Cognitive and Mood Effects of a Nutrient Enriched Breakfast Bar in Healthy Adults: A Randomised, Double-Blind, Placebo-Controlled, Parallel Groups Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
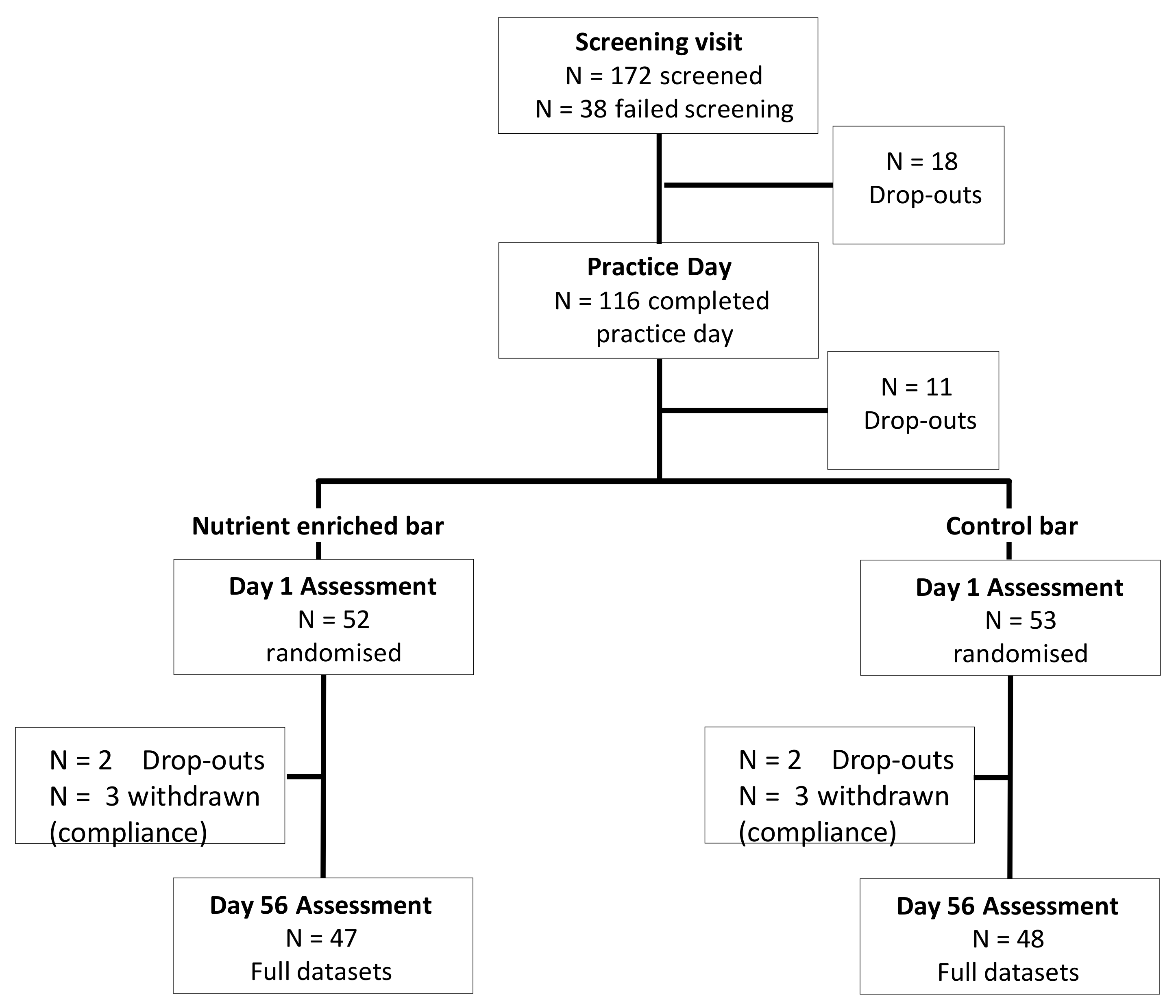
2.2. Participants
2.3. Treatments
2.4. Cognitive and Mood Measures
2.4.1. Picture Presentation
2.4.2. Face Presentation
2.4.3. Word Presentation
2.4.4. Immediate Word Recall
2.4.5. Numeric Working Memory
2.4.6. Choice Reaction Time
2.4.7. Rapid Visual Information Processing (RVIP) Task
2.4.8. Corsi Blocks Task
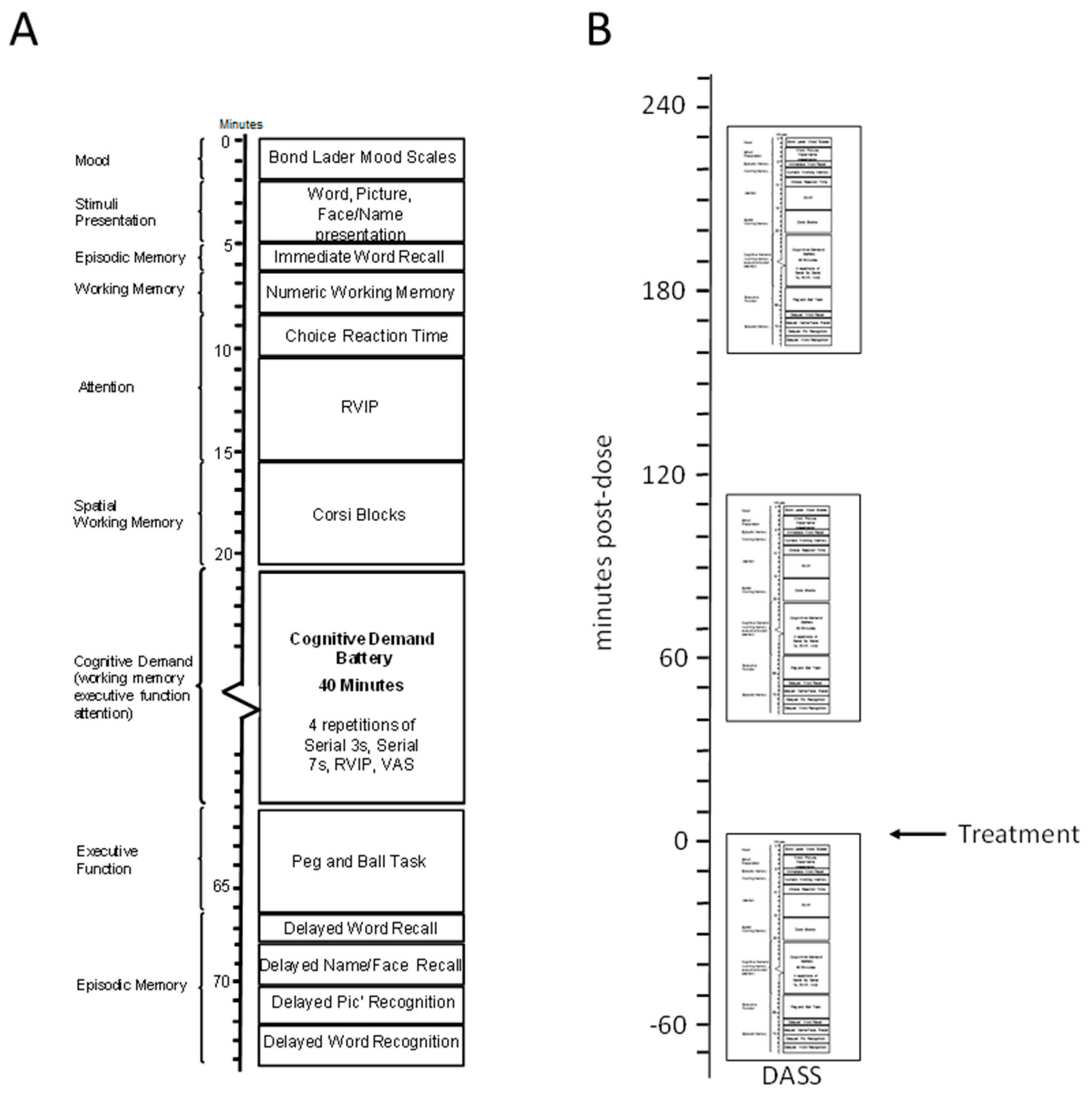
2.4.9. Cognitive Demand Battery
2.4.10. Peg and Ball Task
2.4.11. Delayed Word Recall
2.4.12. Delayed Word Recognition
2.4.13. Delayed Picture Recognition
2.4.14. Names-to-Faces Recall
2.4.15. Mood Measures
2.5. Procedure
2.6. Statistics
2.6.1. Acute and Acute/Chronic Superimposed
2.6.2. Pure Chronic Effects
3. Results
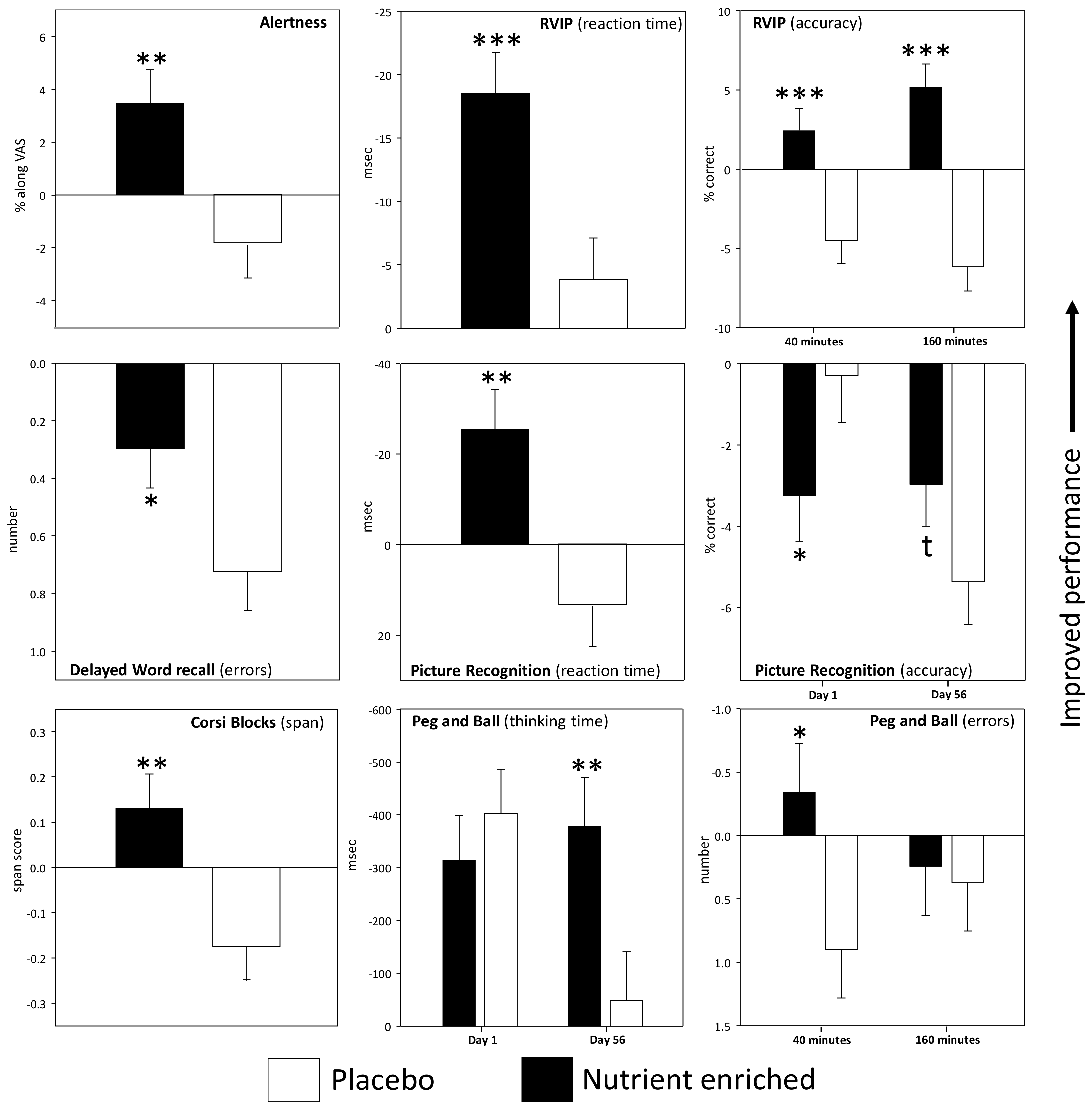
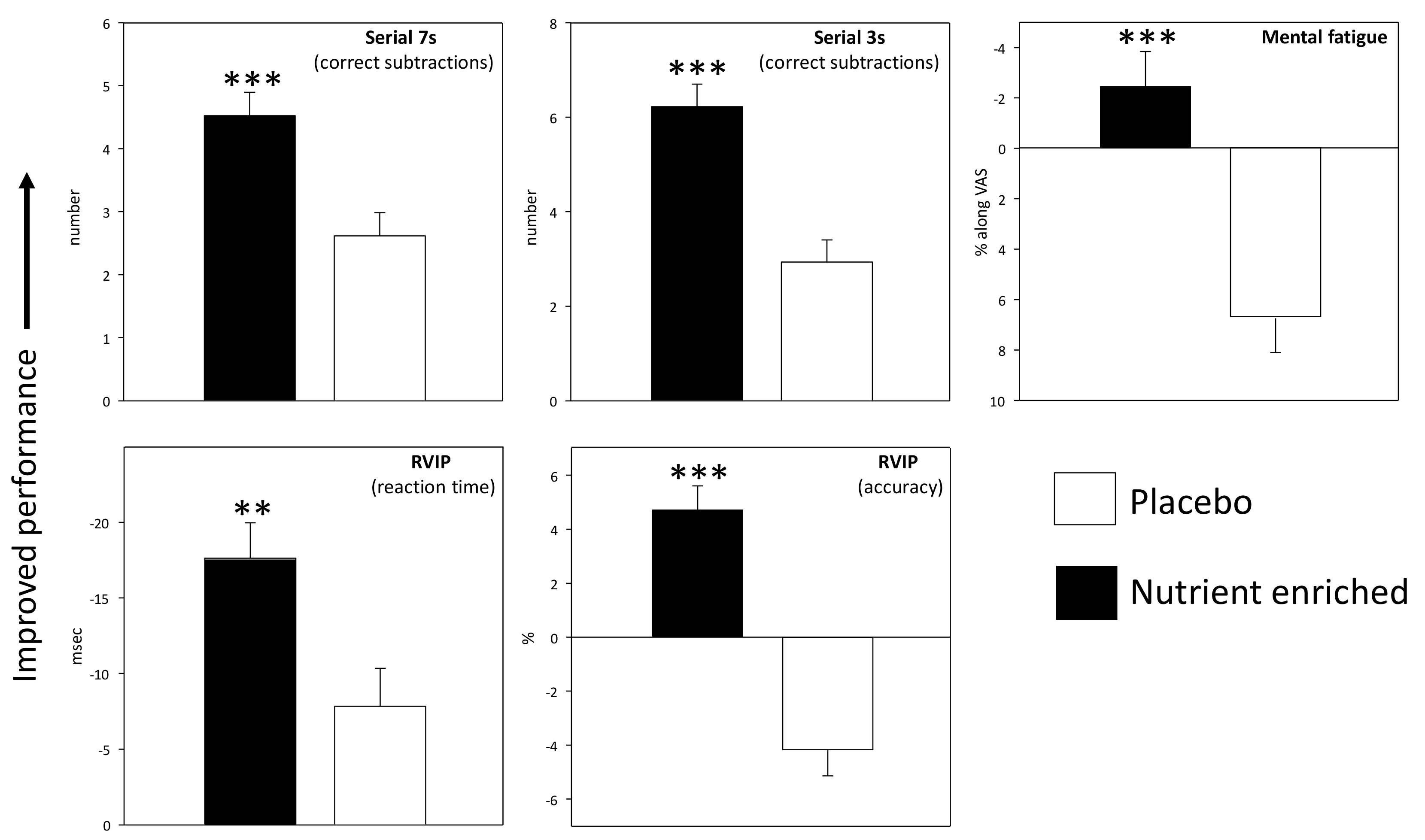
3.1. Acute and Acute/Chronic Superimposed Effects
3.1.1. Individual Cognitive Tasks and Mood Measures
3.1.2. Cognitive Demand Battery (CDB)
3.2. Pure Chronic Effects
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ruston, D.; Hoare, J.; Henderson, L.; Gregory, J.; Bates, C.; Prentice, A.; Birch, M.; Swan, G.; Farron, M. The National Diet and Nutrition Survey: Adults Aged 19 to 64 Years; The Stationery Office: London, UK, 2004. [Google Scholar]
- Bates, B.; Lennox, A.; Prentice, A.; Bates, C.J.; Page, P.; Nicholson, S.; Swan, G. National Diet and Nutrition Survey: Results from Years 1-4 (Combined) of the Rolling Programme (2008/2009-2011/12). Executive Summary; Public Health England: London, UK, 2014. [Google Scholar]
- Kennedy, D.O.; Haskell, C.F. Vitamins and cognition: What is the evidence? Drugs 2011, 71, 1957–1971. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O. B vitamins and the brain: Mechanisms, dose and efficacy—A review. Nutrients 2016, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- La Rue, A.; Koehler, K.; Wayne, S.; Chiulli, S.; Haaland, K.; Garry, P. Nutritional status and cognitive functioning in a normally aging sample: A 6-y reassessment. Am. J. Clin. Nutr. 1997, 65, 20–29. [Google Scholar] [PubMed]
- Perrig, W.J.; Perrig, P.; Stahelin, H.B. The relation between antioxidants and memory performance in the old and very old. J. Am. Geriatr. Soc. 1997, 45, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, A.; Martin, A.; Andres-Lacueva, C.; di Iorio, A.; Lamponi, M.; Mecocci, P.; Bartali, B.; Corsi, A.; Senin, U.; Ferrucci, L. Vitamin E levels, cognitive impairment and dementia in older persons: The InCHIANTI study. Neurobiol. Aging 2005, 26, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Rickard, A.P.; Chatfield, M.P.; Powell, J.; Stephen, A.M.; Richards, M. Dietary iron is associated with memory in midlife: Longitudinal cohort study. J. Pharm. Nutr. Sci. 2012, 2, 57–62. [Google Scholar]
- Beydoun, M.A.; Gamaldo, A.A.; Beydoun, H.A.; Tanaka, T.; Tucker, K.L.; Talegawkar, S.A.; Ferrucci, L.; Zonderman, A.B. Caffeine and alcohol intakes and overall nutrient adequacy are associated with longitudinal cognitive performance among US adults. J. Nutr. 2014, 144, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Dangour, A.D.; Andreeva, V.A.; Sydenham, E.; Uauy, R. Omega 3 fatty acids and cognitive health in older people. Br. J. Nutr. 2012, 107 (Suppl. 2), S152–S158. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Ding, Y.; Wu, F.; Li, R.; Hou, J.; Mao, P. Omega-3 fatty acids intake and risks of dementia and Alzheimer’s disease: A meta-analysis. Neurosci. Biobehav. Rev. 2015, 48, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Valls-Pedret, C.; Lamuela-Raventós, R.M.; Medina-Remón, A.; Quintana, M.; Corella, D.; Pintó, X.; Martínez-González, M.Á.; Estruch, R.; Ros, E. Polyphenol-rich foods in the Mediterranean diet are associated with better cognitive function in elderly subjects at high cardiovascular risk. J. Alzheimers Dis. 2012, 29, 773–782. [Google Scholar] [PubMed]
- Loef, M.; Walach, H. Fruit, vegetables and prevention of cognitive decline or dementia: A systematic review of cohort studies. J. Nutr. Health Aging 2012, 16, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O. Polyphenols and the human brain: Plant “secondary metabolite” ecologic roles and endogenous signaling functions drive benefits. Adv. Nutr. 2014, 5, 515–533. [Google Scholar] [CrossRef] [PubMed]
