Glucose Plus Fructose Ingestion for Post-Exercise Recovery—Greater than the Sum of Its Parts?
Abstract
:1. Introduction
2. Dietary Carbohydrates for Sport Nutrition
3. Endogenous Carbohydrate Stores and Exercise Performance
3.1. Muscle Glycogen
3.2. Liver Glycogen
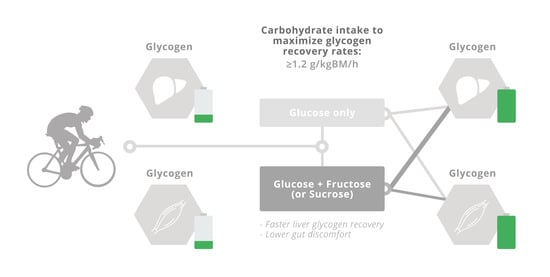
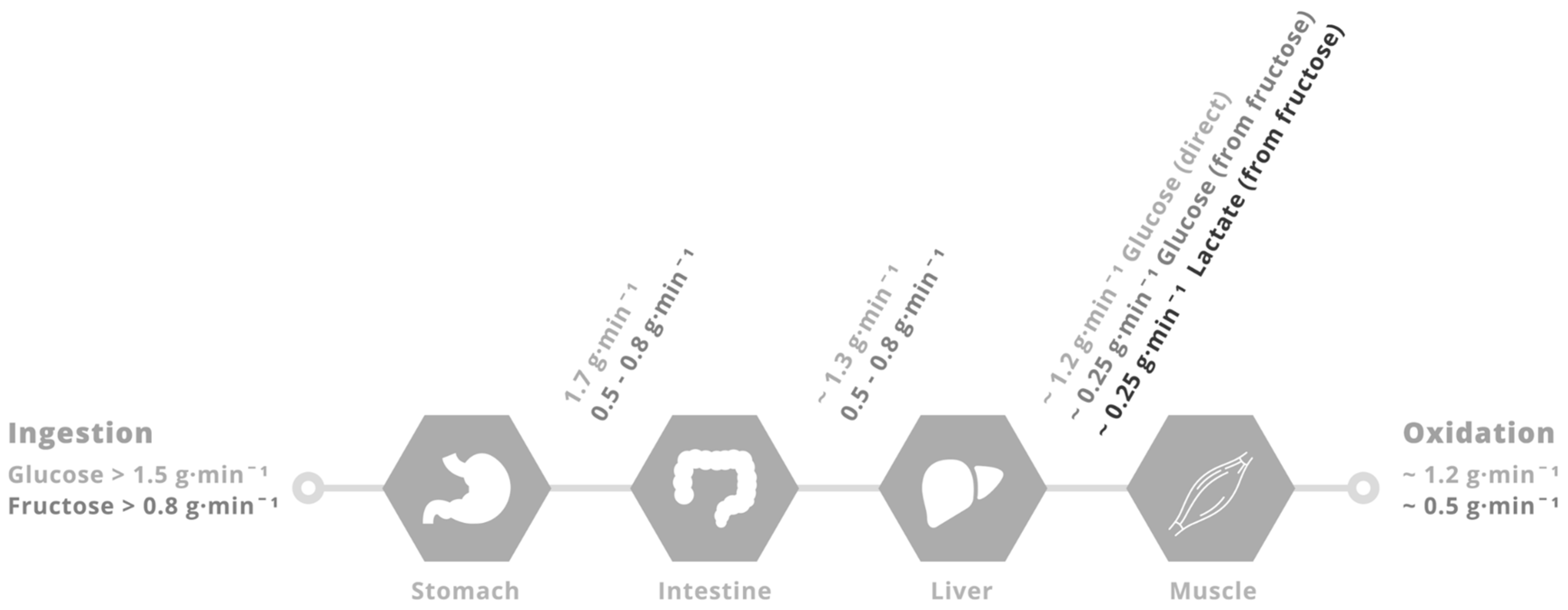
4. Physiological Rationale for Glucose–Fructose Co-Ingestion in Post-Exercise Recovery
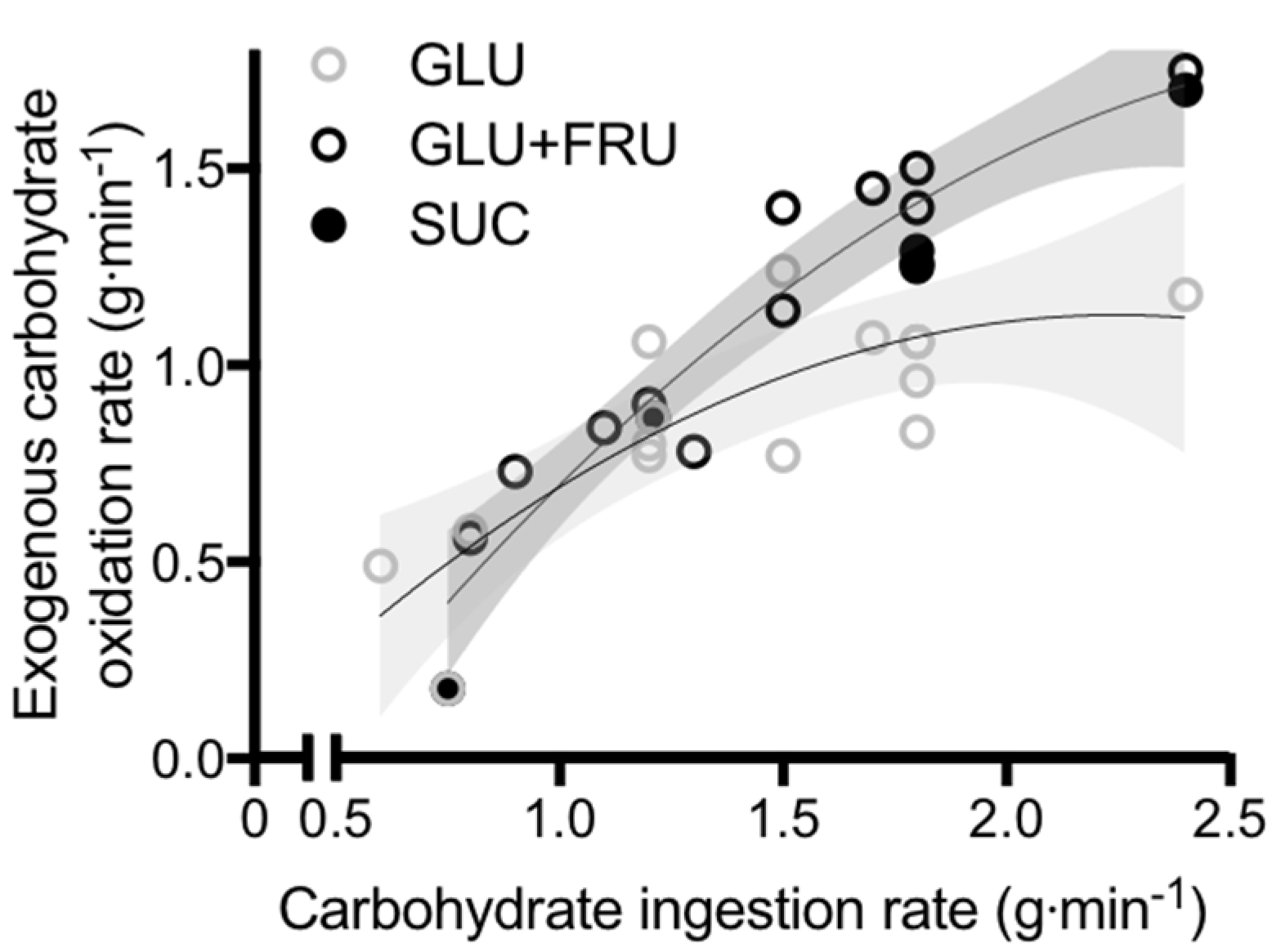
5. Glucose–Fructose Co-Ingestion and Recovery from Exercise
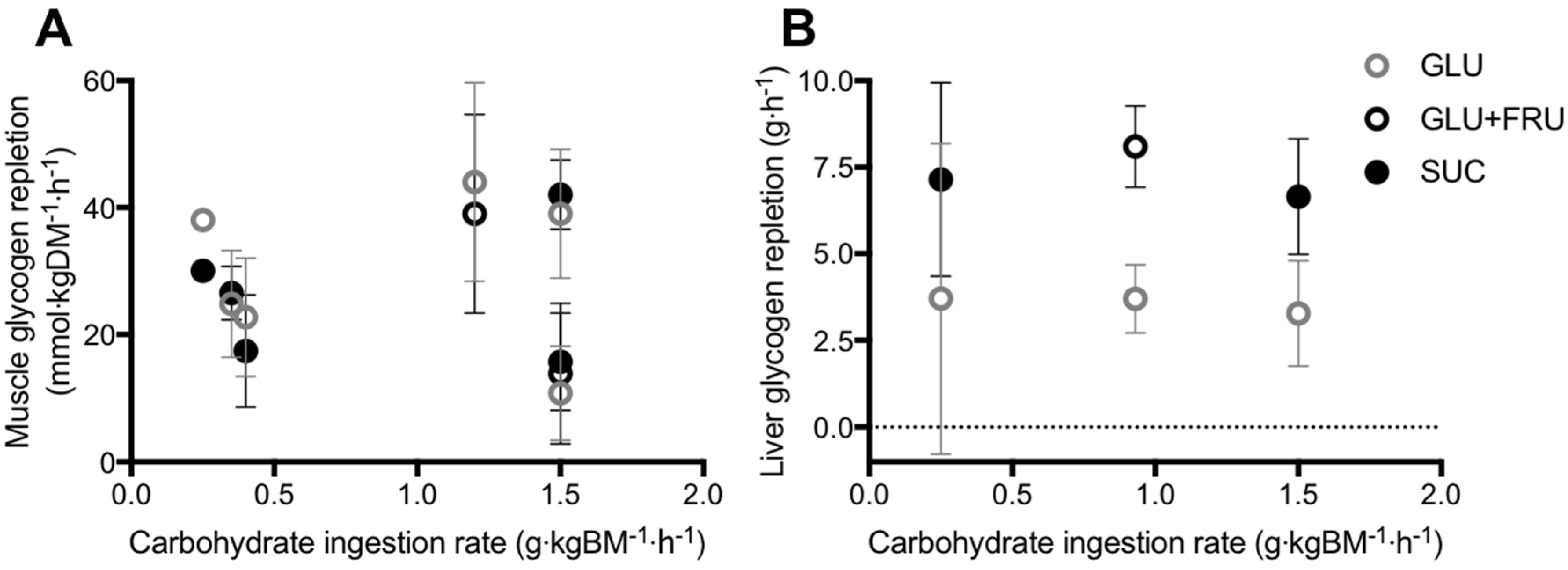
5.1. Muscle Glycogen Repletion
5.2. Liver Glycogen Repletion
6. Conclusions and Recommendations
Conflicts of Interest
References
- Van Loon, L.J.; Greenhaff, P.L.; Constantin-Teodosiu, D.; Saris, W.H.; Wagenmakers, A.J. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J. Physiol. 2001, 536, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Romijn, J.A.; Coyle, E.F.; Sidossis, L.S.; Gastaldelli, A.; Horowitz, J.F.; Endert, E.; Wolfe, R.R. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. 1993, 265, E380–E391. [Google Scholar] [PubMed]
- Gonzalez, J.T.; Veasey, R.C.; Rumbold, P.L.; Stevenson, E.J. Breakfast and exercise contingently affect postprandial metabolism and energy balance in physically active males. Br. J. Nutr. 2013, 110, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.J.; Jeukendrup, A.E.; Saris, W.H.; Wagenmakers, A.J. Effect of training status on fuel selection during submaximal exercise with glucose ingestion. J. Appl. Physiol. 1999, 87, 1413–1420. [Google Scholar] [PubMed]
- Gonzalez, J.T.; Fuchs, C.J.; Betts, J.A.; van Loon, L.J. Liver glycogen metabolism during and after prolonged endurance-type exercise. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E543–E553. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, E.J.; Thelwall, P.E.; Thomas, K.; Smith, F.; Brand-Miller, J.; Trenell, M.I. Dietary glycemic index influences lipid oxidation but not muscle or liver glycogen oxidation during exercise. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1140–E1147. [Google Scholar] [CrossRef] [PubMed]
- Casey, A.; Mann, R.; Banister, K.; Fox, J.; Morris, P.G.; Macdonald, I.A.; Greenhaff, P.L. Effect of carbohydrate ingestion on glycogen resynthesis in human liver and skeletal muscle, measured by (13)c mrs. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E65–E75. [Google Scholar] [PubMed]
- Bergstrom, J.; Hermansen, L.; Hultman, E.; Saltin, B. Diet, muscle glycogen and physical performance. Acta. Physiol. Scand. 1967, 71, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Coyle, E.F.; Coggan, A.R.; Hemmert, M.K.; Ivy, J.L. Muscle glycogen utilization during prolonged strenuous exercise when fed carbohydrate. J. Appl. Physiol. 1986, 61, 165–172. [Google Scholar] [PubMed]
- Alghannam, A.F.; Jedrzejewski, D.; Tweddle, M.G.; Gribble, H.; Bilzon, J.; Thompson, D.; Tsintzas, K.; Betts, J.A. Impact of muscle glycogen availability on the capacity for repeated exercise in man. Med. Sci. Sports Exerc. 2016, 48, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Stellingwerff, T.; Boon, H.; Gijsen, A.P.; Stegen, J.H.; Kuipers, H.; van Loon, L.J. Carbohydrate supplementation during prolonged cycling exercise spares muscle glycogen but does not affect intramyocellular lipid use. Med. Sci. Sports Exer. 2007, 454, 635–647. [Google Scholar] [CrossRef]
