Probiotic, Prebiotic, and Brain Development
Abstract
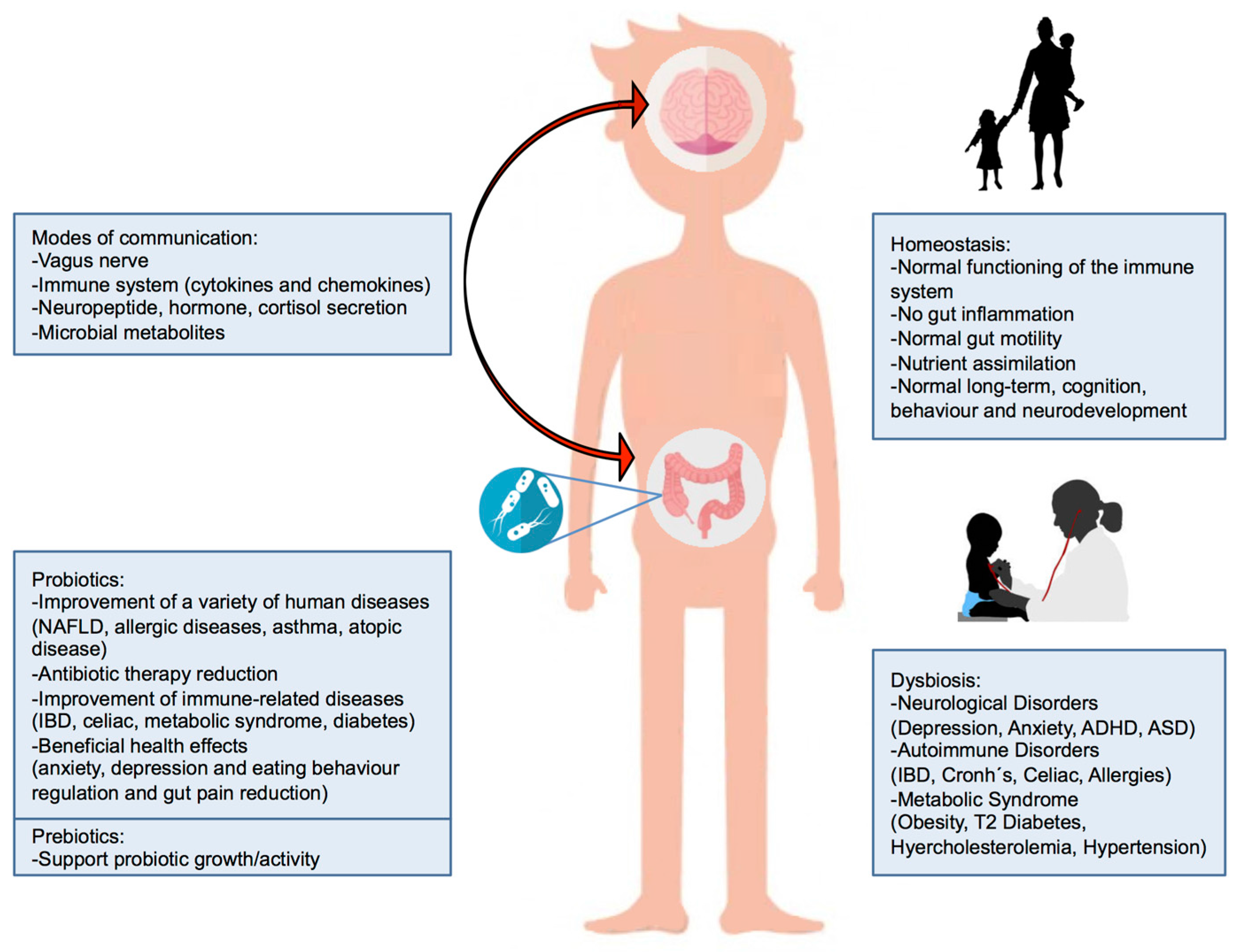
:1. Introduction
2. Establishment of Intestinal Microbiota during Early Neurodevelopmental Windows
3. Gut Microbiota–Brain Axis
4. Probiotics
5. Prebiotics
6. Synbiotics
7. Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rieder, R.; Wisniewski, P.J.; Alderman, B.L.; Campbell, S.C. Microbes and mental health: A review. Brain Behav. Immun. 2017, 66, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Cerdó, T.; Ruiz, A.; Jáuregui, R.; Azaryah, H.; Torres-Espínola, F.J.; García-Valdés, L.; Segura, M.T.; Suárez, A.; Campoy, C. Maternal obesity is associated with gut microbial metabolic potential in offspring during infancy. J. Physiol. Biochem. 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vuong, H.; Yano, J.; Fung, T.; Hsiao, E. The microbiome and host behavior. Annu. Rev. Neurosci. 2017, 40, 21–49. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.M. Microbiota-gut-brain axis, part 1: An integrated system of immunological, neural, and hormonal signals. Holist. Nurs. Pract. 2017, 31, 133–136. [Google Scholar] [PubMed]
- Hennessey, C.H.; Sladek, J.; Miller, E.; Kim, J.; Kaur, M.; Gareau, M.G. Intestinal dysbiosis during neonatal development alters the microbiota-gut-brain axis in adulthood. FASEB J. 2017, 31, 890–895. [Google Scholar]
- Scott, K.A.; Ida, M.; Peterson, V.L.; Prenderville, J.A.; Moloney, G.M.; Izumo, T.; Murphy, K.; Murphy, A.; Ross, R.P.; Stanton, C.; et al. Revisiting metchnikoff: Age-related alterations in microbiota-gut-brain axis in the mouse. Brain Behav. Immun. 2017, 65, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.O.; Bäckhed, F. Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 2016, 22, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of lactobacillus strain regulates emotional behavior and central gaba receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed]
- Bercik, P.; Park, A.; Sinclair, D.; Khoshdel, A.; Lu, J.; Huang, X.; Deng, Y.; Blennerhassett, P.; Fahnestock, M.; Moine, D.; et al. The anxiolytic effect of bifidobacterium longum ncc3001 involves vagal pathways for gut–brain communication. Neurogastroenterol. Motil. 2011, 23, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Bercik, P.; Verdu, E.F.; Foster, J.A.; Macri, J.; Potter, M.; Huang, X.; Malinowski, P.; Jackson, W.; Blennerhassett, P.; Neufeld, K.A.; et al. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology 2010, 139, 2102–2112. [Google Scholar] [CrossRef] [PubMed]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D.; et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 2011, 141, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Kunze, W.A.; Mao, Y.K.; Wang, B.; Huizinga, J.D.; Ma, X.; Forsythe, P.; Bienenstock, J. Lactobacillus reuteri enhances excitability of colonic ah neurons by inhibiting calcium-dependent potassium channel opening. J. Cell. Mol. Med. 2009, 13, 2261–2270. [Google Scholar] [CrossRef] [PubMed]
- McVey Neufeld, K.; Mao, Y.; Bienenstock, J.; Foster, J.; Kunze, W. The microbiome is essential for normal gut intrinsic primary afferent neuron excitability in the mouse. Neurogastroenterol. Motil. 2013, 25, 183. [Google Scholar] [CrossRef] [PubMed]
- Cerdó, T.; García-Valdés, L.; Altmäe, S.; Ruíz, A.; Suárez, A.; Campoy, C. Role of microbiota function during early life on child’s neurodevelopment. Trends Food Sci. Technol. 2016, 57, 273–288. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Zakharova, I.; Dmitrieva, Y. Oligosaccharides in infant formula: More evidence to validate the role of prebiotics. Br. J. Nutr. 2015, 113, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Foster, J.A. Psychobiotics and the gut–brain axis: In the pursuit of happiness. Neuropsychiatr. Disease Treat. 2015, 11, 715. [Google Scholar]
- Borre, Y.E.; O’Keeffe, G.W.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota and neurodevelopmental windows: Implications for brain disorders. Trends Mol. Med. 2014, 20, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Tau, G.Z.; Peterson, B.S. Normal development of brain circuits. Neuropsychopharmacology 2010, 35, 147–168. [Google Scholar] [CrossRef] [PubMed]
- Roca-Saavedra, P.; Mendez-Vilabrille, V.; Miranda, J.M.; Nebot, C.; Cardelle-Cobas, A.; Franco, C.M.; Cepeda, A. Food additives, contaminants and other minor components: Effects on human gut microbiota-a review. J. Phys. Biochem. 2017, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Jost, T.; Lacroix, C.; Braegger, C.P.; Rochat, F.; Chassard, C. Vertical mother–neonate transfer of maternal gut bacteria via breastfeeding. Environ. Microbiol. 2014, 16, 2891–2904. [Google Scholar] [CrossRef] [PubMed]
