A Critical Review on Polyphenols and Health Benefits of Black Soybeans
Abstract
:1. Polyphenols
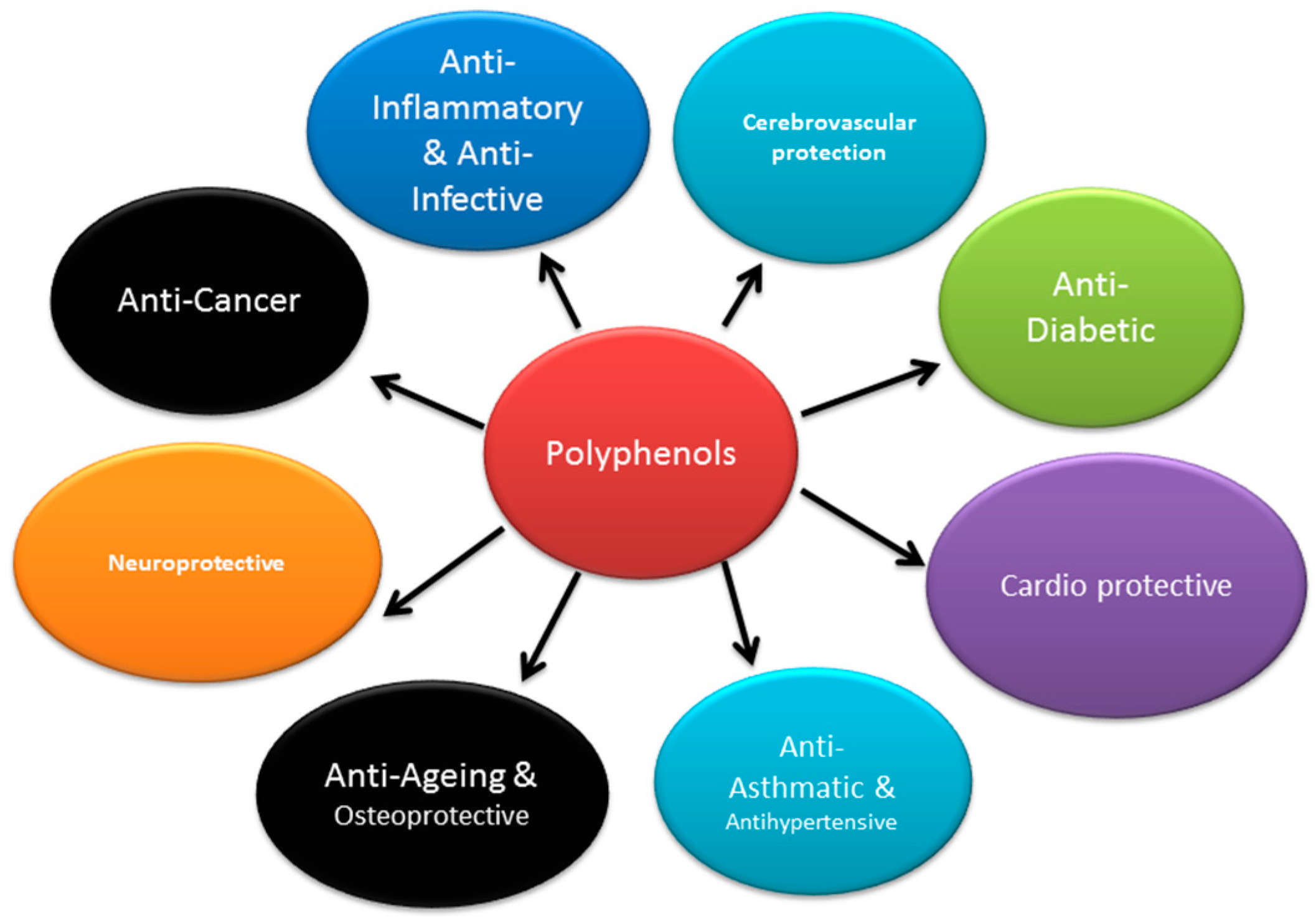
1.1. Types of Polyphenols
- Flavonoids: Have a potential effect on radical scavenging and inflammatory reactions. They are predominantly found in fruits, vegetables, legumes, red wine, and green tea. They are further divided into a number of subgroups namely, flavones, flavonols, flavanones, isoflavones, anthocyanidins, chalcones, and catechins.
- Stilbenes: Found in product of graphs, red wine, and peanuts. Resveratrol is the most well-known compound among the group.
- Lignans: Found in seeds like flax, linseed, legumes, cereals, grains, fruits, algae, and certain vegetables.
- Phenolic acids: Found in coffee, tea, cinnamon, blueberries, kiwis, plums, apples, and cherries and have two subgroups, namely hydroxybenzoic acids, and hydroxycinnamic acids.
1.2. Role of Polyphenols in Plants and Humans
2. Black Soybeans
Nutritional Importance of BSB
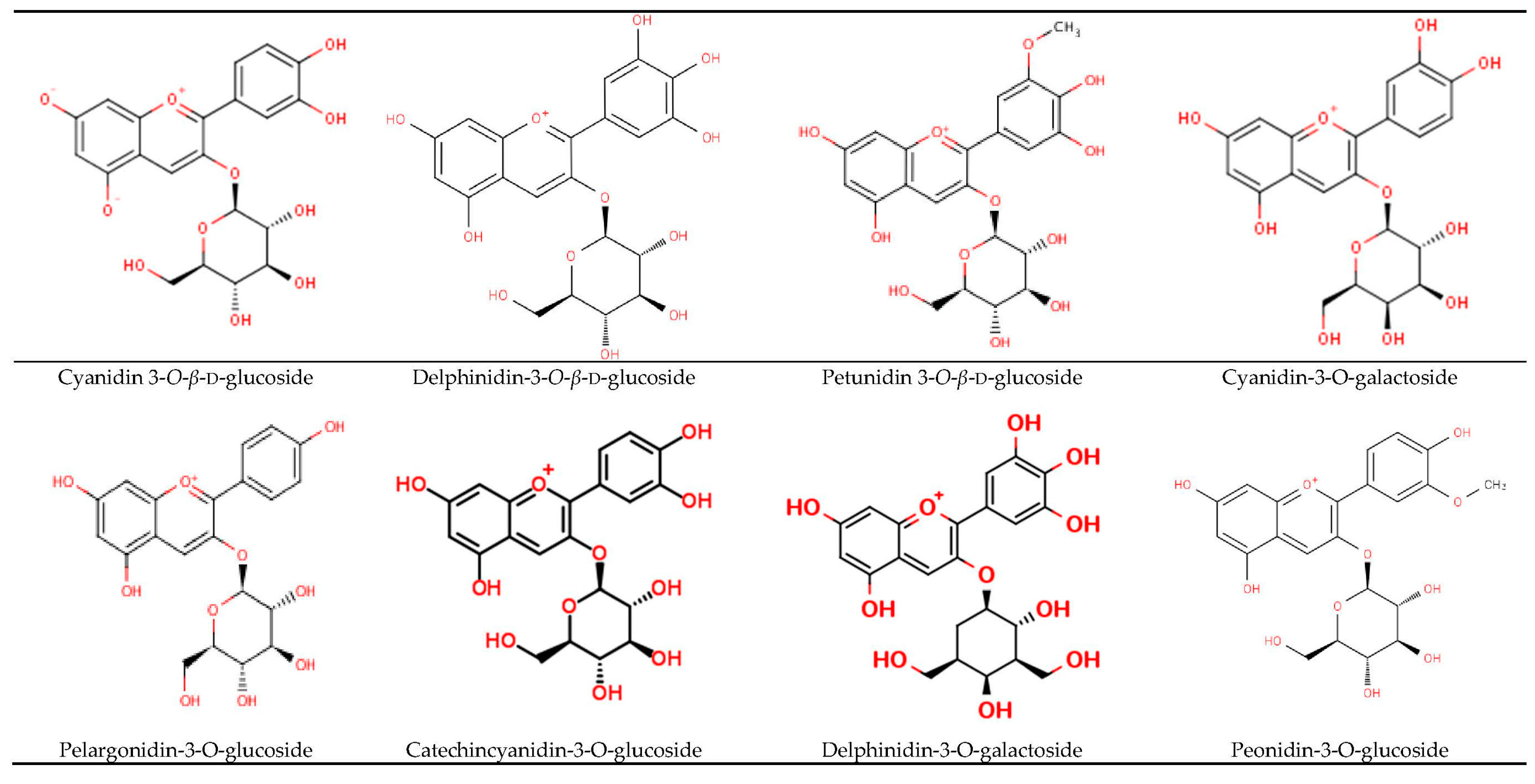
3. Anthocyanins Rich BSB
4. Health Benefits of Anthocyanins Rich BSB
4.1. Enhance Bone Stability
4.2. Reduce Blood Pressure
4.3. Reduce Cardiovascular Complications
4.4. In Managing Diabetes
4.5. Cancer Prevention
4.6. Reduce Body Weight
4.7. Antimicrobial Actions
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Sun, L.M. Dietary intake of flavonoid subclasses and risk of colorectal cancer: Evidence from population studies. Oncotarget 2016, 7, 26617–26627. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Godos, J.; Lamuela-Raventos, R.; Ray, S.; Micek, A.; Pajak, A.; Sciacca, S.; D’Orazio, N.; Del Rio, D.; Galvano, F. A comprehensive meta-analysis on dietary flavonoid and lignan intake and cancer risk: Level of evidence and limitations. Mol. Nutr. Food Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Zhan, J.; Liu, X.L.; Wang, Y.; Ji, J.; He, Q.Q. Dietary flavonoids intake and risk of type 2 diabetes: A meta-analysis of prospective cohort studies. Clin. Nutr. 2014, 33, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.M.; Liu, Y.J.; Huang, Y.; Yu, H.J.; Yuan, S.; Tang, B.W.; Wang, P.G.; He, Q.Q. Dietary total flavonoids intake and risk of mortality from all causes and cardiovascular disease in the general population: A systematic review and meta-analysis of cohort studies. Mol. Nutr. Food Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [PubMed]
- Omodanisi, E.I.; Aboua, Y.G.; Oguntibeju, O.O. Assessment of the anti-hyperglycaemic, anti-inflammatory and antioxidant activities of the methanol extract of Moringa oleifera in diabetes-induced nephrotoxic male wistar rats. Molecules 2017, 22, 439. [Google Scholar] [CrossRef] [PubMed]
- Venkata, K.C.; Bagchi, D.; Bishayee, A. A small plant with big benefits: Fenugreek (Trigonella foenum-graecum Linn.) for disease prevention and health promotion. Mol. Nutr. Food Res 2017. [Google Scholar] [CrossRef]
- Odongo, G.A.; Schlotz, N.; Herz, C.; Hanschen, F.S.; Baldermann, S.; Neugart, S.; Trierweiler, B.; Frommherz, L.; Franz, C.M.; Ngwene, B.; et al. The role of plant processing for the cancer preventive potential of Ethiopian kale (Brassica carinata). Food Nutr. Res. 2017, 61, 1271527. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wei, Z.; Zhang, S.; Peng, X.; Huang, Y.; Zhang, Y.; Lu, J. Phenolic fractions from muscadine grape “Noble” Pomace can inhibit breast cancer cell MDA-MB-231 Better than those from European grape “Cabernet Sauvignon” and induces-phase arrest and apoptosis. Food Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Franceschelli, S.; Pesce, M.; Ferrone, A.; Gatta, D.M.; Patruno, A.; Lutiis, M.A.; Quiles, J.L.; Grilli, A.; Felaco, M.; Speranza, L. Biological effect of licochalcone C on the regulation of PI3K/Akt/eNOS and NF-κB/iNOS/NO Signaling pathways in H9c2 cells in response to LPS stimulation. Int. J. Mol. Sci. 2017, 18, 690. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Khan, M.R.; Shah, S.A.; Majid, M.; Ismail, H.; Maryam, S.; Batool, R.; Younis, T. Investigations on anti-inflammatory and analgesic activities of Alnus nitida Spach (Endl). stem bark in sprague dawley rats. J. Ethnopharmacol. 2017, 198, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, P.R.; Figueiredo-González, M.; González-Barreiro, C.; Simal-Gándara, J.; Salvador, M.D.; Cancho-Grande, B.; Fregapane, G. State of the art on functional virgin olive oils enriched with bioactive compounds and their properties. Int. J. Mol. Sci. 2017, 18, 668. [Google Scholar]
- Léotoing, L.; Wauquier, F.; Davicco, M.J.; Lebecque, P.; Gaudout, D.; Rey, S.; Vitrac, X.; Massenat, L.; Rashidi, S.; Wittrant, Y.; et al. The phenolic acids of Agen prunes (dried plums) or Agen prune juice concentrates do not account for the protective action on bone in a rat model of postmenopausal osteoporosis. Nutr. Res. 2016, 36, 161–173. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Hao, D.; Zhang, Q.; Chen, B.; Zhang, R.; Wang, Y.; Yang, H. Natural products for treatment of bone-erosive diseases: The effects and mechanisms on inhibiting osteoclastogenesis and bone resorption. Int. Immunopharmacol. 2016, 36, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Ben Mansour, R.; Wided, M.K.; Cluzet, S.; Krisa, S.; Richard, T.; Ksouri, R. LC-MS identification and preparative HPLC isolation of Frankenia pulverulenta phenolics with antioxidant and neuroprotective capacities in PC12 cell line. Pharm. Biol. 2017, 55, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Sarrías, A.G.; Núñez-Sánchez, M.Á.; Tomás-Barberán, F.A.; Espín, J.C. Neurorotective effects of bioavailable polyphenol-derived metabolites against oxidative stress-induced cytotoxicity in human neuroblastoma SH-SY5Y Cells. J. Agric. Food Chem. 2017, 65, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Shaw, O.M.; Hurst, R.D.; Harper, J.L. Boysenberry ingestion supports fibrolytic macrophages with the capacity to ameliorate chronic lung remodeling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 311, L628–L638. [Google Scholar] [CrossRef] [PubMed]