- Vandenbogaerde, T.J.; Hopkins, W.G. Effects of acute carbohydrate supplementation on endurance performance: A meta-analysis. Sports Med. 2011, 41, 773–792. [Google Scholar] [CrossRef] [PubMed]
- Van Hall, G.; Shirreffs, S.M.; Calbet, J.A. Muscle glycogen resynthesis during recovery from cycle exercise: No effect of additional protein ingestion. J. Appl. Physiol. 2000, 88, 1631–1636. [Google Scholar] [PubMed]
- Betts, J.A.; Williams, C. Short-term recovery from prolonged exercise: Exploring the potential for protein ingestion to accentuate the benefits of carbohydrate supplements. Sports Med. 2010, 40, 941–959. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; van Loon, L.J.; Hawley, J.A. Post-exercise muscle glycogen resynthesis in humans. J. Appl. Physiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, D.S.; Houltham, S.; Musa-Veloso, K.; Brown, F.; Paulionis, L.; Bailey, D. Fructose-glucose composite carbohydrates and endurance performance: Critical review and future perspectives. Sports Med. 2015, 45, 1561–1576. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Collier, G.R.; Hargreaves, M. Muscle glycogen storage after prolonged exercise: Effect of the glycemic index of carbohydrate feedings. J. Appl. Physiol. 1993, 75, 1019–1023. [Google Scholar] [PubMed]
- Food and Agriculture Organization of the United Nations; The World Health Organization. Carbohydrates in Human Nutrition. Report of a joint FAO/WHO expert consultation. In FAO Food and Nutrition Paper; FAO: Rome, Italy, 1998; Volume 66. [Google Scholar]
- Scientific Advisory Committee on Nutrition. SACN Carbohydrates and Health Report; Public Health England: London, UK, 2015. [Google Scholar]
- Lina, B.A.; Jonker, D.; Kozianowski, G. Isomaltulose (palatinose): A review of biological and toxicological studies. Food Chem. Toxicol. 2002, 40, 1375–1381. [Google Scholar] [CrossRef]
- Drozdowski, L.A.; Thomson, A.B. Intestinal sugar transport. World J. Gastroenterol. 2006, 12, 1657–1670. [Google Scholar] [CrossRef] [PubMed]
- DeBosch, B.J.; Chi, M.; Moley, K.H. Glucose transporter 8 (glut8) regulates enterocyte fructose transport and global mammalian fructose utilization. Endocrinology 2012, 153, 4181–4191. [Google Scholar] [CrossRef] [PubMed]
- Daniel, H.; Zietek, T. Taste and move: Glucose and peptide transporters in the gastrointestinal tract. Exp. Physiol. 2015, 100, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.; Chandler, J.D.; Clarke, A.L.; Petrou, S.; Best, J.D. Glucose transporter glut12-functional characterization in xenopus laevis oocytes. Biochem. Biophys. Res. Commun. 2003, 308, 422–426. [Google Scholar] [CrossRef]