- Long, S.J.; Benton, D. Effects of vitamin and mineral supplementation on stress, mild psychiatric symptoms, and mood in nonclinical samples: A meta-analysis. Psychosom. Med. 2013, 75, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Veasey, R.; Watson, A.; Dodd, F.; Jones, E.; Maggini, S.; Haskell, C.F. Effects of high-dose B vitamin complex with vitamin C and minerals on subjective mood and performance in healthy males. Psychopharmacology 2010, 211, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Haskell, C.F.; Robertson, B.; Jones, E.; Forster, J.; Jones, R.; Wilde, A.; Maggini, S.; Kennedy, D.O. Effects of a multi-vitamin/mineral supplement on cognitive function and fatigue during extended multi-tasking. Hum. Psychopharmacol. Clin. Exp. 2010, 25, 448–461. [Google Scholar] [CrossRef] [PubMed]
- Murray-Kolb, L.E.; Beard, J.L. Iron treatment normalizes cognitive functioning in young women. Am. J. Clin. Nutr. 2007, 85, 778–787. [Google Scholar] [PubMed]
- Scholey, A.B.; French, S.J.; Morris, P.J.; Kennedy, D.O.; Milne, A.L.; Haskell, C.F. Consumption of cocoa flavanols results in acute improvements in mood and cognitive performance during sustained mental effort. J. Psychopharmacol. 2010, 24, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Desideri, G.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Ghiadoni, L.; Mastroiacovo, D.; Raffaele, A.; Ferri, L.; Bocale, R.; Lechiara, M.C. Benefits in cognitive function, blood pressure, and insulin resistance through cocoa flavanol consumption in elderly subjects with mild cognitive impairmentnovelty and significance the cocoa, cognition, and aging (cocoa) study. Hypertension 2012, 60, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Mastroiacovo, D.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Raffaele, A.; Pistacchio, L.; Righetti, R.; Bocale, R.; Lechiara, M.C.; Marini, C.; et al. Cocoa flavanol consumption improves cognitive function, blood pressure control, and metabolic profile in elderly subjects: The cocoa, cognition, and aging (cocoa) study—A randomized controlled trial. Am. J. Clin. Nutr. 2015, 101, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Sinn, N.; Milte, C.M.; Street, S.J.; Buckley, J.D.; Coates, A.M.; Petkov, J.; Howe, P.R. Effects of n-3 fatty acids, EPA v. DHA, on depressive symptoms, quality of life, memory and executive function in older adults with mild cognitive impairment: A 6-month randomised controlled trial. Br. J. Nutr. 2012, 107, 1682–1693. [Google Scholar] [CrossRef] [PubMed]
- Stonehouse, W.; Conlon, C.A.; Podd, J.; Hill, S.R.; Minihane, A.M.; Haskell, C.; Kennedy, D. DHA supplementation improved both memory and reaction time in healthy young adults: A randomized controlled trial. Am. J. Clin. Nutr. 2013, 97, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Nehlig, A. Is caffeine a cognitive enhancer? J. Alzheimers Dis. 2010, 20, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Haskell, C.F.; Kennedy, D.O.; Milne, A.L.; Wesnes, K.A.; Scholey, A.B. The effects of L-theanine, caffeine and their combination on cognition and mood. Biol. Psychol. 2008, 77, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Steenbergen, L.; Sellaro, R.; Hommel, B.; Colzato, L.S. Tyrosine promotes cognitive flexibility: Evidence from proactive vs. reactive control during task switching performance. Neuropsychologia 2015, 69, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Colzato, L.S.; Jongkees, B.J.; Sellaro, R.; van den Wildenberg, W.P.; Hommel, B. Eating to stop: Tyrosine supplementation enhances inhibitory control but not response execution. Neuropsychologia 2014, 62, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Colzato, L.S.; de Haan, A.M.; Hommel, B. Food for creativity: Tyrosine promotes deep thinking. Psychol. Res. 2015, 79, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Colzato, L.S.; Jongkees, B.J.; Sellaro, R.; Hommel, B. Working memory reloaded: Tyrosine repletes updating in the N-back task. Front. Behav. Neurosci. 2013, 7, 200. [Google Scholar] [CrossRef] [PubMed]
- Doshi, S.N.; McDowell, I.F.W.; Moat, S.J.; Payne, N.; Durrant, H.J.; Lewis, M.J.; Goodfellow, J. Folic acid improves endothelial function in coronary artery disease via mechanisms largely independent of homocysteine lowering. Circulation 2002, 105, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Obad, A.; Palada, I.; Valic, Z.; Ivancev, V.; Bakovic, D.; Wisloff, U.; Brubakk, A.O.; Dujic, Z. The effects of acute oral antioxidants on diving-induced alterations in human cardiovascular function. J. Physiol. 2007, 578, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.L.; Nawaz, H.; Boukhalil, J.; Giannamore, V.; Chan, W.; Ahmadi, R.; Sarrel, P.M. Acute effects of oats and vitamin E on endothelial responses to ingested fat. Am. J. Prev. Med. 2001, 20, 124–129. [Google Scholar] [CrossRef]
