C.P.; Chou, D.T.; Tsai, S.H. Evidence for a protective role for adiponectin in osteoarthritis. Biochim. Biophys. Acta 2006, 1762, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Koskinen, A.; Juslin, S.; Nieminen, R.; Moilanen, T.; Vuolteenaho, K.; Moilanen, E. Adiponectin associates with markers of cartilage degradation in osteoarthritis and induces production of proinflammatory and catabolic factors through mitogen-activated protein kinase pathways. Arthritis Res. Ther. 2011, 13, R184. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.M.; Lee, Y.A.; Lee, S.H.; Hong, S.J.; Hahm, D.H.; Choi, S.Y.; Yang, H.I.; Yoo, M.C.; Kim, K.S. Adiponectin may contribute to synovitis and joint destruction in rheumatoid arthritis by stimulating vascular endothelial growth factor, matrix metalloproteinase-1, and matrix metalloproteinase-13 expression in fibroblast-like synoviocytes more than proinflammatory mediators. Arthritis Res. Ther. 2009, 11, R161. [Google Scholar] [PubMed]
- Lago, R.; Gomez, R.; Otero, M.; Lago, F.; Gallego, R.; Dieguez, C.; Gomez-Reino, J.J.; Gualillo, O. A new player in cartilage homeostasis: Adiponectin induces nitric oxide synthase type II and pro-inflammatory cytokines in chondrocytes. Osteoarthr. Cartil. 2008, 16, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Scoditti, E.; Massaro, M.; Carluccio, M.A.; Pellegrino, M.; Wabitsch, M.; Calabriso, N.; Storelli, C.; De Caterina, R. Additive regulation of adiponectin expression by the mediterranean diet olive oil components oleic acid and hydroxytyrosol in human adipocytes. PLoS ONE 2015, 10, e0128218. [Google Scholar] [CrossRef] [PubMed]
- Kabiri, A.; Hosseinzadeh-Attar, M.J.; Haghighatdoost, F.; Eshraghian, M.; Esmaillzadeh, A. Impact of olive oil-rich diet on serum omentin and adiponectin levels: A randomized cross-over clinical trial among overweight women. Int. J. Food Sci. Nutr. 2017, 68, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Tantavisut, S.; Tanavalee, A.; Honsawek, S.; Suantawee, T.; Ngarmukos, S.; Adisakwatana, S.; Callaghan, J.J. Effect of vitamin E on oxidative stress level in blood, synovial fluid, and synovial tissue in severe knee osteoarthritis: A randomized controlled study. BMC Musculoskelet. Disord. 2017, 18, 281. [Google Scholar] [CrossRef] [PubMed]
- Bastiaansen-Jenniskens, Y.M.; Siawash, M.; van de Lest, C.H.; Verhaar, J.A.; Kloppenburg, M.; Zuurmond, A.M.; Stojanovic-Susulic, V.; Van Osch, G.J.; Clockaerts, S. Monounsaturated and saturated, but not n-6 polyunsaturated fatty acids decrease cartilage destruction under inflammatory conditions: A preliminary study. Cartilage 2013, 4, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yin, J.; Gao, J.; Cheng, T.S.; Pavlos, N.J.; Zhang, C.; Zheng, M.H. Subchondral bone in osteoarthritis: Insight into risk factors and microstructural changes. Arthritis Res. Ther. 2013, 15, 223. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.-Y.; Ima-Nirwana, S. Olives and bone: A green osteoporosis prevention option. Int. J. Environ. Res. Public Health 2016, 13, 755. [Google Scholar] [CrossRef] [PubMed]
- Bellido, M.; Lugo, L.; Roman-Blas, J.A.; Castaneda, S.; Calvo, E.; Largo, R.; Herrero-Beaumont, G. Improving subchondral bone integrity reduces progression of cartilage damage in experimental osteoarthritis preceded by osteoporosis. Osteoarthr. Cartil. 2011, 19, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
| Authors | Study Design | Patients, Interventions, Comparisons | Outcomes |
|---|---|---|---|
| Bitler et al. 2007 [102] | Randomized, double-blinded, placebo-controlled trial. | Patients with OA or RA, aged 55 to 75 years, free from other chronic diseases. Treatment group: 13 RAs and 30 OAs; 400 mg of freeze-dried olive water extract per day for 8 weeks Placebo group: 14 RAs and 33 OAs | OA patients receiving treatment showed significant improvements, as indicated by the Health Assessment Questionnaire–Disability Index, Disease Activity Score With 28-Joint Count index. |
| Bhoololi et al. 2012 [103] | Randomized, standard- controlled trial. | Female participants from a clinic in Iran, aged between 40–85 years diagnosed with OA. Treatment: 30 patients, virgin olive oil Control: 30 patients, 0.5% piroxicam 1 g gel, 3 times daily, for 4 weeks. | Both topical piroxicam and olive oil decreased WOMAC pain subscale scores and secondary outcome measures for the subjects. The performance of olive oil was superior compared to piroxicam, starting at week 2. Only one patient suffered a skin allergy after olive oil application. |
| Takeda et al. 2013 [104] | Double-blinded placebo- controlled trial. | Men and women with knee pain (gonarthrosis) Treatment group (13): aged 60.8 ± 7.2 years; 50.1 mg/day olive extract containing 10.04 mg hydroxytyrosol for 4 weeks. Placebo group (12): aged 61.4 ± 8.3 years. | Total improvement, based on JOA scores, was higher in the treated group compared to the placebo group, but not for subscales. Pain scores for pain during sleeping at night was significantly reduced for the treated group compared to the placebo group, pain during walking in flat planes was marginally signficiant, but other reductions in pain were not signficant. |
| Gelmini et al. 2015 [105] | Uncontrolled trial. | 5 humans (men and women), 60.2 ± 8.1 years, diagnosed with symptomatic OA. They applied 5 g of oinment to their painful joints on knee and hands three times a day for 2–3 weeks. The oinment contained a 5% unsaponifiable fraction from unripe olive oil. | Joint pain, oedema and mobility started to improve after week 1. Redness and heat started to improve after week 2. No adverse reactions were reported. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chin, K.-Y.; Pang, K.-L. Therapeutic Effects of Olive and Its Derivatives on Osteoarthritis: From Bench to Bedside. Nutrients 2017, 9, 1060. https://doi.org/10.3390/nu9101060
Chin K-Y, Pang K-L. Therapeutic Effects of Olive and Its Derivatives on Osteoarthritis: From Bench to Bedside. Nutrients. 2017; 9(10):1060. https://doi.org/10.3390/nu9101060
Chicago/Turabian StyleChin, Kok-Yong, and Kok-Lun Pang. 2017. "Therapeutic Effects of Olive and Its Derivatives on Osteoarthritis: From Bench to Bedside" Nutrients 9, no. 10: 1060. https://doi.org/10.3390/nu9101060
APA StyleChin, K.-Y., & Pang, K.-L. (2017). Therapeutic Effects of Olive and Its Derivatives on Osteoarthritis: From Bench to Bedside. Nutrients, 9(10), 1060. https://doi.org/10.3390/nu9101060