- Rudi, K.; Avershina, E. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 2015, 26, 26050. [Google Scholar]
- Rautava, S. Early microbial contact, the breast milk microbiome and child health. J. Dev. Orig. Health Dis. 2016, 7, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Escherich, T. The intestinal bacteria of the neonate and breast-fed infant. Rev. Infect. Dis. 1988, 10, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The placenta harbors a unique microbiome. Sci. Transl. Med. 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Xiao, X.; Zhang, Q.; Mao, L.; Yu, M.; Xu, J. The placental microbiome varies in association with low birth weight in full-term neonates. Nutrients 2015, 7, 6924–6937. [Google Scholar] [CrossRef] [PubMed]
- Perez-Muñoz, M.E.; Arrieta, M.-C.; Ramer-Tait, A.E.; Walter, J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: Implications for research on the pioneer infant microbiome. Microbiome 2017, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N.T.; Bakacs, E.; Combellick, J.; Grigoryan, Z.; Dominguez-Bello, M.G. The infant microbiome development: Mom matters. Trends Mol. Med. 2015, 21, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Rautava, S.; Collado, M.C.; Salminen, S.; Isolauri, E. Probiotics modulate host-microbe interaction in the placenta and fetal gut: A randomized, double-blind, placebo-controlled trial. Neonatology 2012, 102, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Toh, Z.Q.; Anzela, A.; Tang, M.L.; Licciardi, P.V. Probiotic therapy as a novel approach for allergic disease. Front. Pharmacol. 2012, 3, 171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-Q.; Hu, H.-J.; Liu, C.-Y.; Zhang, Q.; Shakya, S.; Li, Z.-Y. Probiotics for prevention of atopy and food hypersensitivity in early childhood: A prisma-compliant systematic review and meta-analysis of randomized controlled trials. Medicine 2016, 95, e2562. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.; Scott, K.P.; Khan, S.; Martin, J.C.; Berry, S.H.; Stevenson, M.; Okpapi, A.; Munro, M.J.; Hold, G.L. First-pass meconium samples from healthy term vaginally-delivered neonates: An analysis of the microbiota. PLoS ONE 2015, 10, e0133320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bright, M.; Bulgheresi, S. A complex journey: Transmission of microbial symbionts. Nat. Rev. Microbiol. 2010, 8, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, H.E.; Abrahamsson, T.R.; Jenmalm, M.C.; Harris, K.; Quince, C.; Jernberg, C.; Björkstén, B.; Engstrand, L.; Andersson, A.F. Decreased gut microbiota diversity, delayed bacteroidetes colonisation and reduced th1 responses in infants delivered by caesarean section. Gut 2014, 63, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Jašarević, E.; Howerton, C.L.; Howard, C.D.; Bale, T.L. Alterations in the vaginal microbiome by maternal stress are associated with metabolic reprogramming of the offspring gut and brain. Endocrinology 2015, 156, 3265–3276. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Concato, J.; Leventhal, J.M. How good is the evidence linking breastfeeding and intelligence? Pediatrics 2002, 109, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, A.R.; Barile, D.; Underwood, M.A.; Mills, D.A. The impact of the milk glycobiome on the neonate gut microbiota. Annu. Rev. Anim. Biosci. 2015, 3, 419–445. [Google Scholar] [CrossRef] [PubMed]
- Hunt, K.M.; Foster, J.A.; Forney, L.J.; Schütte, U.M.; Beck, D.L.; Abdo, Z.; Fox, L.K.; Williams, J.E.; McGuire, M.K.; McGuire, M.A. Characterization of the diversity and temporal stability of bacterial communities in human milk. PLoS ONE 2011, 6, e21313. [Google Scholar] [CrossRef] [PubMed]
- Zivkovic, A.M.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Human milk glycobiome and its impact on the infant gastrointestinal microbiota. Proc. Natl. Acad. Sci. USA 2011, 108, 4653–4658. [Google Scholar] [CrossRef] [PubMed]
- Urbaniak, C.; Cummins, J.; Brackstone, M.; Macklaim, J.M.; Gloor, G.B.; Baban, C.K.; Scott, L.; O’Hanlon, D.M.; Burton, J.P.; Francis, K.P.; et al. Microbiota of human breast tissue. Appl. Environ. Microbiol. 2014, 80, 3007–3014. [Google Scholar] [CrossRef] [PubMed]
