Influence of Genetic Variations in Selenoprotein Genes on the Pattern of Gene Expression after Supplementation with Brazil Nuts
Abstract
:1. Introduction
2. Materials and Methods
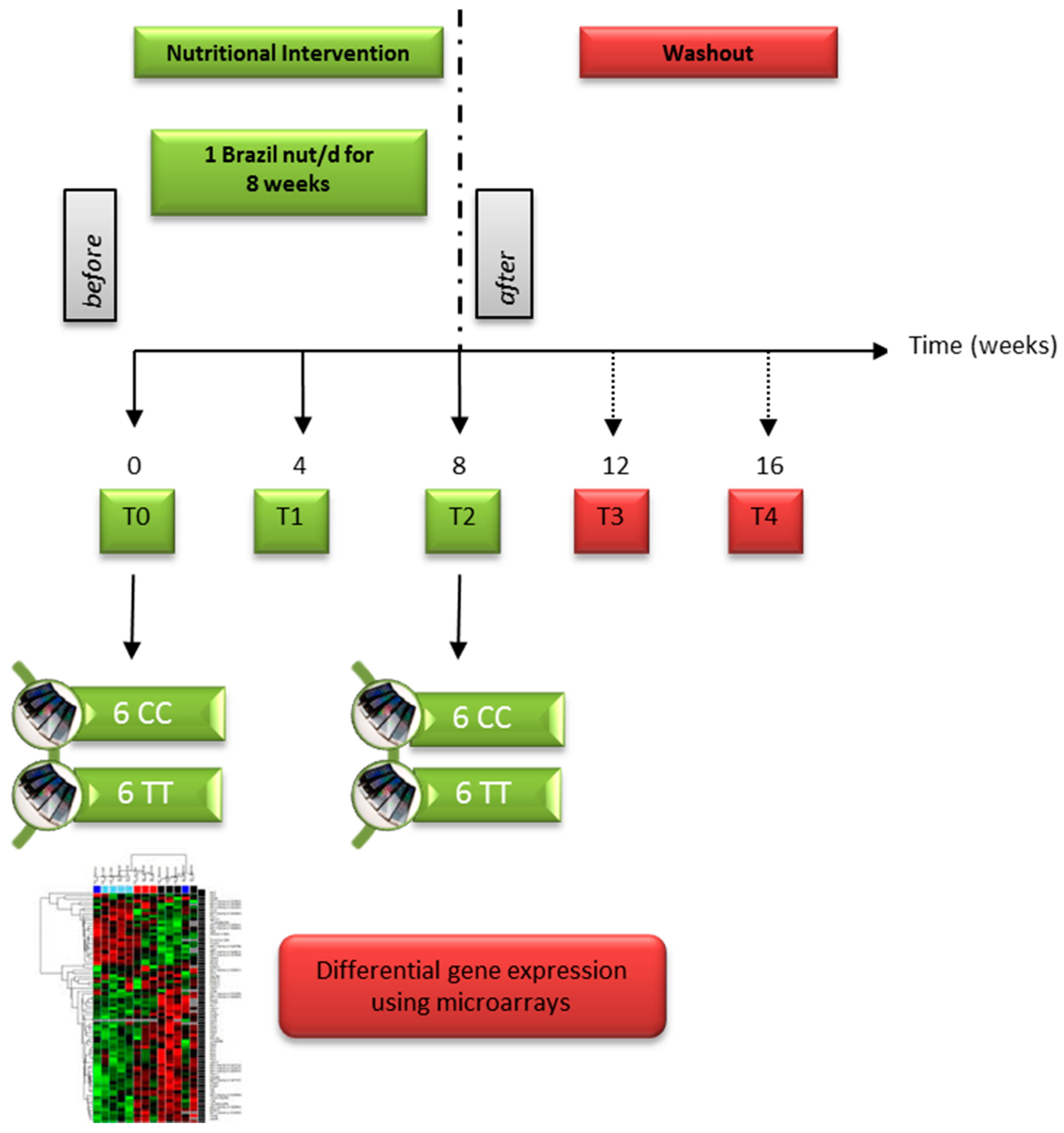
2.1. Brazil Nut Supplementation and Blood Sampling
2.2. Genotyping
2.3. Selenoprotein Gene Expression
2.4. Microarray Analysis
