Kaempherol and Luteolin Decrease Claudin-2 Expression Mediated by Inhibition of STAT3 in Lung Adenocarcinoma A549 Cells
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Cell Culture and Transfection
2.3. SDS-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and Immunoblotting
2.4. Measurement of O2− Scavenging Activity
2.5. RNA Isolation and Polymerase Chain Reaction (PCR)
2.6. Luciferase Reporter Assay
2.7. Chromatin Immunoprecipitation (ChIP) Assay
2.8. Cell Proliferation
2.9. Statistics
3. Results
3.1. Effects of Flavonoids on Claudin-2 Expression in A549 Cells
3.2. Effects of Antioxidant Capacity on Claudin-2 Expression
3.3. Effects of Flavonoids on mRNA Levels of Junctional Protein and Intracellular Signaling Pathway
3.4. Effects of Flavonoids on the Promoter Activity of Claudin-2
3.5. Inhibition of Association between STAT3 and Claudin-2 Promoter by Flavonoids
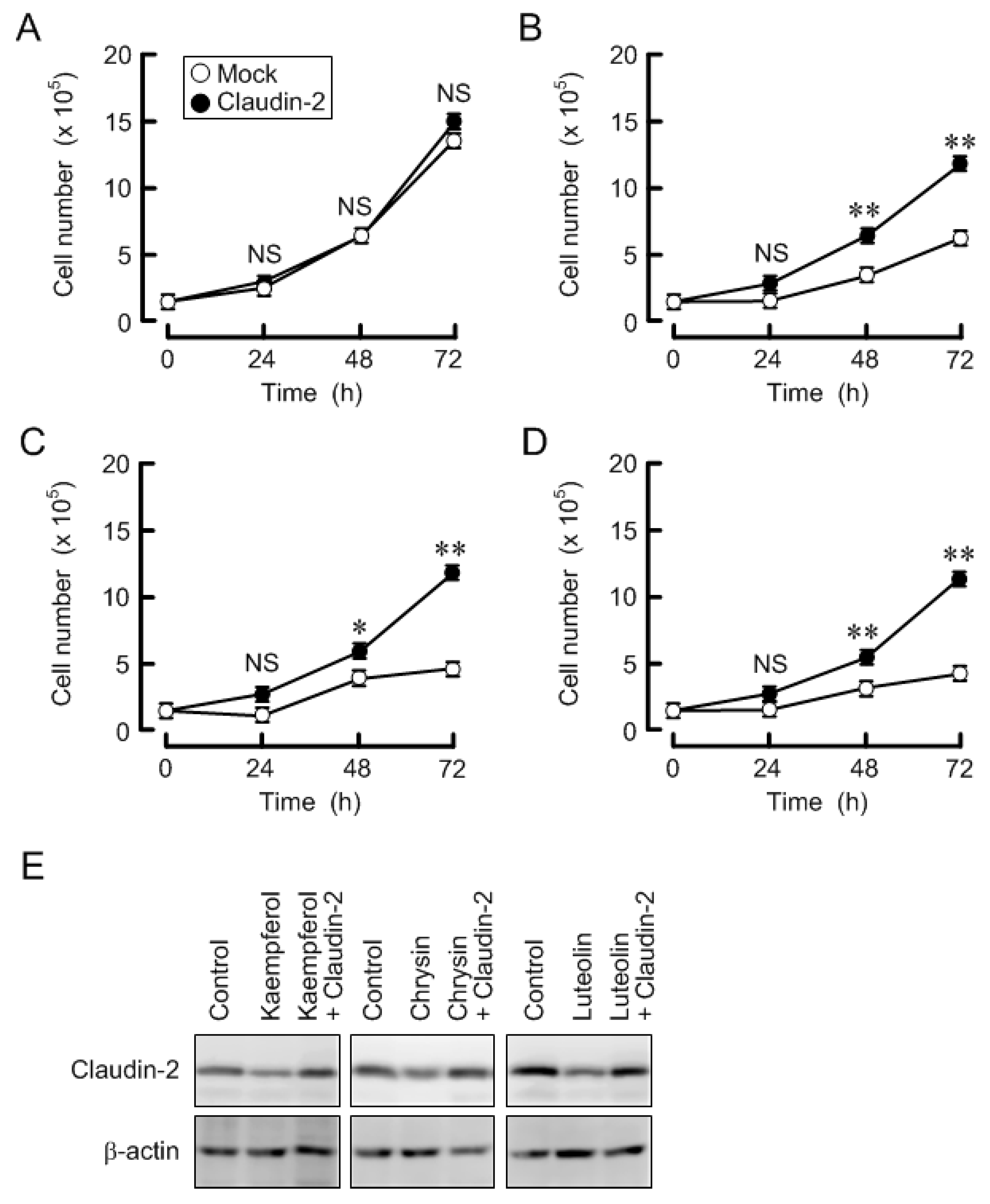
3.6. Effects of Three Flavonoids and Ectopic Claudin-2 Expression on Cell Proliferation
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| cdx2 | caudal-related homeobox 2 |
| ChIP | chromatin immunoprecipitation |
| COX | cyclooxygenase |
| DMEM | Dulbecco’s Modified Eagle’s medium |
| DMSO | Dimethyl sulfoxide |
| ERK | extracellular signal-regulated kinase |
| FCS | fetal calf serum |
| NAC | N-acetyl cysteine |
| MDCK | Madin Darby canine kidney |
| MEK | mitogen-activated protein kinase kinase |
| NF-κB | nuclear factor-κB |
| PCR | polymerase chain reaction |
| PI3K | phosphoinositide 3-kinase |
| SDS-PAGE | SDS-polyacrylamide gel electrophoresis |
| STAT | signal transducers and activators of transcription |
| TJs | tight junctions |
References
- Tsukita, S.; Yamazaki, Y.; Katsuno, T.; Tamura, A. Tight junction-based epithelial microenvironment and cell proliferation. Oncogene 2008, 27, 6930–6938. [Google Scholar] [CrossRef] [PubMed]
- Powell, D.W. Barrier function of epithelia. Am. J. Physiol. Gastrointest. Liver Physiol. 1981, 241, G275–G288. [Google Scholar]
- Matter, K.; Balda, M.S. Signalling to and from tight junctions. Nat. Rev. Mol. Cell Biol. 2003, 4, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Mineta, K.; Yamamoto, Y.; Yamazaki, Y.; Tanaka, H.; Tada, Y.; Saito, K.; Tamura, A.; Igarashi, M.; Endo, T.; Takeuchi, K.; et al. Predicted expansion of the claudin multigene family. FEBS Lett. 2011, 585, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Turksen, K.; Troy, T.C. Barriers built on claudins. J. Cell Sci. 2004, 117, 2435–2447. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Lu, Z.; Lu, Q.; Chen, Y.H. The claudin family of proteins in human malignancy: A clinical perspective. Cancer Manag. Res. 2013, 5, 367–375. [Google Scholar] [PubMed]