- Gueimonde, M.; Laitinen, K.; Salminen, S.; Isolauri, E. Breast milk: A source of bifidobacteria for infant gut development and maturation? Neonatology 2007, 92, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.A.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Bifidobacterium longum subspecies infantis: Champion colonizer of the infant gut. Pediatr. Res. 2015, 77, 229. [Google Scholar] [CrossRef] [PubMed]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Kiely, B.; Cryan, J.; Dinan, T. Effects of the probiotic bifidobacterium infantis in the maternal separation model of depression. Neuroscience 2010, 170, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Bienenstock, J.; Dinan, T.G. The probiotic bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J. Psychiatr. Res. 2008, 43, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Berk, M.; Goehler, L.; Song, C.; Anderson, G.; Gałecki, P.; Leonard, B. Depression and sickness behavior are janus-faced responses to shared inflammatory pathways. BMC Med. 2012, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Bezirtzoglou, E.; Tsiotsias, A.; Welling, G.W. Microbiota profile in feces of breast-and formula-fed newborns by using fluorescence in situ hybridization (fish). Anaerobe 2011, 17, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Fallani, M.; Young, D.; Scott, J.; Norin, E.; Amarri, S.; Adam, R.; Aguilera, M.; Khanna, S.; Gil, A.; Edwards, C.A.; et al. Intestinal microbiota of 6-week-old infants across europe: Geographic influence beyond delivery mode, breast-feeding, and antibiotics. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Tognini, P. Gut microbiota: A potential regulator of neurodevelopment. Front. Cell. Neurosci. 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Cong, X.; Henderson, W.A.; Graf, J.; McGrath, J.M. Early life experience and gut microbiome: The brain-gut-microbiota signaling system. Adv. Neonatal Care Off. J. Natl. Assoc. Neonatal Nurses 2015, 15, 314. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.M.; Bercik, P. Gut microbiota: Intestinal bacteria influence brain activity in healthy humans. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 326–327. [Google Scholar] [CrossRef] [PubMed]
- Palma, G.; Collins, S.M.; Bercik, P.; Verdu, E.F. The microbiota–gut–brain axis in gastrointestinal disorders: Stressed bugs, stressed brain or both? J. Physiol. 2014, 592, 2989–2997. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.M.; Surette, M.; Bercik, P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012, 10, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Knight, R.; Mazmanian, S.K.; Cryan, J.F.; Tillisch, K. Gut microbes and the brain: Paradigm shift in neuroscience. J. Neurosci. 2014, 34, 15490–15496. [Google Scholar] [CrossRef] [PubMed]
- Luczynski, P.; McVey Neufeld, K.-A.; Oriach, C.S.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Growing up in a bubble: Using germ-free animals to assess the influence of the gut microbiota on brain and behavior. Int. J. Neuropsychopharmacol. 2016, 19. [Google Scholar] [CrossRef] [PubMed]
- Tarr, A.J.; Galley, J.D.; Fisher, S.E.; Chichlowski, M.; Berg, B.M.; Bailey, M.T. The prebiotics 3′ sialyllactose and 6′ sialyllactose diminish stressor-induced anxiety-like behavior and colonic microbiota alterations: Evidence for effects on the gut–brain axis. Brain Behav. Immun. 2015, 50, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Buffington, S.A.; Di Prisco, G.V.; Auchtung, T.A.; Ajami, N.J.; Petrosino, J.F.; Costa-Mattioli, M. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell 2016, 165, 1762–1775. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of parkinson’s disease. Cell 2016, 167, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Minamiyama, M.; Katsuno, M.; Adachi, H.; Waza, M.; Sang, C.; Kobayashi, Y.; Tanaka, F.; Doyu, M.; Inukai, A.; Sobue, G. Sodium butyrate ameliorates phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Hum. Mol. Genet. 2004, 13, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Semak, I.; Pisarchik, A.; Sweatman, T.; Szczesniewski, A.; Wortsman, J. Conversion of l-tryptophan to serotonin and melatonin in human melanoma cells. FEBS Lett. 2002, 511, 102–106. [Google Scholar] [CrossRef]
- Alexander, K.S.; Pocivavsek, A.; Wu, H.-Q.; Pershing, M.L.; Schwarcz, R.; Bruno, J.P. Early developmental elevations of brain kynurenic acid impair cognitive flexibility in adults: Reversal with galantamine. Neuroscience 2013, 238, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Khalil, O.S.; Pisar, M.; Forrest, C.M.; Vincenten, M.C.; Darlington, L.G.; Stone, T.W. Prenatal inhibition of the kynurenine pathway leads to structural changes in the hippocampus of adult rat offspring. Eur. J. Neurosci. 2014, 39, 1558–1571. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P.; Farzi, A. Neuropeptides and the microbiota-gut-brain axis. In Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease; Springer: New York, NY, USA, 2014; pp. 195–219. [Google Scholar]