- Gómez, C.I.G.; González-Laredo, R.F.; Gallegos-Infante, J.A.; Pérez, M.D.; Moreno-Jiménez, M.R.; Flores-Rueda, A.G.; Rocha-Guzmán, N.E. Antioxidant and angiotensin-converting enzyme inhibitory activity of Eucalyptus camaldulensis and Litsea glaucescens infusions fermented with kombucha consortium. Food Technol. Biotechnol. 2016, 54, 367–374. [Google Scholar]
- Nobile, V.; Michelotti, A.; Cestone, E.; Caturla, N.; Castillo, J.; Benavente-García, O.; Pérez-Sánchez, A.; Micol, V. Skin photoprotective and antiageing effects of a combination of rosemary (Rosmarinus officinalis) and grapefruit (Citrus paradisi) polyphenols. Food Nutr. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Le Sage, F.; Meilhac, O.; Gonthier, M.P. Anti-inflammatory and antioxidant effects of polyphenols extracted from Antirhea borbonica medicinal plant on adipocytes exposed to Porphyromonas gingivalis and Escherichia coli lipopolysaccharides. Pharmacol. Res. 2017, 119, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Forte, M.; Conti, V.; Damato, A.; Ambrosio, M.; Puca, A.A.; Sciarretta, S.; Frati, G.; Vecchione, C.; Carrizzo, A. Targeting nitric oxide with natural derived compounds as a therapeutic strategy in vascular diseases. Oxid. Med. Cell. Longev. 2016, 2016, 7364138. [Google Scholar] [CrossRef] [PubMed]
- Tenore, G.C.; Caruso, D.; Buonomo, G.; D’Avino, M.; Campiglia, P.; Marinelli, L.; Novellino, E. A healthy balance of plasma cholesterol by a novel Annurca apple-based nutraceutical formulation: Results of a randomized trial. J. Med. Food 2017, 20, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Ren, D.; Nie, Y.; Yang, X. Beneficial effects of apple peel polyphenols on vascular endothelial dysfunction and liver injury in high choline-fed mice. Food Funct. 2017, 8, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Ayub, M.A.; Hussain, A.I.; Hanif, M.A.; Chatha, S.A.; Kamal, G.M.; Shahid, M.; Janneh, O. Variation in phenolic profile, β-carotene and flavonoid contents, biological activities of two Tagetes species from Pakistani flora. Chem. Biodivers. 2017. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Zhang, X.; Ueyama, Y.; Apisada, K.; Nakayama, M.; Suzuki, Y.; Ozawa, T.; Mitani, A.; Shigemune, N.; Shimatani, K.; et al. Development of novel monoclonal antibodies directed against catechins for investigation of antibacterial mechanism of catechins. J. Microbiol. Methods 2017, 137, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.; Parvez, M.K.; Arbab, A.H.; Al-Dosari, M.S. Quantitative analysis of rutin, quercetin, naringenin, and gallic acid by validated RP- and NP-HPTLC methods for quality control of anti-HBV active extract of Guiera senegalensis. Pharm Biol. 2017, 55, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Kim, J.S.; Yoo, H.; Choung, M.G.; Sung, M.K. Effects of black soybean [Glycine max (L.) Merr.] seed coats and its anthocyanidins on colonic inflammation and cell proliferation in vitro and in vivo. J. Agric. Food Chem. 2008, 56, 8427–8433. [Google Scholar] [CrossRef] [PubMed]
- Choung, M.G.; Baek, I.Y.; Kang, S.T.; Han, W.Y.; Shin, D.C.; Moon, H.P.; Kang, K.H. Isolation and determination of anthocyanins in seed coats of black soybean (Glycine max (L.) Merr.). J. Agric. Food Chem. 2001, 49, 5848–5851. [Google Scholar] [CrossRef] [PubMed]
- Omoni, A.O.; Aluko, R.E. Soybean foods and their benefits: Potential mechanisms of action. Nutr. Rev. 2005, 63, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.W. Health effects of soy protein and isoflavones in humans. J. Nutr. 2008, 138, 1244S–1249S. [Google Scholar] [PubMed]
- Zhang, R.F.; Zhang, F.X.; Zhang, M.W.; Wei, Z.C.; Yang, C.Y.; Zhang, Y.; Tang, X.J.; Deng, Y.Y.; Chi, J.W. Phenolic composition and antioxidant activity in seed coats of 60 Chinese black soybean (Glycine max L. Merr.) varieties. J. Agric. Food Chem. 2011, 59, 5935–5944. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Ohmori, R.; Kiyose, C.; Momiyama, Y.; Ohsuzu, F.; Kondo, K. Antioxidant activities of black and yellow soybeans against low density lipoprotein oxidation. J. Agric. Food Chem. 2005, 53, 4578–4582. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chang, S.K. Antioxidant capacity of seed coat, dehulled bean, and whole black soybeans in relation to their distributions of total phenolics, phenolic acids, anthocyanins, and isoflavones. J. Agric. Food Chem. 2008, 56, 8365–8373. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Jung, W.S.; Chun, S.C.; Yu, C.Y.; Ma, K.H.; Gwag, J.G.; Chung, I.M. A correlation between the level of phenolic compounds and the antioxidant capacity in cooked-with-rice and vegetable soybean (Glycine max L.) varieties. Eur. Food Res. Technol. 2006, 224, 259–270. [Google Scholar] [CrossRef]
- Slavin, M.; Kenworthy, W.; Yu, L.L. Antioxidant properties, phytochemical composition, and antiproliferative activity of Maryland grown soybeans with colored seed coats. J. Agric. Food Chem. 2009, 57, 11174–11185. [Google Scholar] [CrossRef] [PubMed]