- Ikari, A.; Sato, T.; Takiguchi, A.; Atomi, K.; Yamazaki, Y.; Sugatani, J. Claudin-2 knockdown decreases matrix metalloproteinase-9 activity and cell migration via suppression of nuclear Sp1 in A549 cells. Life Sci. 2011, 88, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Halasz, J.; Holczbauer, A.; Paska, C.; Kovacs, M.; Benyo, G.; Verebely, T.; Schaff, Z.; Kiss, A. Claudin-1 and claudin-2 differentiate fetal and embryonal components in human hepatoblastoma. Hum. Pathol. 2006, 37, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Kinugasa, T.; Huo, Q.; Higashi, D.; Shibaguchi, H.; Kuroki, M.; Tanaka, T.; Futami, K.; Yamashita, Y.; Hachimine, K.; Maekawa, S.; et al. Selective up-regulation of claudin-1 and claudin-2 in colorectal cancer. Anticancer Res. 2007, 27, 3729–3734. [Google Scholar] [CrossRef]
- Song, X.; Li, X.; Tang, Y.; Chen, H.; Wong, B.; Wang, J.; Chen, M. Expression of Cdx2 and claudin-2 in the multistage tissue of gastric carcinogenesis. Oncology 2007, 73, 357–365. [Google Scholar]
- Ikari, A.; Watanabe, R.; Sato, T.; Taga, S.; Shimobaba, S.; Yamaguchi, M.; Yamazaki, Y.; Endo, S.; Matsunaga, T.; Sugatani, J. Nuclear distribution of claudin-2 increases cell proliferation in human lung adenocarcinoma cells. Biochim. Biophys. Acta 2014, 1843, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Sawicka, D.; Car, H.; Borawska, M.H.; Niklinski, J. The anticancer activity of propolis. Folia Histochem. Cytobiol. 2012, 50, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.Y.; Naidu, A.; Parent, M.E.; Pintos, J.; Abrahamowicz, M.; Siemiatycki, J.; Koushik, A. The risk of lung cancer related to dietary intake of flavonoids. Nutr. Cancer 2012, 64, 964–974. [Google Scholar] [CrossRef] [PubMed]
- Stefani, E.D.; Boffetta, P.; Deneo-Pellegrini, H.; Mendilaharsu, M.; Carzoglio, J.C.; Ronco, A.; Olivera, L. Dietary antioxidants and lung cancer risk: A case-control study in Uruguay. Nutr. Cancer 1999, 34, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Amararathna, M.; Johnston, M.R.; Rupasinghe, H.P. Plant Polyphenols as Chemopreventive Agents for Lung Cancer. Int. J. Mol. Sci. 2016, 17, 1352. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Bolwell, P.G.; Bramley, P.M.; Pridham, J.B. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic. Res. 1995, 22, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Tran, E.; Nguyen, T.H.; Do, P.T.; Huynh, T.H.; Huynh, H. The role of activated MEK-ERK pathway in quercetin-induced growth inhibition and apoptosis in A549 lung cancer cells. Carcinogenesis 2004, 25, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.A.; Kim, J.Y.; Lee, J.Y.; Kang, C.M.; Kwon, H.J.; Yoo, Y.D.; Kim, T.W.; Lee, Y.S.; Lee, S.J. Induction of cell cycle arrest and apoptosis in human breast cancer cells by quercetin. Int. J. Oncol. 2001, 19, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Sakai, T.; Hosokawa, N.; Marui, N.; Matsumoto, K.; Fujioka, A.; Nishino, H.; Aoike, A. The effect of quercetin on cell cycle progression and growth of human gastric cancer cells. FEBS Lett. 1990, 260, 10–13. [Google Scholar] [CrossRef]