- Title, L.M.; Cummings, P.M.; Giddens, K.; Nassar, B.A. Oral glucose loading acutely attenuates endothelium-dependent vasodilation in healthy adults without diabetes: An effect prevented by vitamins C and E. J. Am. Coll. Cardiol. 2000, 36, 2185–2191. [Google Scholar] [CrossRef]
- Usui, M.; Matsuoka, H.; Miyazaki, H.; Ueda, S.; Okuda, S.; Imaizumi, T. Endothelial dysfunction by acute hyperhomocyst (e) inaemia: Restoration by folic acid. Clin. Sci. 1999, 96, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Doshi, S.N.; McDowell, I.F.; Moat, S.J.; Lang, D.; Newcombe, R.G.; Kredan, M.B.; Lewis, M.J.; Goodfellow, J. Folate improves endothelial function in coronary artery disease: An effect mediated by reduction of intracellular superoxide? Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.; Rumbold, P.; Stevenson, E. Effect of calcium intake on fat oxidation in adults: A meta-analysis of randomized, controlled trials. Obes. Rev. 2012, 13, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Chan She Ping-Delfos, W.; Soares, M. Diet induced thermogenesis, fat oxidation and food intake following sequential meals: Influence of calcium and vitamin d. Clin. Nutr. 2011, 30, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Stevenson, E.J.; Jackson, P.A.; Dunn, S.; Wishart, K.; Bieri, G.; Barella, L.; Carne, A.; Dodd, F.L.; Robertson, B.C.; et al. Multivitamins and minerals modulate whole-body energy metabolism and cerebral blood-flow during cognitive task performance: A double-blind, randomised, placebo-controlled trial. Nutr. Metab. 2016, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Haskell, C.F.; Scholey, A.B.; Jackson, P.A.; Elliott, J.M.; Defeyter, M.A.; Greer, J.; Robertson, B.C.; Buchanan, T.; Tiplady, B.; Kennedy, D.O. Cognitive and mood effects in healthy children during 12 weeks’ supplementation with multi-vitamin/minerals. Br. J. Nutr. 2008, 100, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Scholey, A.; Bauer, I.; Neale, C.; Savage, K.; Camfield, D.; White, D.; Maggini, S.; Pipingas, A.; Stough, C.; Hughes, M. Acute effects of different multivitamin mineral preparations with and without Guaraná on mood, cognitive performance and functional brain activation. Nutrients 2013, 5, 3589–3604. [Google Scholar] [CrossRef] [PubMed]
- White, D.J.; Camfield, D.A.; Maggini, S.; Pipingas, A.; Silberstein, R.; Stough, C.; Scholey, A. The effect of a single dose of multivitamin and mineral combinations with and without guaraná on functional brain activity during a continuous performance task. Nutr. Neurosci. 2017, 20, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, A.; MacKay, D. Health habits and other characteristics of dietary supplement users: A review. Nutr. J. 2014, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.L.; Gahche, J.J.; Miller, P.E.; Thomas, P.R.; Dwyer, J.T. Why US adults use dietary supplements. JAMA Intern. Med. 2013, 173, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Rovira, M.-A.; Grau, M.; Castañer, O.; Covas, M.I.; Schröder, H.; Investigators, R. Dietary supplement use and health-related behaviors in a Mediterranean population. J. Nutr. Educ. Behav. 2013, 45, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Dodd, F.L.; Robertson, B.C.; Okello, E.J.; Reay, J.L.; Scholey, A.B.; Haskell, C.F. Monoterpenoid extract of sage (Salvia lavandulaefolia) with cholinesterase inhibiting properties improves cognitive performance and mood in healthy adults. J. Psychopharmacol. 2011, 25, 1088–1100. [Google Scholar] [CrossRef] [PubMed]
- Veasey, R.C.; Gonzalez, J.T.; Kennedy, D.O.; Haskell, C.F.; Stevenson, E.J. Breakfast consumption and exercise interact to affect cognitive performance and mood later in the day. A randomized controlled trial. Appetite 2013, 68, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Scholey, A.; Ossoukhova, A.; Owen, L.; Ibarra, A.; Pipingas, A.; He, K.; Roller, M.; Stough, C. Effects of American ginseng (Panax quinquefolius) on neurocognitive function: An acute, randomised, double-blind, placebo-controlled, crossover study. Psychopharmacology 2010, 212, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Reay, J.L.; Kennedy, D.O.; Scholey, A.B. Single doses of Panax ginseng (G115) reduce blood glucose levels and improve cognitive performance during sustained mental activity. J. Psychopharmacol. 