- Kusunoki, M.; Sato, D.; Tsutsumi, K.; Tsutsui, H.; Nakamura, T.; Oshida, Y. Black soybean extract improves lipid profiles in fenofibrate-treated type 2 diabetics with postprandial hyperlipidemia. J. Med Food. 2015, 18, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Zou, P. Traditional Chinese medicine, food therapy, and hypertension control: A narrative review of Chinese literature. Am. J. Chin. Med. 2016, 44, 1579–1594. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Chang, S.K.; Zhang, Y. Innovative soaking and grinding methods and cooking affect the retention of isoflavones, antioxidant and antiproliferative properties in soymilk prepared from black soybean. J. Food Sci. 2016, 81, H1016–H1023. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, T.; Villareal, M.O.; Motojima, H.; Isoda, H. Increasing cAMP levels of preadipocytes by cyanidin-3-glucoside treatment induces the formation of beige phenotypes in 3T3-L1 adipocytes. J. Nutr. Biochem. 2017, 40, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Ensminger, M.E.; Oldfield, J.E.; Heinemann, W.W. Feeds and Nutrition; The Ensminger Publishing Company: Clovis, CA, USA, 1990. [Google Scholar]
- Fetriyuna, F. The potential of darmo black soybean varieties as an alternative of a promising food for future. Int. J. Adv. Sci. Eng. Inf. Technol. 2015, 5, 44–46. [Google Scholar] [CrossRef]
- National Research Council (NRC). Nutrient Requirements of Swine, 10th ed.; National Academy Press: Washington, DC, USA, 1998. [Google Scholar]
- Katsuzaki, H.; Hibasami, H.; Ohwaki, S.; Ishikawa, K.; Imai, K.; Date, K.; Kimura, Y.; Komiya, T. Cyanidin-3-O-beta-d-glucoside isolated from skin of black Glycine max and other anthocyanins isolated from skin of red grape induce apoptosis in human lymphoid leukemia Molt 4B cells. Oncol. Rep. 2003, 10, 297–300. [Google Scholar] [PubMed]
- Lee, J.H.; Kang, N.S.; Shin, S.-O.; Shin, S.-H.; Lim, S.-G.; Suh, D.-Y.; Baek, I.-Y.; Park, K.-Y.; Ha, T.J. Characterisation of anthocyanins in the black soybean (Glycine max L.) by HPLC-DAD-ESI/MS analysis. Food Chem. 2009, 112, 226–231. [Google Scholar] [CrossRef]
- Koh, K.; Youn, J.E.; Kim, H.S. Identification of anthocyanins in black soybean (Glycine max (L.) Merr.) varieties. J. Food Sci. Technol. 2014, 51, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Fukami, H.; Yano, Y.; Iwashita, T. Isolation of a reduced form of cyanidin 3-O-β-d-glucoside from immature black soybean (Glycine max (L.) Merr.) and its reducing properties. J. Oleo Sci. 2013, 62, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Ali, T.; Kim, M.W.; Jo, M.H.; Jo, M.G.; Badshah, H.; Kim, M.O. Anthocyanins protect against LPS-induced oxidative stress-mediated neuroinflammation and neurodegeneration in the adult mouse cortex. Neurochem. Int. 2016, 100, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Sorn, S.R.; Park, Y.; Park, H.K. Anthocyanin rich-black soybean testa improved visceral fat and plasma lipid profiles in overweight/obese Korean adults: A randomized controlled trial. J. Med. Food 2016, 19, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Min, H.K.; Kim, S.M.; Baek, S.Y.; Woo, J.W.; Park, J.S.; Cho, M.L.; Lee, J.; Kwok, S.K.; Kim, S.W.; Park, S.H. Anthocyanin extracted from black soybean seed coats prevents autoimmune arthritis by suppressing the development of Th17 Cells and synthesis of proinflammatory cytokines by such cells, via inhibition of NF-κB. PLoS ONE 2015, 10, e0138201. [Google Scholar] [CrossRef] [PubMed]
- Park, M.Y.; Kim, J.M.; Kim, J.S.; Choung, M.G.; Sung, M.K. Chemopreventive action of anthocyanin-rich black soybean fraction in APC (Min/+) intestinal polyposis Model. J. Cancer Prev. 2015, 20, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Cooke, D.; Schwarz, M.; Boocock, D.; Winterhalter, P.; Steward, W.P.; Gescher, A.J.; Marczylo, T.H. Effect of cyanidin-3-glucoside and an anthocyanin mixture from bilberry on adenoma development in the ApcMin mouse model of intestinal carcinogenesis--relationship with tissue anthocyanin levels. Int. J. Cancer 2006, 119, 2213–2220. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, N.; Oki, T.; Sasaki, K.; Suda, I.; Okuno, S. Black soybean seed coat extract prevents hydrogen peroxide-mediated cell death via extracellular signal-related kinase signalling in HepG2 cells. J. Nutr. Sci. Vitaminol. 2015, 61, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Chung, M.J.; Ha, T.J.; Choi, H.N.; Jang, S.J.; Kim, S.O.; Chun, M.H.; Do, S.I.; Choo, Y.K.; Park, Y.I. Neuroprotective effects of black soybean anthocyanins via inactivation of ASK1-JNK/p38 pathways and mobilization of cellular sialic acids. Life Sci. 2012, 90, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Jiang, S.; He, C.; Kimura, Y.; Yamashita, Y.; Ashida, H. Black soybean seed coat polyphenols prevent B(a)P-induced DNA damage through modulating drug-metabolizing enzymes in HepG2 cells and ICR mice. Mutat. Res. 2013, 752, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Kim, H.J.; Park, S.K.; Jin, D.E.; Kwon, O.J.; Kim, H.J.; Heo, H.J. An investigation into the ameliorating effect of black soybean extract on learning and memory impairment with assessment of neuroprotective effects. BMC Complement. Altern. Med. 2014, 14, 482. [Google Scholar] [CrossRef] [PubMed]
- Mok, J.W.; Chang, D.J.; Joo, C.K. Antiapoptotic effects of anthocyanin from the seed coat of black soybean against oxidative damage of human lens epithelial cell induced by H2O2. Curr. Eye Res. 2014, 39, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, M.I.; Kim, J.Y.; Ha, T.J.; Kim, S.Y.; Cho, K.O. Anthocyanins extracted from black soybean seed coat protect primary cortical neurons against in vitro ischemia. Biol. Pharm. Bull. 2012, 35, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, T.; Inaguma, T.; Han, J.; Villareal, M.O.; Isoda, H. Cyanidin-3-glucoside derived from black soybeans ameliorate type 2 diabetes through the induction of differentiation of preadipocytes into smaller and insulin-sensitive adipocytes. J. Nutr. Biochem. 