- Tappy, L.; Le, K.A. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010, 90, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Jentjens, R.L.; Venables, M.C.; Jeukendrup, A.E. Oxidation of exogenous glucose, sucrose, and maltose during prolonged cycling exercise. J. Appl. Physiol. 2004, 96, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Moodley, D.; Noakes, T.D.; Bosch, A.N.; Hawley, J.A.; Schall, R.; Dennis, S.C. Oxidation of exogenous carbohydrate during prolonged exercise: The effects of the carbohydrate type and its concentration. Eur. J. Appl. Physiol. Occup. Physiol. 1992, 64, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A.; Dennis, S.C.; Laidler, B.J.; Bosch, A.N.; Noakes, T.D.; Brouns, F. High rates of exogenous carbohydrate oxidation from starch ingested during prolonged exercise. J. Appl. Physiol. 1991, 71, 1801–1806. [Google Scholar] [PubMed]
- Gray, G.M.; Ingelfinger, F.J. Intestinal absorption of sucrose in man: Interrelation of hydrolysis and monosaccharide product absorption. J. Clin. Invest. 1966, 45, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Dahlqvist, A.; Auricchio, S.; Semenza, G.; Prader, A. Human intestinal disaccharidases and hereditary disaccharide intolerance. The hydrolysis of sucrose, isomaltose, palatinose (isomaltulose), and a 1,6-alpha-oligosaccharide (isomalto-oligosaccharide) preparation. J. Clin. Invest. 1963, 42, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Van Can, J.G.; Ijzerman, T.H.; van Loon, L.J.; Brouns, F.; Blaak, E.E. Reduced glycaemic and insulinaemic responses following isomaltulose ingestion: Implications for postprandial substrate use. Br. J. Nutr. 2009, 102, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Oosthuyse, T.; Carstens, M.; Millen, A.M. Ingesting isomaltulose versus fructose-maltodextrin during prolonged moderate-heavy exercise increases fat oxidation but impairs gastrointestinal comfort and cycling performance. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Sunehag, A.L.; Haymond, M.W. Splanchnic galactose extraction is regulated by coingestion of glucose in humans. Metabolism 2002, 51, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Tappy, L.; Egli, L.; Lecoultre, V.; Schneider, P. Effects of fructose-containing caloric sweeteners on resting energy expenditure and energy efficiency: A review of human trials. Nutr. Metab. 2013, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.F.; Fielding, B.A.; Frayn, K.N. Mechanisms for the acute effect of fructose on postprandial lipemia. Am. J. Clin. Nutr. 2007, 85, 1511–1520. [Google Scholar] [PubMed]
- Jentjens, R.L.; Jeukendrup, A.E. Effects of pre-exercise ingestion of trehalose, galactose and glucose on subsequent metabolism and cycling performance. Eur. J. Appl. Physiol. 2003, 88, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Tornheim, K.; Lowenstein, J.M. Control of phosphofructokinase from rat skeletal muscle. Effects of fructose diphosphate, amp, atp, and citrate. J. Biol. Chem. 1976, 251, 7322–7328. [Google Scholar] [PubMed]
- Bergstrom, J.; Hultman, E. Muscle glycogen synthesis after exercise: An enhancing factor localized to the muscle cells in man. Nature 1966, 210, 309–310. [Google Scholar] [CrossRef] [PubMed]