2005, 19, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Reay, J.L.; Kennedy, D.O.; Scholey, A.B. Effects of Panax ginseng, consumed with and without glucose, on blood glucose levels and cognitive performance during sustained ‘mentally demanding’ tasks. J. Psychopharmacol. 2006, 20, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.; Haskell, C.F.; Robertson, B.; Reay, J.; Brewster-Maund, C.; Luedemann, J.; Maggini, S.; Ruf, M.; Zangara, A.; Scholey, A.B. Improved cognitive performance and mental fatigue following a multi-vitamin and mineral supplement with added guarana (Paullinia cupana). Appetite 2008, 50, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Scholey, A.B. A glucose-caffeine ‘energy drink’ ameliorates subjective and performance deficits during prolonged cognitive demand. Appetite 2004, 42, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Bond, A.; Lader, M. Use of analog scales in rating subjective feelings. Br. J. Med. Psychol. 1974, 47, 211–218. [Google Scholar] [CrossRef]
- Lovibond, S.H.; Lovibond, P.F. Manual for the Depression, Anxiety and Stress Scales, 2nd ed.; Psychology Foundation: Sydney, Australia, 1995. [Google Scholar]
- Smit, H.; Rogers, P. Effects of low doses of caffeine on cognitive performance, mood and thirst in low and higher caffeine consumers. Psychopharmacology 2000, 152, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Childs, E.; de Wit, H. Subjective, behavioral, and physiological effects of acute caffeine in light, nondependent caffeine users. Psychopharmacology 2006, 185, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, H.R. The effects of ginseng, ephedrine, and caffeine on cognitive performance, mood and energy. Nutr. Rev. 2001, 59, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, H.R. Nutrition, brain function and cognitive performance. Appetite 2003, 40, 245–254. [Google Scholar] [CrossRef]
- Childs, E. Influence of energy drink ingredients on mood and cognitive performance. Nutr. Rev. 2014, 72 (Suppl. 1), 48–59. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.B.; Nuccio, R.P.; Jeukendrup, A.E. Acute effects of dietary constituents on motor skill and cognitive performance in athletes. Nutr. Rev. 2014, 72, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Deijen, J.; Wientjes, C.; Vullinghs, H.; Cloin, P.; Langefeld, J. Tyrosine improves cognitive performance and reduces blood pressure in cadets after one week of a combat training course. Brain Res. Bull. 1999, 48, 203–209. [Google Scholar] [CrossRef]
- Neri, D.F.; Wiegmann, D.; Stanny, R.R.; Shappell, S.A.; McCardie, A.; McKay, D.L. The effects of tyrosine on cognitive performance during extended wakefulness. Aviat. Space Environ. Med. 1995, 66, 313–319. [Google Scholar] [PubMed]
- Magill, R.A.; Waters, W.F.; Bray, G.A.; Volaufova, J.; Smith, S.R.; Lieberman, H.R.; McNevin, N.; Ryan, D.H. Effects of tyrosine, phentermine, caffeine D-amphetamine, and placebo on cognitive and motor performance deficits during sleep deprivation. Nutr. Neurosci. 2003, 6, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Banderet, L.E.; Lieberman, H.R. Treatment with tyrosine, a neurotransmitter precursor, reduces environmental stress in humans. Brain Res. Bull. 1989, 22, 759–762. [Google Scholar] [CrossRef]
- Mahoney, C.R.; Castellani, J.; Kramer, F.M.; Young, A.; Lieberman, H.R. Tyrosine supplementation mitigates working memory decrements during cold exposure. Physiol. Behav. 2007, 92, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Shurtleff, D.; Thomas, J.R.; Schrot, J.; Kowalski, K.; Harford, R. Tyrosine reverses a cold-induced working memory deficit in humans. Pharmacol. Biochem. Behav. 1994, 47, 935–941. [Google Scholar] [CrossRef]
- Thomas, J.R.; Lockwood, P.A.; Singh, A.; Deuster, P.A. Tyrosine improves working memory in a multitasking environment. Pharmacol. Biochem. Behav. 1999, 64, 495–500. [Google Scholar] [CrossRef]
- Booij, L.; Merens, W.; Markus, C.R.; van der Does, A.W. Diet rich in α-lactalbumin improves memory in unmedicated recovered depressed patients and matched controls. J. Psychopharmacol. 2006, 20, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Markus, C.R.; Olivier, B.; de Haan, E.H. Whey protein rich in α-lactalbumin increases the ratio of plasma tryptophan to the sum of the other large neutral amino acids and improves cognitive performance in stress-vulnerable subjects. Am. J. Clin. Nutr. 2002, 75, 1051–1056. [Google Scholar] [PubMed]
- Haskell, C.; Dodd, F.; Wightman, E.; Kennedy, D. Behavioural effects of compounds co-consumed in dietary forms of caffeinated plants. Nutr. Res. Rev. 2013, 26, 49–70. [Google Scholar] [CrossRef] [PubMed]