2015, 26, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Kim, J.N.; Han, S.N.; Nam, J.H.; Na, H.N.; Ha, T.J. Black soybean anthocyanins inhibit adipocyte differentiation in 3T3-L1 cells. Nutr. Res. 2012, 32, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Badshah, H.; Kim, T.H.; Kim, M.O. Protective effects of anthocyanins against amyloid beta-induced neurotoxicity in vivo and in vitro. Neurochem. Int. 2015, 80, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Ali Shah, S.; Ullah, I.; Lee, H.Y.; Kim, M.O. Anthocyanins protect against ethanol-induced neuronal apoptosis via GABAB1 receptors intracellular signaling in prenatal rat hippocampal neurons. Mol. Neurobiol. 2013, 48, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Yoon, G.H.; Kim, M.O. Protection of the developing brain with anthocyanins against ethanol-induced oxidative stress and neurodegeneration. Mol. Neurobiol. 2015, 51, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Park, H.Y.; Kim, M.O. Anthocyanins protect against kainic acid-induced excitotoxicity and apoptosis via ROS-activated AMPK pathway in hippocampal neurons. CNS Neurosci. Ther. 2014, 20, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Ha, U.S.; Bae, W.J.; Kim, S.J.; Yoon, B.I.; Hong, S.H.; Lee, J.Y.; Hwang, T.K.; Hwang, S.Y.; Wang, Z.; Kim, S.W. Anthocyanin induces apoptosis of DU-145 cells in vitro and inhibits xenograft growth of prostate cancer. Yonsei Med. J. 2015, 56, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Badshah, H.; Ali, T.; Ahmad, A.; Kim, M.J.; Abid, N.B.; Shah, S.A.; Yoon, G.H.; Lee, H.Y.; Kim, M.O. Co-treatment with anthocyanins and vitamin-C ameliorates ethanol-induced neurodegeneration via modulation of gabab receptor signaling in the adult rat brain. CNS Neurol. Disord. Drug Target. 2015, 14, 791–803. [Google Scholar] [CrossRef]
- Sohn, D.W.; Bae, W.J.; Kim, H.S.; Kim, S.W.; Kim, S.W. The anti-inflammatory and antifibrosis effects of anthocyanin extracted from black soybean on a Peyronie disease rat model. Urology 2014, 84, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- De Moraes Filho, M.L.; Hirozawa, S.S.; Prudencio, S.H.; Ida, E.I.; Garcia, S. Petit suisse from black soybean: Bioactive compounds and antioxidant properties during development process. Int. J. Food Sci. Nutr. 2014, 65, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Jo, Y.N.; Kim, H.J.; Jin, D.E.; Kim, D.O.; Heo, H.J. Black soybean extract protects against TMT-induced cognitive defects in mice. J. Med. Food 2014, 17, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.I.; Bae, W.J.; Choi, Y.S.; Kim, S.J.; Ha, U.S.; Hong, S.H.; Sohn, D.W.; Kim, S.W. The anti-inflammatory and antimicrobial effects of anthocyanin extracted from black soybean on chronic bacterial prostatitis rat model. Chin. J. Integr. Med. 2013. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.K.; Lin, W.H.; Yang, H.W. Influence of preheating on antioxidant activity of the water extract from black soybean and color and sensory properties of black soybean decoction. J. Sci. Food Agric. 2013, 93, 3883–3890. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Chou, C.C. Effect of heat treatment on total phenolic and anthocyanin contents as well as antioxidant activity of the extract from Aspergillus awamori-fermented black soybeans, a healthy food ingredient. Int. J. Food Sci. Nutr. 2009, 60, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Malencić, D.; Maksimović, Z.; Popović, M.; Miladinović, J. Polyphenol contents and antioxidant activity of soybean seed extracts. Bioresour. Technol. 2008, 99, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chang, S.K. Total phenolics, phenolic acids, isoflavones, and anthocyanins and antioxidant properties of yellow and black soybeans as affected by thermal processing. J. Agric. Food Chem. 2008, 56, 7165–7175. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chang, S.K. Characterization of phenolic substances and antioxidant properties of food soybeans grown in the North Dakota-Minnesota region. J. Agric. Food Chem. 2008, 56, 9102–9113. [Google Scholar] [CrossRef] [PubMed]
- Badshah, H.; Ullah, I.; Kim, S.E.; Kim, T.H.; Lee, H.Y.; Kim, M.O. Anthocyanins attenuate body weight gain via modulating neuropeptide Y and GABAB1 receptor in rats hypothalamus. Neuropeptides 2013, 47, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.H.; Ahn, I.S.; Kim, S.O.; Kong, C.S.; Chung, H.Y.; Do, M.S.; Park, K.Y. Anti-obesity and hypolipidemic effects of black soybean anthocyanins. J. Med. Food 2007, 10, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.G.; Kang, Y.R.; Lee, H.Y.; Kim, J.H.; Shin, E.H.; Lee, B.G.; Park, S.H.; Moon, D.I.; Kim, O.J.; Lee, I.A.; et al. Ameliorative effects of Monascus pilosus-fermented black soybean (Glycine max L. Merrill) on high-fat diet-induced obesity. J. Med. Food 2014, 17, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Kurimoto, Y.; Shibayama, Y.; Inoue, S.; Soga, M.; Takikawa, M.; Ito, C.; Nanba, F.; Yoshida, T.; Yamashita, Y.; Ashida, H.; et al. Black soybean seed coat extract ameliorates hyperglycemia and insulin sensitivity via the activation of AMP-activated protein kinase in diabetic mice. J. Agric. Food Chem. 2013, 61, 5558–5564. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Kim, K.M.; Park, E.H.; Seo, J.H.; Song, J.Y.; Shin, S.C.; Kang, H.L.; Lee, W.K.; Cho, M.J.; Rhee, K.H.; et al. Anthocyanins from black soybean inhibit Helicobacter pylori-induced inflammation in human gastric epithelial AGS cells. Microbiol. Immunol. 2013, 57, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Choi, T.H.; Kim, S.; Kim, S.H.; Chang, H.W.; Choe, M.; Kwon, S.Y.; Hur, J.A.; Shin, S.C.; Chung, J.I.; et al. Anthocyanins from black soybean seed coat enhance wound healing. Ann. Plast. Surg. 2013, 71, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Nizamutdinova, I.T.; Kim, Y.M.; Chung, J.I.; Shin, S.C.; Jeong, Y.K.; Seo, H.G.; Lee, J.H.; Chang, K.C.; Kim, H.J. Anthocyanins from black soybean seed coats stimulate wound healing in fibroblasts and keratinocytes and prevent inflammation in endothelial cells. Food Chem. Toxicol. 2009, 47, 2806–2812. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Xu, L.; Chang, K.C.; Shin, S.C.; Chung, J.I.; Kang, D.; Kim, S.H.; Hur, J.A.; Choi, T.H.; Kim, S.; et al. Anti-inflammatory effects of anthocyanins from black soybean seed coat on the keratinocytes and ischemia-reperfusion injury in rat skin flaps. Microsurgery 2012, 32, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Tsoy, I.; Park, J.M.; Chung, J.I.; Shin, S.C.; Chang, K.C. Anthocyanins from soybean seed coat inhibit the expression of TNF-alpha-induced genes associated with ischemia/reperfusion in endothelial cell by NF-kappaB-dependent pathway and reduce rat myocardial damages incurred by ischemia and reperfusion in vivo. FEBS Lett. 2006, 580, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.W.; Lee, W.S.; Shin, S.C.; Kim, G.Y.; Choi, B.T.; Choi, Y.H. Anthocyanins down-regulate lipopolysaccharide-induced inflammatory responses in BV2 microglial cells by suppressing the NF-κB and Akt/MAPKs signaling pathways. Int. J. Mol. Sci. 2013, 14, 1502–1515. [Google Scholar] [CrossRef] [PubMed]
- Choe, Y.J.; Ha, T.J.; Ko, K.W.; Lee, S.Y.; Shin, S.J.; Kim, H.S. Anthocyanins in the black soybean (Glycine max L.) protect U2OS cells from apoptosis by inducing autophagy via the activation of adenosyl monophosphate-dependent protein kinase. Oncol. Rep. 2012, 28, 2049–2056. [Google Scholar] [PubMed]
- Jang, H.; Kim, S.J.; Yuk, S.M.; Han, D.S.; Ha, U.S.; Hong, S.H.; Lee, J.Y.; Hwang, T.K.; Hwang, S.Y.; Kim, S.W. Effects of anthocyanin extracted from black soybean seed coat on spermatogenesis in a rat varicocele-induced model. Reprod. Fertil. Dev. 2012, 24, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Ha, U.S.; Kim, S.J.; Yoon, B.I.; Han, D.S.; Yuk, S.M.; Kim, S.W. Anthocyanin extracted from black soybean reduces prostate weight and promotes apoptosis in the prostatic hyperplasia-induced rat model. J. Agric. Food Chem. 2010, 58, 12686–12691. [Google Scholar] [CrossRef] [PubMed]
- Paik, S.S.; Jeong, E.; Jung, S.W.; Ha, T.J.; Kang, S.; Sim, S.; Jeon, J.H.; Chun, M.H.; Kim, I.B. Anthocyanins from the seed coat of black soybean reduce retinal degeneration induced by N-methyl-N-nitrosourea. Exp. Eye Res. 2012, 97, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Wu, P.S.; Liang, D.W.; Kwan, C.C.; Chen, Y.S. Quality, antioxidative ability, and cell proliferation-enhancing activity of fermented black soybean broths with various supplemental culture medium. J. Food Sci. 2012, 77, C95–C101. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Yoon, H.H.; Lee, Y.D.; Youn, D.Y.; Ha, T.J.; Kim, H.S.; Lee, J.H. Anthocyanin extracts from black soybean (Glycine max L.) protect human glial cells against oxygen-glucose deprivation by promoting autophagy. Biomol. Ther. (Seoul) 2012, 20, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Iqbal, M.; Kawakami, N.; Yamamoto, Y.; Toyokuni, S.; Okada, S. A beverage containing fermented black soybean ameliorates ferric nitrilotriacetate-induced renal oxidative damage in rats. J. Clin. Biochem. Nutr. 2010, 47, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Juan, M.Y.; Wu, C.H.; Chou, C.C. Fermentation with Bacillus spp. as a bioprocess to enhance anthocyanin content, the angiotensin converting enzyme inhibitory effect, and the reducing activity of black soybeans. Food Microbiol. 2010, 27, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.H.; Huang, H.Y.; Chou, C.C. Mutagenic and antimutagenic effects of methanol extracts of unfermented and fermented black soybeans. Int. J. Food Microbiol. 2007, 118, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Juan, M.Y.; Chou, C.C. Enhancement of antioxidant activity, total phenolic and flavonoid content of black soybeans by solid state fermentation with Bacillus subtilis BCRC 14715. Food Microbiol. 2010, 27, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Tsoyi, K.; Park, H.B.; Kim, Y.M.; Chung, J.I.; Shin, S.C.; Lee, W.S.; Seo, H.G.; Lee, J.H.; Chang, K.C.; Kim, H.J. Anthocyanins from black soybean seed coats inhibit UVB-induced inflammatory cylooxygenase-2 gene expression and PGE2 production through regulation of the nuclear factor-kappaB and phosphatidylinositol 3-kinase/Akt pathway. J. Agric. Food Chem. 2008, 56, 8969–8974. [Google Scholar] [CrossRef] [PubMed]
- Tsoyi, K.; Park, H.B.; Kim, Y.M.; Chung, J.I.; Shin, S.C.; Shim, H.J.; Lee, W.S.; Seo, H.G.; Lee, J.H.; Chang, K.C.; et al. Protective effect of anthocyanins from black soybean seed coats on UVB-induced apoptotic cell death in vitro and in vivo. J. Agric. Food Chem. 2008, 56, 10600–10605. [Google Scholar] [CrossRef] [PubMed]
- Yamai, M.; Tsumura, K.; Kimura, M.; Fukuda, S.; Murakami, T.; Kimura, Y. Antiviral activity of a hot water extract of black soybean against a human respiratory illness virus. Biosci. Biotechnol. Biochem. 2003, 67, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.C.; Woo, J.; Lam, S.; Chen, Y.; Sham, A.; Lau, J. Soy protein consumption and bone mass in early postmenopausal Chinese women. Osteoporosis Int. 2003, 14, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Hirata, H.; Kitamura, K.; Saito, T.; Kobayashi, R.; Iwasaki, M.; Yoshihara, A.; Watanabe, Y.; Oshiki, R.; Nishiwaki, T.; Nakamura, K. Association between dietary intake and bone mineral density in Japanese postmenopausal women: The Yokogoshi cohort study. Tohoku J. Exp. Med. 2016, 239, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.S.; Lee, S.S. Effect of soybeans and sword beans on bone metabolism in a rat model of osteoporosis. Ann. Nutr. Metab. 2010, 56, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Marini, H.; Minutoli, L.; Polito, F.; Bitto, A.; Altavilla, D.; Atteritano, M.; Gaudio, A.; Mazzaferro, S.; Frisina, A.; Frisina, N.; et al. Effects of the phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women: A randomized trial. Ann. Intern. Med. 2007, 146, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cohn, J.S.; Harvey, I.; Le Cornu, K.A.; Ryder, J.J.; Hall, W.L.; Cassidy, A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2008, 88, 38–50. [Google Scholar] [PubMed]