- Del Zoppo, G.J.; Mabuchi, T. Cerebral microvessel responses to focal ischemia. J. Cereb. Blood Flow Metab. 2003, 23, 879–894. [Google Scholar] [CrossRef] [PubMed]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, E.; Rea, K.; Dinan, T.G.; Cryan, J.F. A gut (microbiome) feeling about the brain. Curr. Opin. Gastroenterol. 2016, 32, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Gao, J.; Gillilland, M.; Wu, X.; Song, I.; Kao, J.Y.; Owyang, C. Rifaximin alters intestinal bacteria and prevents stress-induced gut inflammation and visceral hyperalgesia in rats. Gastroenterology 2014, 146, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Joint, F.A.O. Who expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Córdoba Argentina Oct. 2001, 1, 1–4. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Al-muzafar, H.M.; Amin, K.A. Probiotic mixture improves fatty liver disease by virtue of its action on lipid profiles, leptin, and inflammatory biomarkers. BMC Complement. Altern. Med. 2017, 17, 43. [Google Scholar] [CrossRef] [PubMed]
- West, C.E.; Jenmalm, M.C.; Kozyrskyj, A.L.; Prescott, S.L. Probiotics for treatment and primary prevention of allergic diseases and asthma: Looking back and moving forward. Expert Rev. Clin. Immunol. 2016, 12, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Rayes, N.; Seehofer, D.; Hansen, S.; Boucsein, K.; Müller, A.R.; Serke, S.; Bengmark, S.; Neuhaus, P. Early enteral supply of lactobacillus and fiber versus selective bowel decontamination: A controlled trial in liver transplant recipients. Transplantation 2002, 74, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.I.; Honda, K. Intestinal commensal microbes as immune modulators. Cell Host Microbe 2012, 12, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A novel class of psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, M.; Violle, N.; Bisson, J.-F.; Desor, D.; Javelot, H.; Rougeot, C. Beneficial psychological effects of a probiotic formulation (lactobacillus helveticus r0052 and bifidobacterium longum r0175) in healthy human volunteers. Gut Microbes 2011, 2, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.-F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of psychotropic-like properties of a probiotic formulation (lactobacillus helveticus r0052 and bifidobacterium longum r0175) in rats and human subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Benton, D.; Williams, C.; Brown, A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur. J. Clin. Nutr. 2007, 61, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Steenbergen, L.; Sellaro, R.; van Hemert, S.; Bosch, J.A.; Colzato, L.S. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun. 2015, 48, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Akkasheh, G.; Kashani-Poor, Z.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akbari, H.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z.; Esmaillzadeh, A. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition 2016, 32, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Marcos, A.; Wärnberg, J.; Nova, E.; Gómez, S.; Alvarez, A.; Alvarez, R.; Mateos, J.A.; Cobo, J.M. The effect of milk fermented by yogurt cultures plus lactobacillus casei dn-114001 on the immune response of subjects under academic examination stress. Eur. J. Nutr. 2004, 43, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Romijn, A.R.; Rucklidge, J.J.; Kuijer, R.G.; Frampton, C. A double-blind, randomized, placebo-controlled trial of lactobacillus helveticus and bifidobacterium longum for the symptoms of depression. Aust. N. Z. J. Psychiatry 2017. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.-C.; Jin, H.-M.; Cui, Y.; Kim, D.S.; Jung, J.M.; Park, J.-I.; Jung, E.-S.; Choi, E.-K.; Chae, S.-W. Fermented milk of lactobacillus helveticus idcc3801 improves cognitive functioning during cognitive fatigue tests in healthy older adults. J. Funct. Foods 2014, 10, 465–474. [Google Scholar] [CrossRef]