- Ortenblad, N.; Westerblad, H.; Nielsen, J. Muscle glycogen stores and fatigue. J. Physiol. 2013, 591, 4405–4413. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.N.; Wojtaszewski, J.F.; Haller, R.G.; Hardie, D.G.; Kemp, B.E.; Richter, E.A.; Vissing, J. Role of 5′amp-activated protein kinase in glycogen synthase activity and glucose utilization: Insights from patients with mcardle’s disease. J. Physiol. 2002, 541, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Lucia, A.; Ruiz, J.R.; Santalla, A.; Nogales-Gadea, G.; Rubio, J.C.; Garcia-Consuegra, I.; Cabello, A.; Perez, M.; Teijeira, S.; Vieitez, I.; et al. Genotypic and phenotypic features of mcardle disease: Insights from the spanish national registry. J. Neurol. Neurosurg. Psychiatry 2012, 83, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Walter, G.; Vandenborne, K.; Elliott, M.; Leigh, J.S. In vivo ATP synthesis rates in single human muscles during high intensity exercise. J. Physiol. 1999, 519 Pt 3, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Hultman, E.; Harris, R.C. Carbohydrate metabolism. In Principles of Exercise Biochemistry; Poortmans, J.R., Ed.; S.Karger: Basel, Switzerland, 1988. [Google Scholar]
- Jeukendrup, A.E.; Wallis, G.A. Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int. J. Sports Med. 2005, 26, S28–S37. [Google Scholar] [CrossRef] [PubMed]
- Bangsbo, J.; Graham, T.E.; Kiens, B.; Saltin, B. Elevated muscle glycogen and anaerobic energy production during exhaustive exercise in man. J. Physiol. 1992, 451, 205–227. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.F.; Price, T.; Cline, G.W.; Rothman, D.L.; Shulman, G.I. Contribution of net hepatic glycogenolysis to glucose production during the early postprandial period. Am. J. Physiol. 1996, 270, E186–E191. [Google Scholar] [PubMed]
- Nilsson, L.H.; Hultman, E. Liver glycogen in man--the effect of total starvation or a carbohydrate-poor diet followed by carbohydrate refeeding. Scand. J. Clin. Lab. Invest. 1973, 32, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Bergman, B.C.; Horning, M.A.; Casazza, G.A.; Wolfel, E.E.; Butterfield, G.E.; Brooks, G.A. Endurance training increases gluconeogenesis during rest and exercise in men. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E244–E251. [Google Scholar] [PubMed]
- Gonzalez, J.T.; Fuchs, C.J.; Smith, F.E.; Thelwall, P.E.; Taylor, R.; Stevenson, E.J.; Trenell, M.I.; Cermak, N.M.; van Loon, L.J. Ingestion of glucose or sucrose prevents liver but not muscle glycogen depletion during prolonged endurance-type exercise in trained cyclists. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E1032–E1039. [Google Scholar] [CrossRef] [PubMed]
- Izumida, Y.; Yahagi, N.; Takeuchi, Y.; Nishi, M.; Shikama, A.; Takarada, A.; Masuda, Y.; Kubota, M.; Matsuzaka, T.; Nakagawa, Y.; et al. Glycogen shortage during fasting triggers liver-brain-adipose neurocircuitry to facilitate fat utilization. Nat. Commun. 2013, 4, 2316. [Google Scholar] [PubMed]
- Maehlum, S.; Felig, P.; Wahren, J. Splanchnic glucose and muscle glycogen metabolism after glucose feeding during postexercise recovery. Am. J. Physiol. 1978, 235, E255–E260. [Google Scholar] [PubMed]