- Kim, K.; Lim, K.M.; Kim, C.W.; Shin, H.J.; Seo, D.B.; Lee, S.J.; Noh, J.Y.; Bae, O.N.; Shin, S.; Chung, J.H. Black soybean extract can attenuate thrombosis through inhibition of collagen-induced platelet activation. J. Nutr. Biochem. 2011, 22, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.S.; Han, Y.S.; Lee, S.S. The effects of yellow soybean, black soybean, and sword bean on lipid levels and oxidative stress in ovariectomized rats. Int. J. Vitam. Nutr. Res. 2010, 80, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.L.; Francini, F.; Schinella, G.R. Natural Products for the Treatment of Type 2 Diabetes Mellitus. Planta Med. 2015, 81, 975–994. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.H.; Lee, J.H.; Ahn, C.W.; Park, S.H.; Shim, S.T.; Song, Y.D.; Han, E.N.; Lee, K.H.; Chae, J.S. Black soy peptide supplementation improves glucose control in subjects with prediabetes and newly diagnosed type 2 diabetes mellitus. J. Med. Food 2010, 3, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Kanamoto, Y.; Yamashita, Y.; Nanba, F.; Yoshida, T.; Tsuda, T.; Fukuda, I.; Nakamura-Tsuruta, S.; Ashida, H. A black soybean seed coat extract prevents obesity and glucose intolerance by up-regulating uncoupling proteins and down-regulating inflammatory cytokines in high-fat diet-fed mice. J. Agric. Food Chem. 2011, 59, 8985–8993. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Y.; Liu, H.Y.; Huang, T.C.; Chen, J.H.; Chang, P.Y.; Ho, C.L.; Chao, T.Y. A phase II double-blinded study to evaluate the efficacy of EW02 in reducing chemotherapy-induced neutropenia in breast cancer. Oncol. Lett. 2015, 10, 1793–1798. [Google Scholar] [CrossRef] [PubMed]
- Chia, J.S.; Du, J.L.; Wu, M.S.; Hsu, W.B.; Chiang, C.P.; Sun, A.; Lu, J.J.; Wang, W.B. Fermentation product of soybean, black bean, and green bean mixture induces apoptosis in a wide variety of cancer cells. Integr. Cancer Ther. 2013, 12, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chang, S.K. Comparative study on antiproliferation properties and cellular antioxidant activities of commonly consumed food legumes against nine human cancer cell lines. Food Chem. 2012, 134, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Horn-Ross, P.L.; John, E.M.; Canchola, A.J.; Stewart, S.L.; Lee, M.M. Phytoestrogen intake and endometrial cancer risk. J. Natl. Cancer Inst. 2003, 95, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.J.; Ng, T.B. Antitumor and HIV-1 reverse transcriptase inhibitory activities of a hemagglutinin and a protease inhibitor from mini-black soybean. Evid. Based Complement. Altern. Med. 2011, 2011, 851396. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chang, S.K. Reduction of antiproliferative capacities, cell-based antioxidant capacities and phytochemical contents of common beans and soybeans upon thermal processing. Food Chem. 2011, 129, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Azaïs, H.; Frochot, C.; Grabarz, A.; Khodja Bach, S.; Colombeau, L.; Delhem, N.; Mordon, S.; Collinet, P. Specific folic-acid targeted photosensitizer. The first step toward intraperitoneal photodynamic therapy for epithelial ovarian cancer. Gynecol. Obstet. Fertil. Senol. 2017, 45, 190–196. [Google Scholar] [PubMed]
- Giusti, F.; Caprioli, G.; Ricciutelli, M.; Vittori, S.; Sagratini, G. Determination of fourteen polyphenols in pulses by high performance liquid chromatography-diode array detection (HPLC-DAD) and correlation study with antioxidant activity and colour. Food Chem. 2017, 221, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.; Lee, M.; Cheon, Y.P. A testa extract of black soybean (Glycine max (L.) Merr.) suppresses adipogenic activity of adipose-derived stem cells. Dev. Reprod. 2015, 19, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Sato, D.; Kusunoki, M.; Seino, N.; Nishina, A.; Feng, Z.; Tsutsumi, K.; Nakamura, T. Black soybean extract reduces fatty acid contents in subcutaneous, but not in visceral adipose triglyceride in high-fat fed rats. Int. J. Food Sci. Nutr. 2015, 66, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Abutheraa, R.; Hettiarachchy, N.; Kumar-Phillips, G.; Horax, R.; Chen, P.; Morawicki, R.; Kwon, Y.M. Antimicrobial activities of phenolic extracts derived from seed coats of selected soybean varieties. J. Food Sci. 2017, 82, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Ngai, P.H.; Ng, T.B. Purification of glysojanin, an antifungal protein, from the black soybean Glycine soja. Biochem. Cell Biol. 2003, 81, 387–394. [Google Scholar] [CrossRef] [PubMed]
| Model | Anthocyanin Rich BSB | Dose and Route of Administration | Negative Control | Investigation | Results | Reference |
|---|---|---|---|---|---|---|
| Mouse | Anthocyanin | 24 mg/kg/day PO | Lipopolysaccharide | Assay of phospho-c-JNK1, IL-1β, TNF-α, transcription factor NF-κB, GFAP, Iba-1, Bax, cytosolic cytochrome C, cleaved caspase-3 and PARP-1 | Neuroprotective activity | [48] |
| Obesity human | Anthocyanin-rich BSB testa extracts | 2.5 g/day PO | Obesity human | Assay of TG,LDL-C, non-HDL-C, TC/HDL-C and LDL-C/HDL-C | Anti-obesity | [49] |
| Mouse | Anthocyanin-rich BSB seed coat | 60 mg/kg/PO | Collagen induced arthritis | Assay of histological inflammation, cartilage scores, oxidative stress markers, pro-inflammatory cytokines and NF-κB signaling | Anti-arthritic activity | [50] |
| Apc (Min/+) mice | Anthocyanin-rich BSB seed coat | 0.2% or 0.5% /kg/PO | - | Assay of Number of intestinal tumors, and cellular expression of β-catenin | Anti-cancer activity | [51] |