- Jadrešin, O.; Hojsak, I.; Mišak, Z.; Kekez, A.J.; Trbojevic, T.; Ivkovic, L.; Kolacek, S. Lactobacillus reuteri dsm 17938 in the treatment of functional abdominal pain in children: Rct study. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 925–929. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Bested, A.C.; Beaulne, T.M.; Katzman, M.A.; Iorio, C.; Berardi, J.M.; Logan, A.C. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog. 2009, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Giannetti, E.; Maglione, M.; Alessandrella, A.; Strisciuglio, C.; De Giovanni, D.; Campanozzi, A.; Miele, E.; Staiano, A. A mixture of 3 bifidobacteria decreases abdominal pain and improves the quality of life in children with irritable bowel syndrome: A multicenter, randomized, double-blind, placebo-controlled, crossover trial. J. Clin. Gastroenterol. 2017, 51, e5–e10. [Google Scholar] [CrossRef] [PubMed]
- Tillisch, K.; Labus, J.; Kilpatrick, L.; Jiang, Z.; Stains, J.; Ebrat, B.; Guyonnet, D.; Legrain–Raspaud, S.; Trotin, B.; Naliboff, B.; et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 2013, 144, 1394–1401. [Google Scholar] [CrossRef] [PubMed]
- Kałużna-Czaplińska, J.; Błaszczyk, S. The level of arabinitol in autistic children after probiotic therapy. Nutrition 2012, 28, 124–126. [Google Scholar] [CrossRef] [PubMed]
- West, R.; Roberts, E.; Sichel, L.; Sichel, J. Improvements in gastrointestinal symptoms among children with autism spectrum disorder receiving the delpro® probiotic and immunomodulator formulation. J. Prob. Health 2013, 1, 2. [Google Scholar]
- Tomova, A.; Husarova, V.; Lakatosova, S.; Bakos, J.; Vlkova, B.; Babinska, K.; Ostatnikova, D. Gastrointestinal microbiota in children with autism in slovakia. Physiol. Behav. 2015, 138, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Santocchi, E.; Guiducci, L.; Fulceri, F.; Billeci, L.; Buzzigoli, E.; Apicella, F.; Calderoni, S.; Grossi, E.; Morales, M.A.; Muratori, F. Gut to brain interaction in autism spectrum disorders: A randomized controlled trial on the role of probiotics on clinical, biochemical and neurophysiological parameters. BMC Psychiatry 2016, 16, 183. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, F.B.; Stallings, C.; Origoni, A.; Katsafanas, E.; Savage, C.L.; Schweinfurth, L.A.; Goga, J.; Khushalani, S.; Yolken, R.H. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: A randomized, placebo-controlled trial. Prim. Care Companion CNS Disord. 2014, 16. [Google Scholar] [CrossRef] [PubMed]
- Tomasik, J.; Yolken, R.H.; Bahn, S.; Dickerson, F.B. Immunomodulatory effects of probiotic supplementation in schizophrenia patients: A randomized, placebo-controlled trial. Biomark. Insights 2015, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Severance, E.G.; Gressitt, K.L.; Stallings, C.R.; Katsafanas, E.; Schweinfurth, L.A.; Savage, C.L.; Adamos, M.B.; Sweeney, K.M.; Origoni, A.E.; Khushalani, S.; et al. Candida albicans exposures, sex specificity and cognitive deficits in schizophrenia and bipolar disorder. NPJ Schizophr. 2016, 2, 16018. [Google Scholar] [CrossRef] [PubMed]
- Culligan, E.P.; Hill, C.; Sleator, R.D. Probiotics and gastrointestinal disease: Successes, problems and future prospects. Gut Pathog. 2009, 1, 19. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.W.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D. The international scientific association for probiotics and prebiotics (isapp) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Mancilha, I.M. Non-digestible oligosaccharides: A review. Carbohydr. Polym. 2007, 68, 587–597. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed]
- Page, A.; Kentish, S. Plasticity of gastrointestinal vagal afferent satiety signals. Neurogastroenterol. Motil. 2016. [Google Scholar] [CrossRef] [PubMed]
- Theije, C.G.; Bavelaar, B.M.; Lopes da Silva, S.; Korte, S.M.; Olivier, B.; Garssen, J.; Kraneveld, A.D. Food allergy and food-based therapies in neurodevelopmental disorders. Pediatr. Allergy Immunol. 2014, 25, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Hume, M.P.; Nicolucci, A.C.; Reimer, R.A. Prebiotic supplementation improves appetite control in children with overweight and obesity: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 105, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Cowen, P.J.; Harmer, C.J.; Tzortzis, G.; Errington, S.; Burnet, P.W. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology 2015, 232, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Mannie, Z.N.; Harmer, C.J.; Cowen, P.J. Increased waking salivary cortisol levels in young people at familial risk of depression. Am. J. Psychiatry 2007, 164, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T. Cognitive Therapy and the Emotional Disorders; Penguin: London, UK, 1979. [Google Scholar]