- Dodd, F.L.; Kennedy, D.O.; Riby, L.M.; Haskell-Ramsay, C.F. A double-blind, placebo-controlled study evaluating the effects of caffeine and l-theanine both alone and in combination on cerebral blood flow, cognition and mood. Psychopharmacology 2015, 232, 2563–2576. [Google Scholar] [CrossRef] [PubMed]
- Rogers, P.J.; Smith, J.E.; Heatherley, S.V.; Pleydell-Pearce, C.W. Time for tea: Mood, blood pressure and cognitive performance effects of caffeine and theanine administered alone and together. Psychopharmacology 2008, 195, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Giles, G.E.; Mahoney, C.R.; Brunyé, T.T.; Gardony, A.L.; Taylor, H.A.; Kanarek, R.B. Differential cognitive effects of energy drink ingredients: Caffeine, taurine, and glucose. Pharmacol. Biochem. Behav. 2012, 102, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Peacock, A.; Martin, F.H.; Carr, A. Energy drink ingredients. Contribution of caffeine and taurine to performance outcomes. Appetite 2013, 64, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Misaizu, A.; Kokubo, T.; Tazumi, K.; Kanayama, M.; Miura, Y. The Combined effect of caffeine and ornithine on the mood of healthy office workers. Prev. Nutr. Food Sci. 2014, 19, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Pomportes, L.; Davranche, K.; Brisswalter, I.; Hays, A.; Brisswalter, J. Heart rate variability and cognitive function following a multi-vitamin and mineral supplementation with added guarana (Paullinia cupana). Nutrients 2014, 7, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Adan, A.; Serra-Grabulosa, J.M. Effects of caffeine and glucose, alone and combined, on cognitive performance. Hum. Psychopharmacol. 2010, 25, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Scholey, A.B.; Kennedy, D.O. Cognitive and physiological effects of an “energy drink”: An evaluation of the whole drink and of glucose, caffeine and herbal flavouring fractions. Psychopharmacology 2004, 176, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.G.; Jacobs, I.; Zamecnik, J. Effects of caffeine, ephedrine and their combination on time to exhaustion during high-intensity exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1998, 77, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.A.; Deary, M.E.; Reay, J.L.; Scholey, A.B.; Kennedy, D.O. No effect of 12 weeks’ supplementation with 1 g DHA-rich or EPA-rich fish oil on cognitive function or mood in healthy young adults aged 18–35 years. Br. J. Nutr. 2012, 107, 1232–1243. [Google Scholar] [CrossRef] [PubMed]
- Stough, C.; Downey, L.; Silber, B.; Lloyd, J.; Kure, C.; Wesnes, K.; Camfield, D. The effects of 90-day supplementation with the Omega-3 essential fatty acid docosahexaenoic acid (DHA) on cognitive function and visual acuity in a healthy aging population. Neurobiol. Aging 2012, 33, 824.e1–824.e3. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.A.; Reay, J.L.; Scholey, A.B.; Kennedy, D.O. Docosahexaenoic acid-rich fish oil modulates the cerebral hemodynamic response to cognitive tasks in healthy young adults. Biol. Psychol. 2012, 89, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.A.; Reay, J.L.; Scholey, A.B.; Kennedy, D.O. DHA-rich oil modulates the cerebral haemodynamic response to cognitive tasks in healthy young adults: A near IR spectroscopy pilot study. Br. J. Nutr. 2012, 107, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Dis. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
| Placebo Bar | Nutrient Enriched Bar | |
|---|---|---|
| Number | 48 | 47 |
| Female/male | 31/17 | 27/20 |
| Age | 24.5 | 25.1 |
| Education (year) | 17.2 | 17.5 |
| Vegetables (portion/day) | 3.5 | 3.3 |
| Caffeine consumption (mg/day) | 133 | 150 |
| Blood pressure (sys/dia) | 122/76 | 124/77 |
| Heart rate (bpm) | 69 | 69 |
| Weight (Kg) | 67.5 | 70.3 |
| Height (cm) | 170.5 | 170.6 |
| Body mass index | 23.2 | 24.1 |
| N | Day 1 | Day 56 | ANOVA Sig. Effects | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Dose Baseline | 40 min Post (Change) | 160 min Post (Change) | Pre-Dose Baseline | 40 min Post (Change) | 160 min Post (Change) | ||||||||||
| Alert % along VAS | Placebo | 48 | 55.50 | 2.15 | −1.61 | 1.29 | −4.64 | 1.69 | 54.59 | 2.29 | 0.79 | 1.58 | −2.02 | 2.00 | Tr p = 0.004 |
| Nutrient+ | 47 | 54.20 | 2.17 | 4.49 | 1.31 | 4.62 | 1.71 | 54.72 | 2.32 | 3.42 | 1.60 | 1.36 | 2.02 | ||
| Content % along VAS | Placebo | 48 | 59.85 | 1.92 | 0.49 | 0.98 | −0.78 | 1.39 | 61.05 | 1.96 | −0.31 | 1.03 | −0.88 | 1.49 | |