- Hurren, N.M.; Balanos, G.M.; Blannin, A.K. Is the beneficial effect of prior exercise on postprandial lipaemia partly due to redistribution of blood flow? Clin. Sci. 2011, 120, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, J.; Hultman, E. Synthesis of muscle glycogen in man after glucose and fructose infusion. Acta Med. Scand. 1967, 182, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Roch-Norlund, A.E.; Bergstrom, J.; Hultman, E. Muscle glycogen and glycogen synthetase in normal subjects and in patients with diabetes mellitus. Effect of intravenous glucose and insulin administration. Scand. J. Clin. Lab. Invest. 1972, 30, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, D.J.; Lessard, S.J.; Coffey, V.G.; Churchley, E.G.; Wootton, A.M.; Ng, T.; Watt, M.J.; Hawley, J.A. High rates of muscle glycogen resynthesis after exhaustive exercise when carbohydrate is coingested with caffeine. J. Appl. Physiol. 2008, 105, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A.E. Carbohydrate and exercise performance: The role of multiple transportable carbohydrates. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A.; Bosch, A.N.; Weltan, S.M.; Dennis, S.C.; Noakes, T.D. Glucose kinetics during prolonged exercise in euglycaemic and hyperglycaemic subjects. Pflugers Archiv. Eur. J. Physiol. 1994, 426, 378–386. [Google Scholar] [CrossRef]
- Hulston, C.J.; Wallis, G.A.; Jeukendrup, A.E. Exogenous cho oxidation with glucose plus fructose intake during exercise. Med. Sci. Sports Exerc. 2009, 41, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Jentjens, R.L.; Jeukendrup, A.E. High rates of exogenous carbohydrate oxidation from a mixture of glucose and fructose ingested during prolonged cycling exercise. Br. J. Nutr. 2005, 93, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Jentjens, R.L.; Shaw, C.; Birtles, T.; Waring, R.H.; Harding, L.K.; Jeukendrup, A.E. Oxidation of combined ingestion of glucose and sucrose during exercise. Metabolism 2005, 54, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Jentjens, R.L.; Achten, J.; Jeukendrup, A.E. High oxidation rates from combined carbohydrates ingested during exercise. Med. Sci. Sports Exerc. 2004, 36, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Jentjens, R.L.; Moseley, L.; Waring, R.H.; Harding, L.K.; Jeukendrup, A.E. Oxidation of combined ingestion of glucose and fructose during exercise. J. Appl. Physiol. 2004, 96, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Jentjens, R.L.; Underwood, K.; Achten, J.; Currell, K.; Mann, C.H.; Jeukendrup, A.E. Exogenous carbohydrate oxidation rates are elevated after combined ingestion of glucose and fructose during exercise in the heat. J. Appl. Physiol. 2006, 100, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A.E.; Moseley, L.; Mainwaring, G.I.; Samuels, S.; Perry, S.; Mann, C.H. Exogenous carbohydrate oxidation during ultraendurance exercise. J. Appl. Physiol. 2006, 100, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, D.S.; Thorburn, M.S.; Thorp, R.M.; Broadbent, S.; Shi, X. Effect of graded fructose coingestion with maltodextrin on exogenous 14c-fructose and 13c-glucose oxidation efficiency and high-intensity cycling performance. J. Appl. Physiol. 2008, 104, 1709–1719. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.D.; Tarpey, M.D.; Kass, L.S.; Tarpey, R.J.; Roberts, M.G. Assessing a commercially available sports drink on exogenous carbohydrate oxidation, fluid delivery and sustained exercise performance. J. Int. Soc. Sports Nutr. 2014, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Wagenmakers, A.J.; Brouns, F.; Saris, W.H.; Halliday, D. Oxidation rates of orally ingested carbohydrates during prolonged exercise in men. J. Appl. Physiol. 1993, 75, 2774–2780. [Google Scholar] [PubMed]