- Ironside, M.; O’Shea, J.; Cowen, P.J.; Harmer, C.J. Frontal cortex stimulation reduces vigilance to threat: Implications for the treatment of depression and anxiety. Biol. Psychiatry 2016, 79, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, J.P.; Westerbeek, E.A.; Bröring-Starre, T.; Garssen, J.; van Elburg, R.M. Neurodevelopment of preterm infants at 24 months after neonatal supplementation of a prebiotic mix: A randomized trial. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 270–276. [Google Scholar] [CrossRef] [PubMed]
- LeCouffe, N.E.; Westerbeek, E.A.; van Schie, P.E.; Schaaf, V.A.; Lafeber, H.N.; van Elburg, R.M. Neurodevelopmental outcome during the first year of life in preterm infants after supplementation of a prebiotic mixture in the neonatal period: A follow-up study. Neuropediatrics 2014, 45, 022–029. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, M.; Greco, F.; Barone, G.; Gargante, M.P.; Malaguarnera, M.; Toscano, M.A. Bifidobacterium longum with fructo-oligosaccharide (fos) treatment in minimal hepatic encephalopathy: A randomized, double-blind, placebo-controlled study. Dig. Dis. Sci. 2007, 52, 3259. [Google Scholar] [CrossRef] [PubMed]
- Firmansyah, A.; Dwipoerwantoro, P.G.; Kadim, M.; Alatas, S.; Conus, N.; Lestarina, L.; Bouisset, F.; Steenhout, P. Improved growth of toddlers fed a milk containing synbiotics. Asia Pac. J. Clin. Nutr. 2011, 20, 69–76. [Google Scholar] [PubMed]
- Schrezenmeir, J.; de Vrese, M. Probiotics, prebiotics, and synbiotics—Approaching a definition. Am. J. Clin. Nutr. 2001, 73, 361s–364s. [Google Scholar] [PubMed]
- Islek, A.; Sayar, E.; Yilmaz, A.; Baysan, B.O.; Mutlu, D.; Artan, R. The role of bifidobacterium lactis b94 plus inulin in the treatment of acute infectious diarrhea in children. Turk. J. Gastroenterol. 2014, 25, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Asemi, Z.; Khorrami-Rad, A.; Alizadeh, S.-A.; Shakeri, H.; Esmaillzadeh, A. Effects of synbiotic food consumption on metabolic status of diabetic patients: A double-blind randomized cross-over controlled clinical trial. Clin. Nutr. 2014, 33, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Steed, H.; Macfarlane, G.T.; Blackett, K.L.; Bahrami, B.; Reynolds, N.; Walsh, S.V.; Cummings, J.H.; Macfarlane, S. Clinical trial: The microbiological and immunological effects of synbiotic consumption—A randomized double-blind placebo-controlled study in active crohn’s disease. Aliment. Pharmacol. Ther. 2010, 32, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Dilli, D.; Aydin, B.; Fettah, N.D.; Özyazıcı, E.; Beken, S.; Zenciroğlu, A.; Okumuş, N.; Özyurt, B.M.; İpek, M.Ş.; Akdağ, A. The propre-save study: Effects of probiotics and prebiotics alone or combined on necrotizing enterocolitis in very low birth weight infants. J. Pediatr. 2015, 166, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Eslamparast, T.; Poustchi, H.; Zamani, F.; Sharafkhah, M.; Malekzadeh, R.; Hekmatdoost, A. Synbiotic supplementation in nonalcoholic fatty liver disease: A randomized, double-blind, placebo-controlled pilot study. Am. J. Clin. Nutr. 2014, 99, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Daliri, E.B.-M.; Lee, B.H. New perspectives on probiotics in health and disease. Food Sci. Hum. Wellness 2015, 4, 56–65. [Google Scholar] [CrossRef]
- Kamada, N.; Chen, G.Y.; Inohara, N.; Núñez, G. Control of pathogens and pathobionts by the gut microbiota. Nat. Immunol. 2013, 14, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.V.; Morch, M.G.; Morthorst, T.H.; Lykkemark, S.; Olsen, A. Microbiota, probiotic bacteria and ageing. In Ageing: Lessons from C. elegans; Springer: New York, NY, USA, 2017; pp. 411–429. [Google Scholar]
- Paton, A.W.; Jennings, M.P.; Morona, R.; Wang, H.; Focareta, A.; Roddam, L.F.; Paton, J.C. Recombinant probiotics for treatment and prevention of enterotoxigenic escherichia coli diarrhea. Gastroenterology 2005, 128, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Focareta, A.; Paton, J.C.; Morona, R.; Cook, J.; Paton, A.W. A recombinant probiotic for treatment and prevention of cholera. Gastroenterology 2006, 130, 1688–1695. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.A.; Sultana, S.; Reuteler, G.; Moine, D.; Descombes, P.; Charton, F.; Bourdin, G.; McCallin, S.; Ngom-Bru, C.; Neville, T.; et al. Oral phage therapy of acute bacterial diarrhea with two coliphage preparations: A randomized trial in children from bangladesh. EBioMedicine 2016, 4, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M.K.; Maurice, C.F. Menage a trois in the human gut: Interactions between host, bacteria and phages. Nat. Rev. Microbiol. 2017, 15, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.J.; Mirzaei, M.K.; Nilsson, A.S. Adapting drug approval pathways for bacteriophage-based therapeutics. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