| Nutrient+ | 47 | 61.07 | 1.94 | 1.93 | 0.99 | 2.59 | 1.41 | 60.41 | 1.98 | 1.03 | 1.04 | 0.72 | 1.50 | ||
| Calm % along VAS | Placebo | 48 | 61.21 | 1.70 | −0.83 | 1.29 | −2.12 | 1.15 | 61.91 | 1.96 | −3.08 | 1.35 | −2.15 | 1.51 | |
| Nutrient+ | 47 | 62.33 | 1.72 | −2.23 | 1.30 | 0.11 | 1.16 | 63.13 | 1.98 | −4.60 | 1.37 | −4.34 | 1.52 | ||
| Immediate word recall number correct | Placebo | 48 | 7.23 | 0.29 | −0.25 | 0.29 | −0.62 | 0.30 | 7.71 | 0.33 | −0.55 | 0.32 | −0.65 | 0.26 | |
| Nutrient+ | 47 | 7.51 | 0.30 | −0.62 | 0.29 | −0.55 | 0.30 | 7.84 | 0.33 | −0.31 | 0.32 | −0.85 | 0.27 | ||
| Immediate word recall errors | Placebo | 48 | 0.35 | 0.09 | 0.08 | 0.12 | 0.29 | 0.13 | 0.40 | 0.11 | 0.06 | 0.14 | 0.25 | 0.15 | |
| Nutrient+ | 47 | 0.40 | 0.09 | 0.19 | 0.12 | 0.11 | 0.14 | 0.49 | 0.11 | −0.11 | 0.14 | 0.09 | 0.15 | ||
| Numeric working memory % correct | Placebo | 48 | 95.72 | 0.54 | −0.37 | 0.53 | −0.33 | 0.55 | 95.51 | 0.61 | −1.07 | 0.64 | −0.32 | 0.63 | Tr × Ass p = 0.04 |
| Nutrient+ | 47 | 95.68 | 0.55 | −0.38 | 0.54 | −0.40 | 0.56 | 95.93 | 0.62 | 0.12 | 0.65 | −1.28 | 0.64 | ||
| Numeric working memory reaction time (msec) | Placebo | 48 | 724.60 | 21.16 | −33.93 | 11.23 | −40.55 | 12.09 | 703.96 | 19.24 | −16.01 | 10.60 | −44.00 | 11.81 | |
| Nutrient+ | 47 | 710.97 | 21.38 | −25.55 | 11.35 | −40.75 | 12.21 | 711.91 | 19.44 | −24.64 | 10.71 | −41.06 | 11.93 | ||
| Choice reaction time % correct | Placebo | 48 | 95.67 | 0.46 | 0.00 | 0.46 | 0.33 | 0.48 | 95.83 | 0.44 | −0.17 | 0.46 | 0.66 | 0.50 | |
| Nutrient+ | 46 | 96.74 | 0.47 | −0.44 | 0.47 | −0.26 | 0.49 | 97.13 | 0.45 | −0.31 | 0.47 | −1.53 | 0.51 | ||
| Choice reaction time reaction time (msec) | Placebo | 48 | 387.44 | 6.81 | 2.42 | 5.22 | −2.23 | 5.56 | 386.11 | 6.67 | 10.23 | 5.26 | 13.93 | 5.48 | |
| Nutrient+ | 46 | 395.42 | 6.96 | 4.47 | 5.34 | −4.07 | 5.68 | 398.75 | 6.81 | 3.69 | 5.37 | −5.21 | 5.60 | ||
| Rapid Visual Inf Processing % correct | Placebo | 44 | 61.31 | 2.91 | −2.91 | 1.93 | −3.95 | 1.94 | 59.26 | 3.23 | −6.16 | 1.78 | −8.43 | 2.17 | Tr p < 0.001 Tr × Ass p = 0.014 |
| Nutrient+ | 46 | 58.91 | 2.84 | 2.94 | 1.87 | 6.47 | 1.88 | 56.36 | 3.16 | 1.96 | 1.72 | 3.91 | 2.10 | ||
| Rapid Visual Inf Processing reaction time (msec) | Placebo | 44 | 470.3 | 6.51 | 3.16 | 5.57 | −3.14 | 5.56 | 463.8 | 10.57 | −10.10 | 5.17 | −5.28 | 5.45 | Tr p = 0.002 |
| Nutrient+ | 46 | 477.8 | 6.36 | −21.68 | 5.38 | −19.04 | 5.37 | 474.3 | 10.33 | −15.98 | 5.00 | −17.45 | 5.27 | ||
| Corsi Span span | Placebo | 48 | 6.35 | 0.13 | −0.42 | 0.12 | −0.16 | 0.13 | 6.22 | 0.14 | −0.06 | 0.12 | −0.06 | 0.13 | Tr p = 0.004 |
| Nutrient+ | 47 | 6.25 | 0.13 | 0.16 | 0.12 | 0.06 | 0.13 | 6.05 | 0.14 | 0.16 | 0.12 | 0.16 | 0.13 | ||
| Peg and Ball thinking time (msec) | Placebo | 48 | 3049 | 176.3 | −415.5 | 86.2 | −389.6 | 102.2 | 2682 | 170.5 | 64.8 | 100.2 | −160.9 | 105.6 | Tr × Day p = 0.014 |
| Nutrient+ | 47 | 2586 | 178.2 | −255.3 | 87.1 | −372.5 | 103.2 | 2661 | 172.3 | −345.2 | 101.3 | −410.6 | 106.7 | ||
| Peg and Ball completion time (msec) | Placebo | 48 | 7597 | 260.8 | −775.7 | 150.0 | −990.2 | 173.2 | 7145 | 195.5 | −100.9 | 117.0 | −469.5 | 141.1 | |
| Nutrient+ | 47 | 7438 | 263.6 | −818.7 | 151.6 | −1034.2 | 175.1 | 7070 | 197.6 | −498.0 | 118.2 | −742.3 | 142.6 | ||
| Peg and Ball number of errors | Placebo | 48 | 2.40 | 0.44 | 0.48 | 0.55 | 0.08 | 0.56 | 2.81 | 0.37 | 1.33 | 0.50 | 0.67 | 0.51 | Tr × Ass p = 0.036 |
| Nutrient+ | 47 | 3.62 | 0.44 | −1.32 | 0.56 | −0.45 | 0.57 | 2.23 | 0.37 | 0.64 | 0.50 | 0.94 | 0.51 | ||
| Delayed word recall number correct | Placebo | 47 | 4.19 | 0.31 | −1.46 | 0.33 | −2.28 | 0.34 | 5.25 | 0.34 | −2.21 | 0.33 | −2.36 | 0.33 | |
| Nutrient+ | 47 | 4.69 | 0.31 | −2.38 | 0.33 | −2.14 | 0.34 | 5.05 | 0.34 | −1.83 | 0.33 | −2.00 | 0.33 | ||
| Delayed word recall errors | Placebo | 47 | 0.96 | 0.15 | 0.70 | 0.21 | 0.94 | 0.23 | 0.75 | 0.14 | 0.68 | 0.21 | 0.57 | 0.20 | Tr p = 0.029 |
| Nutrient+ | 47 | 0.83 | 0.15 | 0.55 | 0.21 | 0.47 | 0.23 | 0.92 | 0.14 | −0.02 | 0.21 | 0.19 | 0.20 | ||
| Name to Face % correct | Placebo | 48 | 37.50 | 3.06 | −7.20 | 2.86 | −9.47 | 3.24 | 47.57 | 3.37 | −7.96 | 3.70 | −11.55 | 3.12 | |
| Nutrient+ | 45 | 36.11 | 3.16 | −4.06 | 3.04 | −6.84 | 3.44 | 42.96 | 3.48 | −4.91 | 3.93 | −9.40 | 3.32 | ||
| Name to Face reaction time (msec) | Placebo | 48 | 5427 | 320.3 | −377.7 | 327.0 | −376.0 | 375.8 | 5442 | 221.3 | −177.4 | 207.9 | −461.1 | 214.5 | |