| Apc(Min) mouse | Cyanidin-3-glucoside | 0.03%, 0.1% or 0.3% | - | Assay of Plasma, urine and intestinal mucosaanthocyaninswere determined by HPLC, UV spectrophotometry and tandem MS | Anti-cancer activity | [52] |
| Human hepatoma (HepG2) cells | BSB seed coats | 67 µg/mL | Hydrogen peroxide | Assay of ERK, intracellular total protein phosphatase activity | Anti-cancer activity | [53] |
| Human brain neuroblastoma SK-N-SH cells | Cyanidin-3-O-glucoside, Delphinidin-3-O-glucoside, and petunidin-3-O-glucoside | 67 µg/mL | Hydrogen peroxide | Assay of cell viability, ROS, expression of heme oxygenase (HO)-1 ,MAP kinase, ASK1-JNK/p38 pathways by MTT assay, DCF-DA assay, RT-PCR, and Western blotting | Anti-cancer activity | [54] |
| Humanhepatoma (HepG2) cells and ICR mice | BSB seed coats extracts | 25 μg/mL | Benzo[a]pyrene | Assay of cytochrome P4501A1 expression, Nrf2 to antioxidant response elements | Anti-cancer activity | [55] |
| Ratpheochromocytoma (PC12 cell line) | non-anthocyanin fraction | 3, 6, 12, and 25 μg/mL | Amyloid β peptide | Assay of cellular oxidative stress by using DCF-DA, MTT, LDH, MDA level, acetylcholinesterase activity | Anti-amnesic effect | [56] |
| Human lens epithelial cell line (HLE-B3) | BSB seed coats extracts | 0, 50, 100 and 200 μg/mL | Hydrogen peroxide | Assay of apoptosis by Annexin V assay and APO-BrdU TUNEL assay; Western blot and immunostaining of apoptosis-related molecules; Bcl2, Bax, p53 and caspase-3. | Anti- cataract effect | [57] |
| Rat primary cortical neuron cells | BSB (cv. Cheongja 3, Glycine max (L.) MERR.) seed coat | 50 mg/mL | Glutamate | Assay of LDH, MTT, Intracellular ROS and immunofluorescence | Neuroprotective effect | [58] |
| 3T3-Ll cells db/db mice | Anthocyanin cyanidin-3-glucoside | 60 mg/kg/PO | - | Assay of PPARγ and C/EBPα gene expressions, TNF-α, PGC-1α, SIRT1 and UCP-3 | Antiobesity and antidiabetic effects | [59] |
| 3T3-Ll cells db/db mice | Anthocyanin cyanidin-3-glucoside | 12.5 and 50 μg/mL | - | Assay of MTT, expression of the peroxisome proliferator-activated receptor γ and measurement of lipolysis | Antiobesity and antidiabetic effects | [60] |
| Wistar albino rats | Anthocyanin cyanidin-3-glucoside | Anthocyanins (24 mg/kg) along with and vitC (100 mg/kg) | 10% (v/v) ethanol | Assay of MTT, expression of GABAB1 receptor,Bax/Bcl-2 ratio, release of cytochrome C and activation of caspase-3 and caspase-9 | Neuroprotective effect | [61] |
| Wistar albino rats | Anthocyanin cyanidin-3-glucoside | Anthocyanins (24 mg/kg) along with and vitamin c (100 mg/kg) | 10% (v/v) ethanol | Assay of GABAB1 receptor, cellular levels of proapoptotic proteins such as Bax, activated caspase-3, and cleaved poly (ADP-ribose) polymerase 1 (PARP-1) intracellular free Ca (2+) level and CaMKII | Neuroprotective effect | [62] |
| Wistar albino rats | Anthocyanin cyanidin-3-glucoside | Anthocyanins (24 mg/kg) along with and vitamin c (100 mg/kg) | 10% (v/v) ethanol | Assay of expression of glutamate receptors, intracellular signaling molecules, and various synaptic, inflammatory, and apoptotic markers | Neuroprotective effect | [63] |
| Mouse hippocampal cell line (HT22) and primary prenatal rat hippocampal neurons | Anthocyanin cyanidin-3-glucoside | 12.5 and 50 μg/mL | Kainic acid | intracellular Ca2+ level, ROS, AMPK, Bcl-2, cytochrome-c, and caspase-3 | Antioxidant activity | [64] |
| Human | BSB seed coat | 60 mg/kg/PO | STZ | Assay of glycemic control and lipid metabolism parameters | Anti-hyperlipidemic effect | [37] |
| in vitro (prostate cancer- DU-145 cells) and in vivo (in athymic nude mouse xenograft model) | Anthocyanin | 8 mg/kg | - | Assay of MTT, p53, Bax, Bcl, androgen receptor (AR), and prostate specific antigen | Anti-cancer activity | [65] |
| HT22 cell lines and adult wister male rats | Anthocyanin | 0.2 mg/kg | Amyloid beta 1-42 | Assay of MTT, mitochondrial membrane potential, intracellular free Ca2+ and apoptotic cells (fluoro-jade B and TUNEL),Western blot analyses were performed | Neuroprotective effect | [66] |
| Wistar albino rats | Anthocyanin | 50 mg/kg/PO | Human fibrin and thrombin solutions | Assay of Masson trichrome and transforming growth factor | Anti-inflammatory and antifibrosis effects | [67] |
| In vitro | BSB seed coat | 388 mg/100 g | - | Assay of DPPH and ABTS+ | Antioxidant properties | [68] |
| Wistar albino rats and rat pheochromocytoma PC12 cell line | Non-anthocyanins | 10, 20 mg/kg/PO | H2O2 and trimethyltin | Assay of MTT, LDH, AChE in vitro inhibition, Y-maze test, Passive avoidance test and MDA levels | Beneficial for neurodegenerative disorders | [69] |
| Sprague-Dawley rats | BSB | 10, 20 mg/kg PO | ciprofloxacin, | Assay of prostate tissue, urine culture, and histological analysis | Anti-inflammatory and antimicrobial effects | [70] |
| In vitro | Black soybean tea | 10, 20 mg/kg PO | - | Assay of DPPH, ferrous ion chelating ability and reducing power | Antioxidant activity | [71] |
| In vitro | Aspergillus awamori-fermented BSB | 10, 20 mg/kg PO | - | Assay of DPPH, ferrous ion chelating ability and reducing power | Antioxidant activity | [72] |
| In vitro | 20 soybean hybrids | 10, 20 mg/kg PO | - | Assay of DPPH | Antioxidant activity | [73] |
| In vitro | BSB hybrids | 10, 20 mg/kg PO | - | Assay of DPPH, ferric reducing antioxidant power, oxygen radical absorbance capacity | Antioxidant activity | [34] |
| In vitro | BSB hybrids | 10, 20 mg/kg PO | - | Assay of total phenolic content, total flavonoid content, condensed tannin content, monomeric anthocyanin content, DPPH free radical scavenging activity, ferric reducing antioxidant power, and oxygen radical absorbing capacity | Antioxidant activity | [74] |