| Study (Reference) | Cohort Population | Probiotic Used | Key Findings |
|---|---|---|---|
| Messaoudi et al. (2011) [75,76] | 55 healthy human volunteers plus 25 subjects with urinary free cortisol (UFC) levels less than 50 ng/mL (less stressed subjects), 10 subjects received the probiotic and 15 placebo. | Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 (PF) | Beneficial effects on anxiety and depression related behaviors in healthy human volunteers and volunteers with lower levels of cortisol |
| Benton et al. (2007) [77] | 124 healthy adults volunteers were randomly allocated to a group that consumed, on a daily basis, a probiotic-containing milk drink or a placebo | Lactobacillus casei Shirota | The consumption of a probiotic-containing yoghurt improved the mood of those whose mood was initially poor. However, there was not an increased frequency of defaecation. |
| Steenbergen et al. (2015) [78] | 40 healthy young adults were randomly assigned to receive a 4-week intervention of either placebo or multispecies probiotics in a triple-blind intervention assessment design. | Bifidobacterium bifidum W23, Bifidobacterium lactis W52, Lactobacillus acidophilus W37, Lactobacillus brevis W63, Lactobacillus casei W56, Lactobacillus salivarius W24, and Lactococcus lactis (W19 and W58) | Participants who received multispecies probiotics showed a significantly reduced overall cognitive reactivity to sad mood, which was largely accounted for by reduced rumination and aggressive thoughts. |
| Akkasheh et al. (2016) [79] | 40 patients with a diagnosis of major depressive disorder (MDD) whose age ranged between 20 and 55 years were randomized. | Lactobacillus acidophilus, Lactobacillus casei, and Bifidobacterium bifidum. | Patients who received probiotic supplements had significantly decreased Beck Depression Inventory total scores |
| Marcos et al. (2004) [80] | 136 university students were randomized. | Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus salivarius subsp. thermophilus plus Lactobacillus casei DN-114001 | There was no significant treatment effect on anxiety. |
| Romijn et al. (2017) [81] | 79 participants not currently taking psychotropic medications with at least moderate scores on self-report mood measures. Participants were randomly allocated to receive a probiotic preparation or placebo. | Lactobacillus helveticus and Bifidobacterium longum | No significant difference was found between the probiotic and placebo groups on any psychological outcome measured. |
| Jadrešin et al. (2017) [83] | 55 children with age between 4 and 18 years old, diagnosed as functional abdominal pain (FAP) or irritable bowel syndrome (IBS) were randomly allocated. | Lactobacillus reuteri DSM 17938 | Administration of L. reuteri DSM 17938 was associated with a possible reduction of the intensity of pain and significantly more days without pain in children with FAP and IBS |
| Giannetti et al. (2016) [85] | 48 children with IBS aged between 8 and 17.9 years and 25 with functional dyspepsia (FD) with age between 8 and 16.6 years were randomized. | Bifidobacterium infantis M-63, breve M-16V, and longum BB536 | In children with IBS a mixture of Bifidobacteria is associated with improvement in abdominal pain (AP) and quality of life (QoL). |
| Kałużna-Czaplińska et al. (2012) [87] | 22 autistic children. | Lactobacillus acidophilus | The probiotic supplementation let to a significant decrease in d-arabinitol (DA) and the ratio of d-/l-arabinitol (DA/LA) and to a significant improvement in ability of concentration and carrying out orders |
| West et al. (2013) [88] | 33 ASD children. | Delpro® (Lactocillus acidophilus, Lactobacillus casei, Lactobacillus delbruecki, Bifidobacteria longum, Bifidobacteria bifidum) | 88% reported a decrease in total autism treatment evaluation checklist (ATEC) score, an improvement of ASD symptoms. Participants also had significant improvements in all ATEC domains (speech/language/communication, sociability, sensory/cognitive awareness, and health/physical/behavior) |
| Tomova et al. (2015) [89] | 10 children with autism, 9 siblings and 10 healthy children. | “Children Dophilus” containing three strains of Lactobacillus (60%), two strains of Bifidobacterium (25%), and one strain of Streptococcus (15%) | Probiotic diet supplementation normalized the Bacteroidetes/Firmicutes ratio, Desulfovibrio spp. and the amount of Bifidobacterium spp. in feces of autistic children. No significant difference was found to reduce symptom severity in patients with autism. |