- Vijayababu, M.R.; Kanagaraj, P.; Arunkumar, A.; Ilangovan, R.; Aruldhas, M.M.; Arunakaran, J. Quercetin-induced growth inhibition and cell death in prostatic carcinoma cells (PC-3) are associated with increase in p21 and hypophosphorylated retinoblastoma proteins expression. J. Cancer Res. Clin. Oncol. 2005, 131, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Sonoki, H.; Sato, T.; Endo, S.; Matsunaga, T.; Yamaguchi, M.; Yamazaki, Y.; Sugatani, J.; Ikari, A. Quercetin Decreases Claudin-2 Expression Mediated by Up-Regulation of microRNA miR-16 in Lung Adenocarcinoma A549 Cells. Nutrients 2015, 7, 4578–4592. [Google Scholar] [CrossRef] [PubMed]
- Benekli, M.; Baumann, H.; Wetzler, M. Targeting signal transducer and activator of transcription signaling pathway in leukemias. J. Clin. Oncol. 2009, 27, 4422–4432. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Jove, R. The STATs of cancer—New molecular targets come of age. Nat. Rev. Cancer 2004, 4, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Darnell, J.E., Jr. STATs and gene regulation. Science 1997, 277, 1630–1635. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.; Yang, B.; Gendelman, H.E.; Persidsky, Y.; Kanmogne, G.D. STAT1 signaling modulates HIV-1-induced inflammatory responses and leukocyte transmigration across the blood-brain barrier. Blood 2008, 111, 2062–2072. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Hernandez, V.; Flores-Maldonado, C.; Rincon-Heredia, R.; Verdejo-Torres, O.; Bonilla-Delgado, J.; Meneses-Morales, I.; Gariglio, P.; Contreras, R.G. EGF regulates claudin-2 and -4 expression through Src and STAT3 in MDCK cells. J. Cell. Physiol. 2015, 230, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, O.; Wu, C.; Murai, A.; Nakamura, H. Evaluation of five imidazopyrazinone-type chemiluminescent superoxide probes and their application to the measurement of superoxide anion generated by Listeria monocytogenes. Anal. Biochem. 1998, 258, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Ikari, A.; Sato, T.; Watanabe, R.; Yamazaki, Y.; Sugatani, J. Increase in claudin-2 expression by an EGFR/MEK/ERK/c-Fos pathway in lung adenocarcinoma A549 cells. Biochim. Biophys. Acta 2012, 1823, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, M.; Tanaka, T.; Ito, T.; Nishikawa, A.; Yamazaki, N.; Nakaya, K.; Matsuura, N.; Tsuchiya, H.; Nagayama, M.; Iinuma, M. Antioxidant activity of hydroxyflavonoids. J. Health Sci. 2005, 51, 376–378. [Google Scholar] [CrossRef]
- Hichino, A.; Okamoto, M.; Taga, S.; Akizuki, R.; Endo, S.; Matsunaga, T.; Ikari, A. Down-regulation of Claudin-2 Expression and Proliferation by Epigenetic Inhibitors in Human Lung Adenocarcinoma A549 Cells. J. Biol. Chem. 2017, 292, 2411–2421. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Turkson, J.; Karras, J.G.; Jove, R.; Haura, E.B. Activation of Stat3 by receptor tyrosine kinases and cytokines regulates survival in human non-small cell carcinoma cells. Oncogene 2003, 22, 4150–4165. [Google Scholar] [CrossRef] [PubMed]