| In vitro | 30 BSB hybrids | 10, 20 mg/kg PO | - | Assay of total phenolic content, total flavonoid content, condensed tannin content, monomeric anthocyanin content, DPPH free radical scavenging activity, ferric reducing antioxidant power, and oxygen radical absorbing capacity | Antioxidant activity | [75] |
| Male Sprague-Dawley rats | Anthocyanins | 6 mg/kg and 24 mg/kg PO | - | Assay of body weight and daily food intake, neuropeptide Y, GABAB1 receptor, protein kinase A-α, and phosphorylated cAMP-response element binding protein | Hypolipidemic and anti-obesity effects | [76] |
| Wistar albino rats | BSB seed coats | 0.037%/PO | High fat diet—16% lard oil | Assay of body weight, adipose tissue weight, and serum lipids | Anti-obesity effect | [77] |
| C57BL/6 mice | Monascus pilosus-fermented BSB | 0.5 and 1.0 g/kg/PO | High fat diet—16% lard oil | Assay of blood glucose, TC, leptin and measurement of epididymal, retroperitoneal, and perirenal fat pads | Anti-obesity effect | [78] |
| Male KK-Aydiabetic mice and L6 myotubes | BSB seed coat extract | 22.0 g of BE/kg diet/PO | - | Assay of blood glucose, insulin, AMP-activated protein kinase, glucose transporter 4 | Anti-diabetic effects | [79] |
| Gastric adenocarcinoma, ATCC CRL 1739 | Anthocyanin | 50 µg/mL | - | Assay of cell viability, ROS, Western blot analyses, RT-PCR were performed to assess gene and protein expression | Anti-oxidative, antibacterial and anti-inflammatory effects | [80] |
| Immortalized epidermal keratinocyte cell line (HaCaT) and human neonatal dermal fibroblasts | Anthocyanin | 50 µg/mL | H2O2 | Assay of tissue VEGF, TSP1, CD31, NF-κB, and phosphorylation of IκBα | Wound healing properties | [81] |
| Human dermal fibroblasts and keratinocytes cell lines | BSB seed coat extracts | 100 µg/mL | - | Assay of TNF-alpha, NF-kB, p65, VEGF in in fibroblasts and keratinocytes | Anti-inflammatory effects | [82] |
| Wistar albino rats | BSB seed coat extracts | 50 and 100 mg/kg/PO | - | Assay of TNF-alpha, ICAM, NF-kB,cyclooxygenase-2, VEGF in in fibroblasts and keratinocytes | Anti-inflammatory properties against ischemia-reperfusion injury | [83] |
| Bovine aortic endothelial cells and male Sprague-Dawley rats | Anthocyanin BSB seed coat | 25, 50 and 100 mg/kg/PO | LAD occlusion and reperfusion | Assay of MTT, Luciferase, TNF-alpha, ICAM, NF-kB, cyclooxygenase-2, vascular endothelial growth factor | Cardioprotective effect | [84] |
| Murine BV2 microglial cells | Anthocyanin BSB seed coat | 100 µg/mL | Lipopolysaccharides | Assay of NO, prostaglandin E(2), and pro-inflammatory cytokines, including TNF-α IL-1β, NO synthase, cyclooxygenase-2, NF-κB, ERK, c-JNK, p38 MAP kinase, and Akt. | Anti-inflammatory and potent neurodegenerative diseases | [85] |
| U2OS cells | Anthocyanin BSB seed coat | 200 μg/mL | - | Assay of extracellular signal-regulated kinase 1/2, p38 mitogen-activated protein kinase, c-Jun N-terminal kinase, protein kinase B and adenosyl mono-phosphate-dependent protein kinase | Anticancer effects | [86] |
| Wistar albino rats | Anthocyanin | 40 or 80 mg/kg PO | Varicocele-induced rats | Histological examination and semen analysis | Anti-infertility effects | [87] |
| Wistar albino rats | Anthocyanin | 40, 80, and 160 mg/kg PO | benign prostatic hyperplasia-induced rats | Assay of apoptosis in the prostates by the TUNEL assay | Anti-infertility effects | [88] |
| Wistar albino rats | Anthocyanin BSB seed coat | 50 mg/kg PO | N-methyl-N-nitrosourea | Electro-retinographic recordings and morphological analyses | Anti-blindness | [89] |
| Detroit 551 cells | fermented BSB broth | 200 μg/mL | - | Assay of DPPH radical scavenging effect, reducing power and ferrous ion chelating effect. | Antioxidant effect | [90] |
| Human U87 glioma cells | Anthocyanin BSB seed coat | 100 μg/mL | - | Assay of autophagy, Atg5 expression | Anti- stroke effect | [91] |
| Wistar albino rats | citric acid fermented of BSB | 10 mL/kg | Ferricnitrilotriacetate | Assay of antioxidative enzymes including catalase, glutathione peroxidase, glutathione reductase, glutathione S-transferase, glucose-6-phosphate dehydrogenase, quinone reductase, serum creatinine and urea nitrogen | Anti- renal tubular oxidative damage | [92] |
| In vitro | BSB fermented with either Bacillus subtilis BCRC 14715 or Bacillus sp. CN11 | 2, 4, 6 mL | - | Assay of ACE inhibitory activity and the reducing power of the fermented BSB | Antioxidant activity | [93] |
| In vitro | solid fermentation of steamed BSB | 100 μg/mL | 4-nitroquinoline-N-oxide and Benzo[a]pyrene | Assay of mutagenicity | Mutagenicity and antimutagenicity effects | [94] |
| In vitro | BSB with Bacillus subtilis BCRC 14715 | 100 μg/mL | water, 80% methanol, 80% ethanol, 80% acetone | Assay of DPPH radical-scavenging effect, and Fe2+-chelating activity | Antioxidant activity | [95] |
| Wistar albino rats and In vitro | Anthocyanin BSB seed coat | 100 μg/mL | UVB-induced apoptotic cell death | Assay of caspase-3, Bax, NF-κB, cylooxygenase-2 | Anti-skin cancer | [96,97] |
| In vitro | Hot water extracts of BSB | 100 μg/mL | Human adenovirus type 1 and coxsackievirus B1 | WST assay and in vitro antiviral assay | Antiviral activity | [98] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganesan, K.; Xu, B. A Critical Review on Polyphenols and Health Benefits of Black Soybeans. Nutrients 2017, 9, 455. https://doi.org/10.3390/nu9050455
Ganesan K, Xu B. A Critical Review on Polyphenols and Health Benefits of Black Soybeans. Nutrients. 2017; 9(5):455. https://doi.org/10.3390/nu9050455
Chicago/Turabian StyleGanesan, Kumar, and Baojun Xu. 2017. "A Critical Review on Polyphenols and Health Benefits of Black Soybeans" Nutrients 9, no. 5: 455. https://doi.org/10.3390/nu9050455
APA StyleGanesan, K., & Xu, B. (2017). A Critical Review on Polyphenols and Health Benefits of Black Soybeans. Nutrients, 9(5), 455. https://doi.org/10.3390/nu9050455