- Wallis, G.A.; Rowlands, D.S.; Shaw, C.; Jentjens, R.L.; Jeukendrup, A.E. Oxidation of combined ingestion of maltodextrins and fructose during exercise. Med. Sci. Sports Exerc. 2005, 37, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Trommelen, J.; Fuchs, C.J.; Beelen, M.; Lenaerts, K.; Jeukendrup, A.E.; Cermak, N.M.; van Loon, L.J. Fructose and sucrose intake increase exogenous carbohydrate oxidation during exercise. Nutrients 2017, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Rehrer, N.J.; Wagenmakers, A.J.; Beckers, E.J.; Halliday, D.; Leiper, J.B.; Brouns, F.; Maughan, R.J.; Westerterp, K.; Saris, W.H. Gastric emptying, absorption, and carbohydrate oxidation during prolonged exercise. J. Appl. Physiol. 1992, 72, 468–475. [Google Scholar] [PubMed]
- Duchman, S.M.; Ryan, A.J.; Schedl, H.P.; Summers, R.W.; Bleiler, T.L.; Gisolfi, C.V. Upper limit for intestinal absorption of a dilute glucose solution in men at rest. Med. Sci. Sports Exerc. 1997, 29, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Fordtran, J.S.; Saltin, B. Gastric emptying and intestinal absorption during prolonged severe exercise. J. Appl. Physiol. 1967, 23, 331–335. [Google Scholar] [PubMed]
- Jeukendrup, A.E.; Jentjens, R. Oxidation of carbohydrate feedings during prolonged exercise: Current thoughts, guidelines and directions for future research. Sports Med. 2000, 29, 407–424. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, W.J.; Stannard, S.R.; Clarke, J.A.; Rowlands, D.S. Fructose-maltodextrin ratio governs exogenous and other cho oxidation and performance. Med. Sci. Sports Exerc. 2013, 45, 1814–1824. [Google Scholar] [CrossRef] [PubMed]
- Rosset, R.; Lecoultre, V.; Egli, L.; Cros, J.; Dokumaci, A.S.; Zwygart, K.; Boesch, C.; Kreis, R.; Schneiter, P.; Tappy, L. Postexercise repletion of muscle energy stores with fructose or glucose in mixed meals. Am. J. Clin. Nutr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Rikmenspoel, R.; Caputo, R. The michaelis-menten constant for fructose and for glucose of hexokinase in bull spermatozoa. J. Reprod. Fertil. 1966, 12, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Ahlborg, G.; Bjorkman, O. Splanchnic and muscle fructose metabolism during and after exercise. J. Appl. Physiol. 1990, 69, 1244–1251. [Google Scholar] [PubMed]
- Lecoultre, V.; Benoit, R.; Carrel, G.; Schutz, Y.; Millet, G.P.; Tappy, L.; Schneiter, P. Fructose and glucose co-ingestion during prolonged exercise increases lactate and glucose fluxes and oxidation compared with an equimolar intake of glucose. Am. J. Clin. Nutr. 2010, 92, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Truswell, A.S.; Seach, J.M.; Thorburn, A.W. Incomplete absorption of pure fructose in healthy subjects and the facilitating effect of glucose. Am. J. Clin. Nutr. 1988, 48, 1424–1430. [Google Scholar] [PubMed]
- Patel, C.; Douard, V.; Yu, S.; Gao, N.; Ferraris, R.P. Transport, metabolism, and endosomal trafficking-dependent regulation of intestinal fructose absorption. FASEB J. 2015, 29, 4046–4058. [Google Scholar] [CrossRef] [PubMed]