- Ansari, J.; Shackelford, R.E.; El-Osta, H. Epigenetics in non-small cell lung cancer: From basics to therapeutics. Transl. Lung Cancer Res. 2016, 5, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Amasheh, M.; Andres, S.; Amasheh, S.; Fromm, M.; Schulzke, J.D. Barrier effects of nutritional factors. Ann. N. Y. Acad. Sci. 2009, 1165, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Hara, H. Quercetin enhances intestinal barrier function through the assembly of zonnula occludens-2, occludin, and claudin-1 and the expression of claudin-4 in Caco-2 cells. J. Nutr. 2009, 139, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Kiatprasert, P.; Deachapunya, C.; Benjanirat, C.; Poonyachoti, S. Soy isoflavones improves endometrial barrier through tight junction gene expression. Reproduction 2015, 149, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Scrima, M.; De Marco, C.; Fabiani, F.; Franco, R.; Pirozzi, G.; Rocco, G.; Ravo, M.; Weisz, A.; Zoppoli, P.; Ceccarelli, M.; et al. Signaling networks associated with AKT activation in non-small cell lung cancer (NSCLC): New insights on the role of phosphatydil-inositol-3 kinase. PLoS ONE 2012, 7, e30427. [Google Scholar] [CrossRef] [PubMed]
- Mossman, B.T.; Lounsbury, K.M.; Reddy, S.P. Oxidants and signaling by mitogen-activated protein kinases in lung epithelium. Am. J. Respir. Cell Mol. Biol. 2006, 34, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Lipschutz, J.H.; Li, S.; Arisco, A.; Balkovetz, D.F. Extracellular signal-regulated kinases 1/2 control claudin-2 expression in Madin-Darby canine kidney strain I and II cells. J. Biol. Chem. 2005, 280, 3780–3788. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yoshinaga, N.; Tanabe, S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J. Biol. Chem. 2011, 286, 31263–31271. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.H.; Seok, J.S.; Petriello, M.C.; Han, S.G. Arsenic downregulates tight junction claudin proteins through p38 and NF-kappaB in intestinal epithelial cell line, HT-29. Toxicology 2017, 379, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.E.; DiGeronimo, R.J.; Arthur, D.E.; King, J.M. Remodeling of the tight junction during recovery from exposure to hydrogen peroxide in kidney epithelial cells. Free Radic. Biol. Med. 2009, 47, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.T. Overproduction of nitric oxide intensifies brain infarction and cerebrovascular damage through reduction of claudin-5 and ZO-1 expression in striatum of ischemic brain. Pathol. Res. Pract. 2016, 212, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Chang, C.C.; Mori, T.; Sato, K.; Ohtsuki, K.; Upham, B.L.; Trosko, J.E. Augmentation of differentiation and gap junction function by kaempferol in partially differentiated colon cancer cells. Carcinogenesis 2005, 26, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Lirdprapamongkol, K.; Sakurai, H.; Abdelhamed, S.; Yokoyama, S.; Maruyama, T.; Athikomkulchai, S.; Viriyaroj, A.; Awale, S.; Yagita, H.; Ruchirawat, S.; et al. A flavonoid chrysin suppresses hypoxic survival and metastatic growth of mouse breast cancer cells. Oncol. Rep. 2013, 30, 2357–2364. [Google Scholar] [CrossRef] [PubMed]