| Santocchi et al. (2016) [90] | 100 preschoolers with ASD on the basis of a symptom severity index specific to gastrointestinal (GI) disorders. Patients with and without GI disorders were blind randomized to regular diet with probiotics or with placebo | “Vivomixx®” (one strain of Streptococcus thermophilus DSM 24731, three strains of Bifidobacterium (Bifidobacterium breve DSM 24732, B. longum DSM 24736, B. infantis DSM 24737), and four strains of Lactobacillus (Lactobacillus acidophilus DSM 24735, Lactobacillus plantarum DSM 24730, Lactobacillus paracasei DSM 24733, Lactobacillus delbrueckii subsp. bulgaricus DSM 24734)) | Ongoing study |
| Dickerson et al. (2014) [91] and Tomasik et al. (2015) [92] | 32 patients healthy and 33 patients with schizophrenia meeting DSM-IV criteria and with at least moderately severe psychotic symptoms | Lactobacillus rhamnosus strain GG and Bifidobacterium animalis subsp. lactis strain Bb12 | No significant difference was found to reduce symptom severity in patients with schizophrenia. Probiotic regulate immune and intestinal epithelial cells through the IL-17 family of cytokines |
| Study (Reference) | Cohort Population | Prebiotic Used | Key Findings |
|---|---|---|---|
| Prebiotics | |||
| Hume et al. (2017) [100] | 42 boys and girls, ages 7–12 years, with a body mass index (BMI) of ≥85th percentile | Oligofructose-enriched inulin/d | Prebiotic supplementation in children with overweight and obesity significantly increased feelings of fullness and reduced prospective food consumption in older but not in younger children |
| Schmidt et al. (2105) [101] | 45 adults healthy volunteers | FOS and Bimuno®-galactooligosaccharides, B-GOS | B-GOS reduced waking-cortisol response and decreased attentional vigilance to negative versus positive information |
| van den Berg et al. (2016) [105] | 77 preterm infants (gestational age <32 weeks and/or birth weight <1500 g), admitted to the level-III neonatal intensive care unit (NICU) | scGOS/lcFOS/pAOS | Neurodevelopmental outcomes were not different in the scGOS/lcFOS/pAOS and placebo group. Infections, lower bifidobacteria counts, and higher serum cytokine levels during the neonatal period were associated with lower neurodevelopmental outcomes at 24 months of age |
| LeCouffe et al. (2014) [106] | 93 Infants, with a gestational age (GA) of less than 32 weeks and/or birth weight of less than 1500 g, participed in the study (prebiotic mixture group (n = 48) and placebo group (n = 45) | 80% scGOS/lcFOS and 20% pAO | Short-term enteral supplementation of a prebiotic mixture in the neonatal period had no effect on neurodevelopmental outcome in preterm infants in the first year of life |
| Synbiotics | |||
| Malaguarnera et al. (2007) [107] | 60 cirrhotic patients (30 with synbiotics and 30 with placebo) | Bifidobacterium longum plus fructo-oligosaccharides | Patients with minimal hepatic encephalopathy (MHE) treated with Bifidobacterium + FOS, showed an improvement and a recovery of neuropsychological activities related to short-term memory, attention and computing ability, language, orientation ability, and cognitive activities |
| Firmansyah et al. (2011) [108] | 393 healthy 12 month-old toddlers | The probiotic Bifidobacterium longum BL999 (ATCC: BAA 999) and Lactobacillus rhamonosus, LPR (CGMCC 1.3724), the prebiotics inulin (30%) and fructo-oligosaccharide (70%), and the LCPUFA, arachidonic acid (AA) and docosahexaenoic acid (DHA) | Changes in cognitive and adaptive behaviour scores between 12 and 16 months were higher but not significantly different in the synbiotics group compared with the control group |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerdó, T.; Ruíz, A.; Suárez, A.; Campoy, C. Probiotic, Prebiotic, and Brain Development. Nutrients 2017, 9, 1247. https://doi.org/10.3390/nu9111247
Cerdó T, Ruíz A, Suárez A, Campoy C. Probiotic, Prebiotic, and Brain Development. Nutrients. 2017; 9(11):1247. https://doi.org/10.3390/nu9111247
Chicago/Turabian StyleCerdó, Tomás, Alicia Ruíz, Antonio Suárez, and Cristina Campoy. 2017. "Probiotic, Prebiotic, and Brain Development" Nutrients 9, no. 11: 1247. https://doi.org/10.3390/nu9111247
APA StyleCerdó, T., Ruíz, A., Suárez, A., & Campoy, C. (2017). Probiotic, Prebiotic, and Brain Development. Nutrients, 9(11), 1247. https://doi.org/10.3390/nu9111247