- Stocks, B.; Betts, J.A.; McGawley, K. Effects of carbohydrate dose and frequency on metabolism, gastrointestinal discomfort, and cross-country skiing performance. Scand. J. Med. Sci. Sports 2016, 26, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Bangsbo, J.; Gollnick, P.D.; Graham, T.E.; Saltin, B. Substrates for muscle glycogen synthesis in recovery from intense exercise in man. J. Physiol. 1991, 434, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Kruszynska, Y.T.; Mulford, M.I.; Baloga, J.; Yu, J.G.; Olefsky, J.M. Regulation of skeletal muscle hexokinase ii by insulin in nondiabetic and niddm subjects. Diabetes 1998, 47, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.K.; Chibalin, A.V.; Koistinen, H.A.; Yang, J.; Koumanov, F.; Wallberg-Henriksson, H.; Zierath, J.R.; Holman, G.D. Kinetics of glut4 trafficking in rat and human skeletal muscle. Diabetes 2009, 58, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Laakso, M.; Edelman, S.V.; Brechtel, G.; Baron, A.D. Effects of epinephrine on insulin-mediated glucose uptake in whole body and leg muscle in humans: Role of blood flow. Am. J. Physiol. 1992, 263, E199–E204. [Google Scholar] [PubMed]
- Yki-Jarvinen, H.; Mott, D.; Young, A.A.; Stone, K.; Bogardus, C. Regulation of glycogen synthase and phosphorylase activities by glucose and insulin in human skeletal muscle. J. Clin. Invest. 1987, 80, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.; Ruge, T.; Lai, Y.C.; Svensson, M.K.; Eriksson, J.W. Effects of adrenaline on whole-body glucose metabolism and insulin-mediated regulation of glycogen synthase and pkb phosphorylation in human skeletal muscle. Metabolism 2011, 60, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Bowtell, J.L.; Gelly, K.; Jackman, M.L.; Patel, A.; Simeoni, M.; Rennie, M.J. Effect of different carbohydrate drinks on whole body carbohydrate storage after exhaustive exercise. J. Appl. Physiol. 2000, 88, 1529–1536. [Google Scholar] [PubMed]
- Blom, P.C.; Hostmark, A.T.; Vaage, O.; Kardel, K.R.; Maehlum, S. Effect of different post-exercise sugar diets on the rate of muscle glycogen synthesis. Med. Sci. Sports Exerc. 1987, 19, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.J.; Gonzalez, J.T.; Beelen, M.; Cermak, N.M.; Smith, F.E.; Thelwall, P.E.; Taylor, R.; Trenell, M.I.; Stevenson, E.J.; van Loon, L.J. Sucrose ingestion after exhaustive exercise accelerates liver, but not muscle glycogen repletion when compared to glucose ingestion in trained athletes. J. Appl. Physiol. 2016, 120, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Wallis, G.A.; Hulston, C.J.; Mann, C.H.; Roper, H.P.; Tipton, K.D.; Jeukendrup, A.E. Postexercise muscle glycogen synthesis with combined glucose and fructose ingestion. Med. Sci. Sports Exerc. 2008, 40, 1789–1794. [Google Scholar] [CrossRef] [PubMed]
- Trommelen, J.; Beelen, M.; Pinckaers, P.J.; Senden, J.M.; Cermak, N.M.; van Loon, L.J. Fructose coingestion does not accelerate postexercise muscle glycogen repletion. Med. Sci. Sports Exerc. 2016, 48, 907–912. [Google Scholar] [CrossRef] [PubMed]
- McLane, J.A.; Holloszy, J.O. Glycogen synthesis from lactate in the three types of skeletal muscle. J. Biol. Chem. 1979, 254, 6548–6553. [Google Scholar] [PubMed]