| Nutrient+ | 45 | 5514 | 330.8 | −422.7 | 347.3 | −805.7 | 399.1 | 5035 | 228.5 | −300.8 | 220.9 | −493.7 | 227.9 | ||
| Picture recognition % correct | Placebo | 45 | 90.59 | 1.29 | −0.37 | 1.28 | −0.22 | 1.29 | 94.82 | 1.05 | −5.41 | 1.23 | −5.33 | 1.24 | Tr × Ass p = 0.015 |
| Nutrient+ | 47 | 89.43 | 1.26 | −2.65 | 1.25 | −3.83 | 1.26 | 91.12 | 1.02 | −2.96 | 1.21 | −2.98 | 1.21 | ||
| Picture recognition reaction time (msec) | Placebo | 45 | 767.9 | 16.09 | 20.48 | 15.01 | 18.75 | 13.54 | 788.8 | 17.39 | 17.79 | 14.57 | −2.63 | 14.02 | Tr p = 0.002 |
| Nutrient+ | 47 | 798.4 | 15.75 | −15.03 | 14.68 | −30.96 | 13.24 | 809.6 | 17.02 | −29.32 | 14.26 | −26.74 | 13.72 | ||
| Word recognition % correct | Placebo | 46 | 74.71 | 1.42 | −1.20 | 1.41 | −4.34 | 1.46 | 79.06 | 1.51 | −1.18 | 1.40 | −2.08 | 1.51 | |
| Nutrient+ | 44 | 77.17 | 1.46 | −3.76 | 1.44 | −2.93 | 1.49 | 80.23 | 1.55 | −0.99 | 1.43 | −2.27 | 1.55 | ||
| Word recognition reaction time (msec) | Placebo | 46 | 801.2 | 18.23 | −17.48 | 14.55 | −29.54 | 17.10 | 786.1 | 20.86 | 7.54 | 18.72 | −58.10 | 21.75 | |
| Nutrient+ | 44 | 800.6 | 18.64 | 3.45 | 14.88 | −32.04 | 17.48 | 780.7 | 21.33 | 14.21 | 19.14 | 14.35 | 22.24 | ||
| Pre-Dose Baseline | 40 min Post-Dose (Change) | 60 min Post-Dose (Change) | ANOVA Sig. Effects | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | Rep 1 | Rep 2 | Rep 3 | Rep 4 | Rep 1 | Rep 2 | Rep 3 | Rep 4 | Rep 1 | Rep 2 | Rep 3 | Rep 4 | |||||||||||||||
| RVIP % correct | Day 1 | Plac | 63.33 | 3.34 | 60.1 | 3.34 | 57.92 | 3.41 | 55.5 | 3.65 | −4.31 | 1.92 | −3.82 | 1.81 | −3.33 | 1.77 | −2.50 | 2.38 | −4.44 | 1.95 | −5.76 | 1.96 | −5.63 | 2.12 | 0.35 | 2.08 | Treatment p < 0.001 |
| Nut+ | 60.18 | 3.13 | 58.7 | 3.13 | 56.10 | 3.20 | 52.8 | 3.42 | 3.35 | 1.80 | 5.30 | 1.70 | 6.28 | 1.66 | 7.44 | 2.23 | 4.27 | 1.82 | −0.55 | 1.84 | 0.73 | 1.98 | 3.90 | 1.95 | |||
| Day 56 | Plac | 59.17 | 3.28 | 54.4 | 3.38 | 54.31 | 3.63 | 51.1 | 3.36 | −7.08 | 2.11 | −2.22 | 1.91 | −7.36 | 2.04 | −2.01 | 2.24 | −6.67 | 1.92 | −4.83 | 2.13 | −3.82 | 1.99 | −3.54 | 2.44 | ||
| Nut+ | 55.49 | 3.08 | 51.6 | 3.17 | 50.37 | 3.41 | 49.1 | 3.15 | 6.77 | 1.98 | 4.15 | 1.79 | 3.96 | 1.92 | 8.05 | 2.09 | 3.41 | 1.80 | 6.83 | 1.99 | 5.49 | 1.87 | 6.16 | 2.28 | |||
| RVIP Reaction time msec | Day 1 | Plac | 489.54 | 8.75 | 489 | 8.32 | 490 | 8.89 | 495 | 7.99 | −3.07 | 6.22 | −8.94 | 5.83 | −11.7 | 6.74 | −7.21 | 6.20 | −9.49 | 6.44 | −17.8 | 5.64 | −12.8 | 6.46 | −9.38 | 7.15 | Treatment p < 0.006 |
| Nut+ | 486.76 | 8.20 | 483 | 7.80 | 483 | 8.33 | 483 | 7.49 | −16.2 | 5.83 | −18.3 | 5.46 | −12.7 | 6.32 | −7.7 | 5.81 | −20.8 | 6.04 | −17.8 | 5.29 | −13.5 | 6.06 | −8.32 | 6.70 | |||
| Day 56 | Plac | 486.55 | 7.84 | 486 | 8.48 | 491 | 8.37 | 495 | 7.65 | 0.05 | 5.36 | −1.91 | 5.48 | 3.20 | 6.35 | −8.26 | 6.65 | −10.3 | 5.94 | −6.47 | 5.22 | −3.93 | 6.70 | −17.4 | 7.14 | ||
| Nut+ | 488.00 | 7.34 | 497 | 7.95 | 502 | 7.84 | 493 | 7.17 | −13.3 | 5.02 | −23.5 | 5.14 | −28.3 | 5.95 | −14.6 | 6.23 | −19.5 | 5.57 | −29.6 | 4.89 | −19.6 | 6.27 | −17.9 | 6.69 | |||
| RVIP False alarms | Day 1 | Plac | 0.81 | 0.16 | 0.81 | 0.15 | 0.92 | 0.18 | 0.78 | 0.14 | −0.08 | 0.16 | 0.10 | 0.20 | −0.08 | 0.18 | −0.08 | 0.16 | −0.06 | 0.18 | 0.00 | 0.19 | −0.28 | 0.21 | −0.19 | 0.18 | Treatment p < 0.005 |
| Nut+ | 0.42 | 0.15 | 0.46 | 0.14 | 0.34 | 0.17 | 0.34 | 0.13 | 0.24 | 0.15 | 0.15 | 0.19 | 0.02 | 0.17 | 0.27 | 0.15 | 0.17 | 0.17 | 0.37 | 0.18 | 0.37 | 0.20 | 0.49 | 0.17 | |||
| Day 56 | Plac | 0.92 | 0.18 | 0.83 | 0.14 | 0.78 | 0.15 | 0.69 | 0.14 | −0.03 | 0.21 | −0.08 | 0.19 | −0.08 | 0.24 | −0.14 | 0.20 | −0.28 | 0.21 | −0.28 | 0.15 | −0.14 | 0.18 | 0.22 | 0.18 | ||
| Nut+ | 0.73 | 0.17 | 0.56 | 0.13 | 0.56 | 0.14 | 0.59 | 0.13 | −0.12 | 0.20 | 0.07 | 0.17 | 0.27 | 0.22 | 0.15 | 0.18 | −0.05 | 0.20 | 0.00 | 0.14 | 0.07 | 0.16 | −0.05 | 0.17 | |||
| Serial 3s Number correct | Day 1 | Plac | 44.60 | 2.17 | 46.42 | 2.20 | 45.69 | 2.03 | 44.90 | 2.18 | 4.71 | 1.00 | 1.46 | 1.04 | 1.60 | 1.07 | 3.06 | 1.09 | 3.60 | 1.10 | 1.46 | 1.04 | 3.15 | 1.30 | 1.52 | 1.25 | Treatment p < 0.001 |
| Nut+ | 46.68 | 2.20 | 49.30 | 2.22 | 47.77 | 2.05 | 48.70 | 2.20 | 7.60 | 1.01 | 3.94 | 1.05 | 5.28 | 1.08 | 5.02 | 1.10 | 7.09 | 1.11 | 4.45 | 1.05 | 4.36 | 1.31 | 4.34 | 1.26 | |||
| Day 56 | Plac | 43.48 | 2.40 | 47.65 | 2.28 | 45.73 | 2.30 | 45.58 | 2.36 | 6.48 | 1.52 | 2.06 | 1.27 | 3.33 | 1.11 | −0.79 | 1.38 | 7.90 | 1.64 | 1.81 | 1.16 | 3.15 | 1.40 | 2.46 | 1.31 | ||
| Nut+ | 45.75 | 2.42 | 48.11 | 2.31 | 48.49 | 2.32 | 48.38 | 2.39 | 9.85 | 1.54 | 7.74 | 1.28 | 6.15 | 1.12 | 5.13 | 1.39 | 11.30 | 1.66 | 5.26 | 1.17 | 5.81 | 1.41 | 6.28 | 1.32 | |||