- Anso, E.; Zuazo, A.; Irigoyen, M.; Urdaci, M.C.; Rouzaut, A.; Martinez-Irujo, J.J. Flavonoids inhibit hypoxia-induced vascular endothelial growth factor expression by a HIF-1 independent mechanism. Biochem. Pharmacol. 2010, 79, 1600–1609. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.C.; Tsai, S.H.; Tsai, D.C.; Lin-Shiau, S.Y.; Lin, J.K. Suppression of inducible cyclooxygenase and nitric oxide synthase through activation of peroxisome proliferator-activated receptor-gamma by flavonoids in mouse macrophages. FEBS Lett. 2001, 496, 12–18. [Google Scholar] [CrossRef]
- Woo, K.J.; Jeong, Y.J.; Inoue, H.; Park, J.W.; Kwon, T.K. Chrysin suppresses lipopolysaccharide-induced cyclooxygenase-2 expression through the inhibition of nuclear factor for IL-6 (NF-IL6) DNA-binding activity. FEBS Lett. 2005, 579, 705–711. [Google Scholar] [CrossRef] [PubMed]








| Name | Direction | Sequence |
|---|---|---|
| Claudin-1 | Forward | 5′-ATGAGGATGGCTGTCATTGG-3′ |
| Claudin-1 | Reverse | 5′-ATTGACTGGGGTCATAGGGT-3′ |
| Claudin-2 | Forward | 5′-ATTGTGACAGCAGTTGGCTT-3′ |
| Claudin-2 | Reverse | 5′-CTATAGATGTCACACTGGGTGATG-3′ |
| Occludin | Forward | 5′-TTTGTGGGACAAGGAACACA-3′ |
| Occludin | Reverse | 5′-TCATTCACTTTGCCATTGGA-3′ |
| E-cadherin | Forward | 5′-ACCCCCTGTTGGTGTCTTT-3′ |
| E-cadherin | Reverse | 5′-TTCGGGCTTGTTGTCATTCT-3′ |
| β-actin | Forward | 5′-CCTGAGGCACTCTTCCAGCCTT-3′ |
| β-actin | Reverse | 5′-TGCGGATGTCCACGTCACACTTC-3′ |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonoki, H.; Tanimae, A.; Endo, S.; Matsunaga, T.; Furuta, T.; Ichihara, K.; Ikari, A. Kaempherol and Luteolin Decrease Claudin-2 Expression Mediated by Inhibition of STAT3 in Lung Adenocarcinoma A549 Cells. Nutrients 2017, 9, 597. https://doi.org/10.3390/nu9060597
Sonoki H, Tanimae A, Endo S, Matsunaga T, Furuta T, Ichihara K, Ikari A. Kaempherol and Luteolin Decrease Claudin-2 Expression Mediated by Inhibition of STAT3 in Lung Adenocarcinoma A549 Cells. Nutrients. 2017; 9(6):597. https://doi.org/10.3390/nu9060597
Chicago/Turabian StyleSonoki, Hiroyuki, Asami Tanimae, Satoshi Endo, Toshiyuki Matsunaga, Takumi Furuta, Kenji Ichihara, and Akira Ikari. 2017. "Kaempherol and Luteolin Decrease Claudin-2 Expression Mediated by Inhibition of STAT3 in Lung Adenocarcinoma A549 Cells" Nutrients 9, no. 6: 597. https://doi.org/10.3390/nu9060597
APA StyleSonoki, H., Tanimae, A., Endo, S., Matsunaga, T., Furuta, T., Ichihara, K., & Ikari, A. (2017). Kaempherol and Luteolin Decrease Claudin-2 Expression Mediated by Inhibition of STAT3 in Lung Adenocarcinoma A549 Cells. Nutrients, 9(6), 597. https://doi.org/10.3390/nu9060597