- Ullrich, S.S.; Fitzgerald, P.C.; Schober, G.; Steinert, R.E.; Horowitz, M.; Feinle-Bisset, C. Intragastric administration of leucine or isoleucine lowers the blood glucose response to a mixed-nutrient drink by different mechanisms in healthy, lean volunteers. Am. J. Clin. Nutr. 2016, 104, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Decombaz, J.; Jentjens, R.; Ith, M.; Scheurer, E.; Buehler, T.; Jeukendrup, A.; Boesch, C. Fructose and galactose enhance postexercise human liver glycogen synthesis. Med. Sci. Sports Exerc. 2011, 43, 1964–1971. [Google Scholar] [PubMed]
- Gentilcore, D.; Chaikomin, R.; Jones, K.L.; Russo, A.; Feinle-Bisset, C.; Wishart, J.M.; Rayner, C.K.; Horowitz, M. Effects of fat on gastric emptying of and the glycemic, insulin, and incretin responses to a carbohydrate meal in type 2 diabetes. J. Clin. Endocrinol. Metab. 2006, 91, 2062–2067. [Google Scholar] [CrossRef] [PubMed]
- Rigden, D.J.; Jellyman, A.E.; Frayn, K.N.; Coppack, S.W. Human adipose tissue glycogen levels and responses to carbohydrate feeding. Eur. J. Clin. Nutr. 1990, 44, 689–692. [Google Scholar] [PubMed]
- Oz, G.; Henry, P.G.; Seaquist, E.R.; Gruetter, R. Direct, noninvasive measurement of brain glycogen metabolism in humans. Neurochem. Int. 2003, 43, 323–329. [Google Scholar] [CrossRef]
- Biava, C.; Grossman, A.; West, M. Ultrastructural observations on renal glycogen in normal and pathologic human kidneys. Lab. Invest. 1966, 15, 330–356. [Google Scholar] [PubMed]
| Carbohydrate | Chain Length | Constituent Monomers | Bonds | Apical Membrane Intestinal Transport Protein(s) |
|---|---|---|---|---|
| Glucose | 1 | - | - | SGLT1; GLUT2; GLUT12 |
| Fructose | 1 | - | - | GLUT5; GLUT2; GLUT7; GLUT8; GLUT12 |
| Galactose | 1 | - | - | SGLT1; GLUT2 |
| Maltose | 2 | Glucose + Glucose | α-1,4-glycosidic | SGLT1; GLUT2; GLUT8/12 |
| Sucrose | 2 | Glucose + Fructose | α-1,2-glycosidic | SGLT1; GLUT5; GLUT2; GLUT7; GLUT8 GLUT12 |
| Isomaltulose | 2 | Glucose + Fructose | α-1,6-glycosidic | SGLT1; GLUT5; GLUT2; GLUT7; GLUT8 GLUT12 |
| Lactose | 2 | Glucose + Galactose | β-1,4-glycosidic | SGLT1; GLUT2; GLUT12 |
| Maltodextrin | ~3–9 | Glucose + Glucose… | α-1,4-glycosidic | SGLT1; GLUT2; GLUT12 |
| Starch | >9 (typically >300) | Glucose + Glucose… | α-1,4- and α-1,6-glycosidic | SGLT1; GLUT2; GLUT12 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez, J.T.; Fuchs, C.J.; Betts, J.A.; Van Loon, L.J.C. Glucose Plus Fructose Ingestion for Post-Exercise Recovery—Greater than the Sum of Its Parts? Nutrients 2017, 9, 344. https://doi.org/10.3390/nu9040344
Gonzalez JT, Fuchs CJ, Betts JA, Van Loon LJC. Glucose Plus Fructose Ingestion for Post-Exercise Recovery—Greater than the Sum of Its Parts? Nutrients. 2017; 9(4):344. https://doi.org/10.3390/nu9040344
Chicago/Turabian StyleGonzalez, Javier T., Cas J. Fuchs, James A. Betts, and Luc J. C. Van Loon. 2017. "Glucose Plus Fructose Ingestion for Post-Exercise Recovery—Greater than the Sum of Its Parts?" Nutrients 9, no. 4: 344. https://doi.org/10.3390/nu9040344
APA StyleGonzalez, J. T., Fuchs, C. J., Betts, J. A., & Van Loon, L. J. C. (2017). Glucose Plus Fructose Ingestion for Post-Exercise Recovery—Greater than the Sum of Its Parts? Nutrients, 9(4), 344. https://doi.org/10.3390/nu9040344