| Serial 3s Number of errors | Day 1 | Plac | 2.15 | 0.30 | 2.06 | 0.37 | 2.42 | 0.33 | 2.50 | 0.38 | 0.73 | 0.44 | 0.63 | 0.46 | 0.02 | 0.42 | 0.00 | 0.40 | 0.42 | 0.37 | 0.69 | 0.38 | 0.19 | 0.39 | 0.29 | 0.49 | |
| Nut+ | 2.77 | 0.30 | 2.64 | 0.38 | 2.72 | 0.33 | 2.72 | 0.39 | 0.00 | 0.45 | 0.15 | 0.46 | 0.11 | 0.42 | 0.28 | 0.41 | −0.13 | 0.37 | −0.26 | 0.38 | −0.68 | 0.40 | −0.11 | 0.50 | |||
| Day 56 | Plac | 2.96 | 0.37 | 2.46 | 0.35 | 2.67 | 0.36 | 2.56 | 0.35 | 0.63 | 0.47 | 0.19 | 0.37 | −0.17 | 0.46 | 0.58 | 0.47 | −0.06 | 0.47 | −0.17 | 0.39 | −0.33 | 0.39 | −0.19 | 0.43 | ||
| Nut+ | 2.30 | 0.37 | 2.45 | 0.35 | 2.36 | 0.36 | 2.57 | 0.35 | 0.94 | 0.47 | −0.30 | 0.38 | 0.55 | 0.47 | 0.40 | 0.48 | 0.49 | 0.47 | 0.38 | 0.39 | −0.02 | 0.40 | −0.15 | 0.44 | |||
| Serial 7s number correct | Day 1 | Plac | 25.94 | 1.51 | 26.42 | 1.52 | 26.81 | 1.58 | 26.40 | 1.53 | 2.83 | 0.75 | 2.38 | 0.79 | 1.56 | 0.84 | 1.58 | 0.79 | 2.50 | 0.79 | 2.90 | 0.88 | 3.17 | 0.88 | 3.92 | 0.81 | Treatment p < 0.001 |
| Nut+ | 28.00 | 1.53 | 28.43 | 1.53 | 29.40 | 1.60 | 29.64 | 1.55 | 4.68 | 0.76 | 4.00 | 0.80 | 4.21 | 0.85 | 4.06 | 0.80 | 5.87 | 0.80 | 4.40 | 0.89 | 3.49 | 0.89 | 3.83 | 0.82 | |||
| Day 56 | Plac | 26.58 | 1.48 | 26.17 | 1.57 | 26.98 | 1.50 | 27.15 | 1.61 | 2.25 | 0.88 | 2.52 | 0.91 | 1.21 | 0.91 | 1.13 | 1.06 | 4.33 | 0.97 | 4.10 | 1.09 | 2.92 | 0.90 | 2.54 | 0.95 | ||
| Nut+ | 27.96 | 1.50 | 29.53 | 1.59 | 29.92 | 1.52 | 28.51 | 1.62 | 5.21 | 0.89 | 3.62 | 0.92 | 3.11 | 0.91 | 4.68 | 1.07 | 7.23 | 0.98 | 4.89 | 1.10 | 3.79 | 0.91 | 5.30 | 0.96 | |||
| Serial 7s number of errors | Day 1 | Plac | 2.25 | 0.34 | 2.33 | 0.34 | 2.71 | 0.35 | 2.31 | 0.37 | 0.94 | 0.44 | 0.10 | 0.43 | 0.38 | 0.42 | 0.69 | 0.46 | 0.75 | 0.36 | 0.60 | 0.42 | −0.17 | 0.43 | 0.50 | 0.42 | |
| Nut+ | 2.26 | 0.35 | 2.81 | 0.35 | 2.89 | 0.35 | 3.21 | 0.38 | 0.70 | 0.45 | 0.68 | 0.44 | 0.23 | 0.43 | −0.26 | 0.46 | 0.32 | 0.36 | −0.32 | 0.43 | 0.38 | 0.44 | 0.17 | 0.42 | |||
| Day 56 | Plac | 2.44 | 0.32 | 2.69 | 0.34 | 2.50 | 0.30 | 2.83 | 0.40 | 0.88 | 0.35 | −0.10 | 0.41 | 0.83 | 0.40 | 0.15 | 0.40 | 0.31 | 0.35 | −0.27 | 0.39 | 0.58 | 0.35 | 0.23 | 0.41 | ||
| Nut+ | 2.32 | 0.32 | 2.62 | 0.34 | 2.92 | 0.30 | 3.75 | 0.41 | 0.79 | 0.35 | 0.04 | 0.41 | 0.09 | 0.41 | −0.53 | 0.41 | 0.38 | 0.36 | 0.53 | 0.39 | 0.02 | 0.35 | −0.87 | 0.41 | |||
| Fatigue % along VAS | Day 1 | Plac | 56.29 | 2.48 | 59.35 | 2.80 | 62.29 | 2.84 | 65.08 | 2.93 | 3.81 | 2.42 | 6.58 | 2.27 | 5.19 | 2.36 | 3.90 | 2.16 | 8.40 | 2.43 | 10.48 | 2.22 | 10.04 | 2.14 | 7.10 | 1.90 | Treatment p < 0.001 |
| Nut+ | 57.89 | 2.51 | 62.83 | 2.83 | 67.38 | 2.87 | 70.45 | 2.96 | −5.60 | 2.44 | −7.09 | 2.30 | −8.96 | 2.39 | −11.5 | 2.18 | −1.43 | 2.45 | 0.79 | 2.24 | 0.94 | 2.16 | −0.28 | 1.92 | |||
| Day 56 | Plac | 51.88 | 2.51 | 58.73 | 2.46 | 63.31 | 2.55 | 66.25 | 2.72 | 8.10 | 2.40 | 6.19 | 2.19 | 4.79 | 2.05 | 3.85 | 1.97 | 10.67 | 2.47 | 9.71 | 2.31 | 5.15 | 2.39 | 3.90 | 2.27 | ||
| Nut+ | 57.87 | 2.53 | 65.45 | 2.49 | 70.47 | 2.57 | 74.87 | 2.75 | −0.98 | 2.42 | −3.40 | 2.21 | −4.53 | 2.07 | −6.28 | 1.99 | 5.17 | 2.50 | 3.49 | 2.34 | 1.53 | 2.41 | −1.28 | 2.30 | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kennedy, D.O.; Wightman, E.L.; Forster, J.; Khan, J.; Haskell-Ramsay, C.F.; Jackson, P.A. Cognitive and Mood Effects of a Nutrient Enriched Breakfast Bar in Healthy Adults: A Randomised, Double-Blind, Placebo-Controlled, Parallel Groups Study. Nutrients 2017, 9, 1332. https://doi.org/10.3390/nu9121332
Kennedy DO, Wightman EL, Forster J, Khan J, Haskell-Ramsay CF, Jackson PA. Cognitive and Mood Effects of a Nutrient Enriched Breakfast Bar in Healthy Adults: A Randomised, Double-Blind, Placebo-Controlled, Parallel Groups Study. Nutrients. 2017; 9(12):1332. https://doi.org/10.3390/nu9121332
Chicago/Turabian StyleKennedy, David O., Emma L. Wightman, Joanne Forster, Julie Khan, Crystal F. Haskell-Ramsay, and Philippa A. Jackson. 2017. "Cognitive and Mood Effects of a Nutrient Enriched Breakfast Bar in Healthy Adults: A Randomised, Double-Blind, Placebo-Controlled, Parallel Groups Study" Nutrients 9, no. 12: 1332. https://doi.org/10.3390/nu9121332
APA StyleKennedy, D. O., Wightman, E. L., Forster, J., Khan, J., Haskell-Ramsay, C. F., & Jackson, P. A. (2017). Cognitive and Mood Effects of a Nutrient Enriched Breakfast Bar in Healthy Adults: A Randomised, Double-Blind, Placebo-Controlled, Parallel Groups Study. Nutrients, 9(12), 1332. https://doi.org/10.3390/nu9121332