2.5. Gene Set Enrichment Analysis (GSEA)
2.6. Statistical Analysis
3. Results
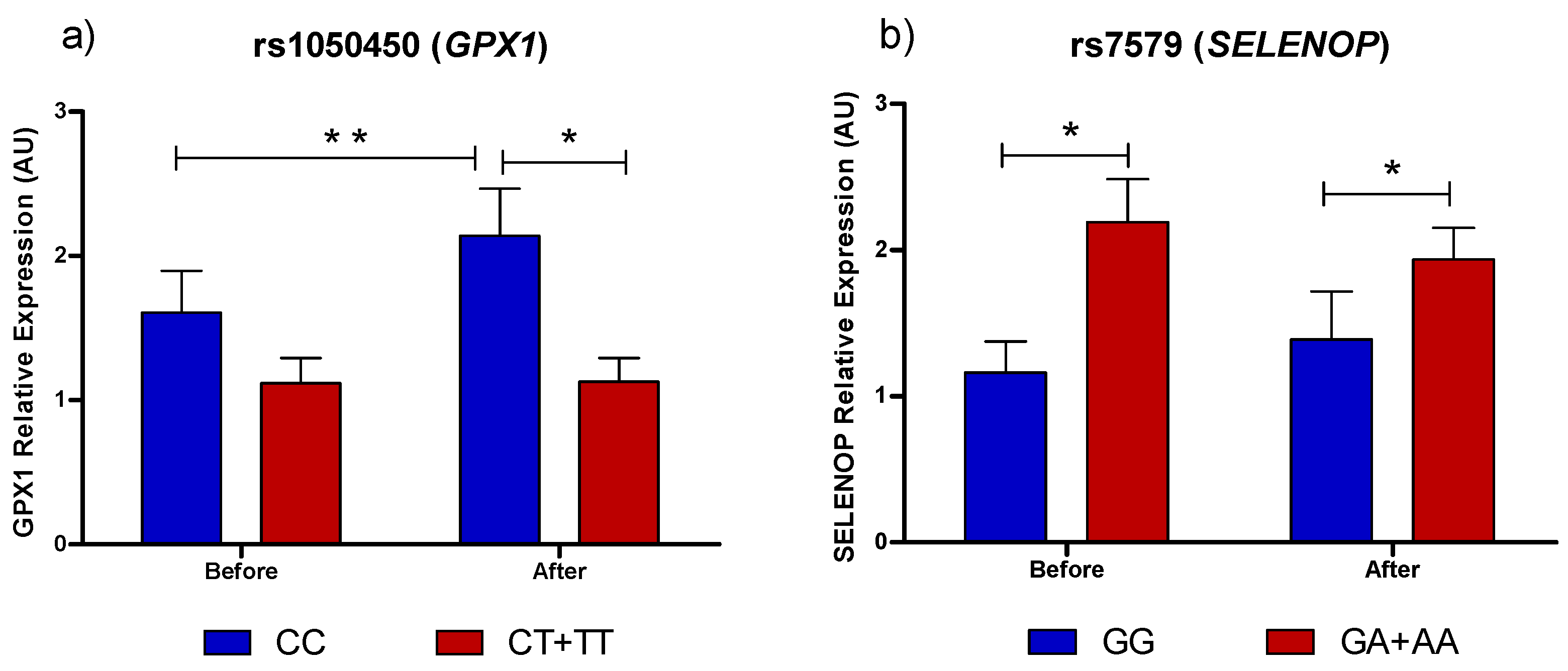
3.1. Selenoprotein Gene Expression
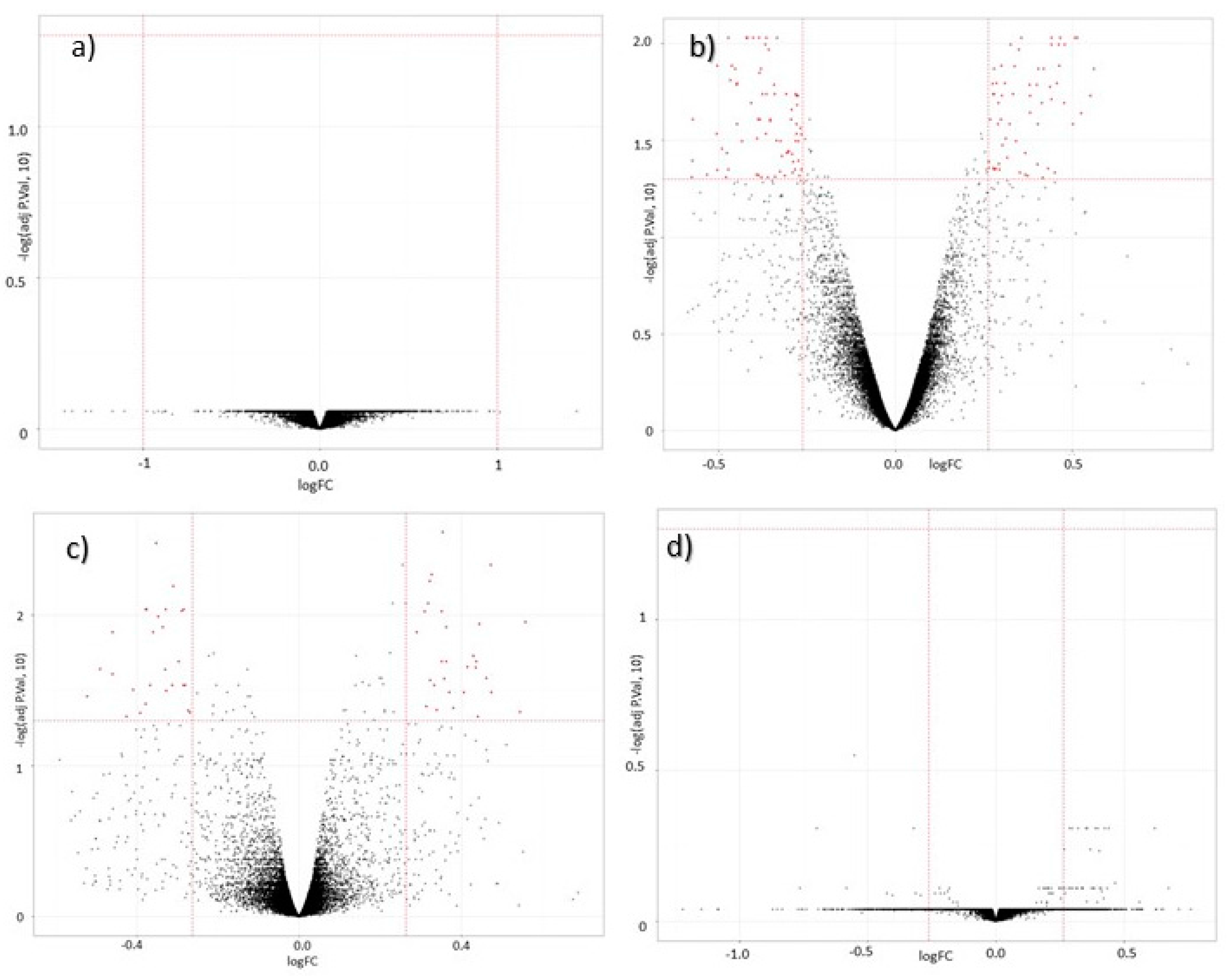
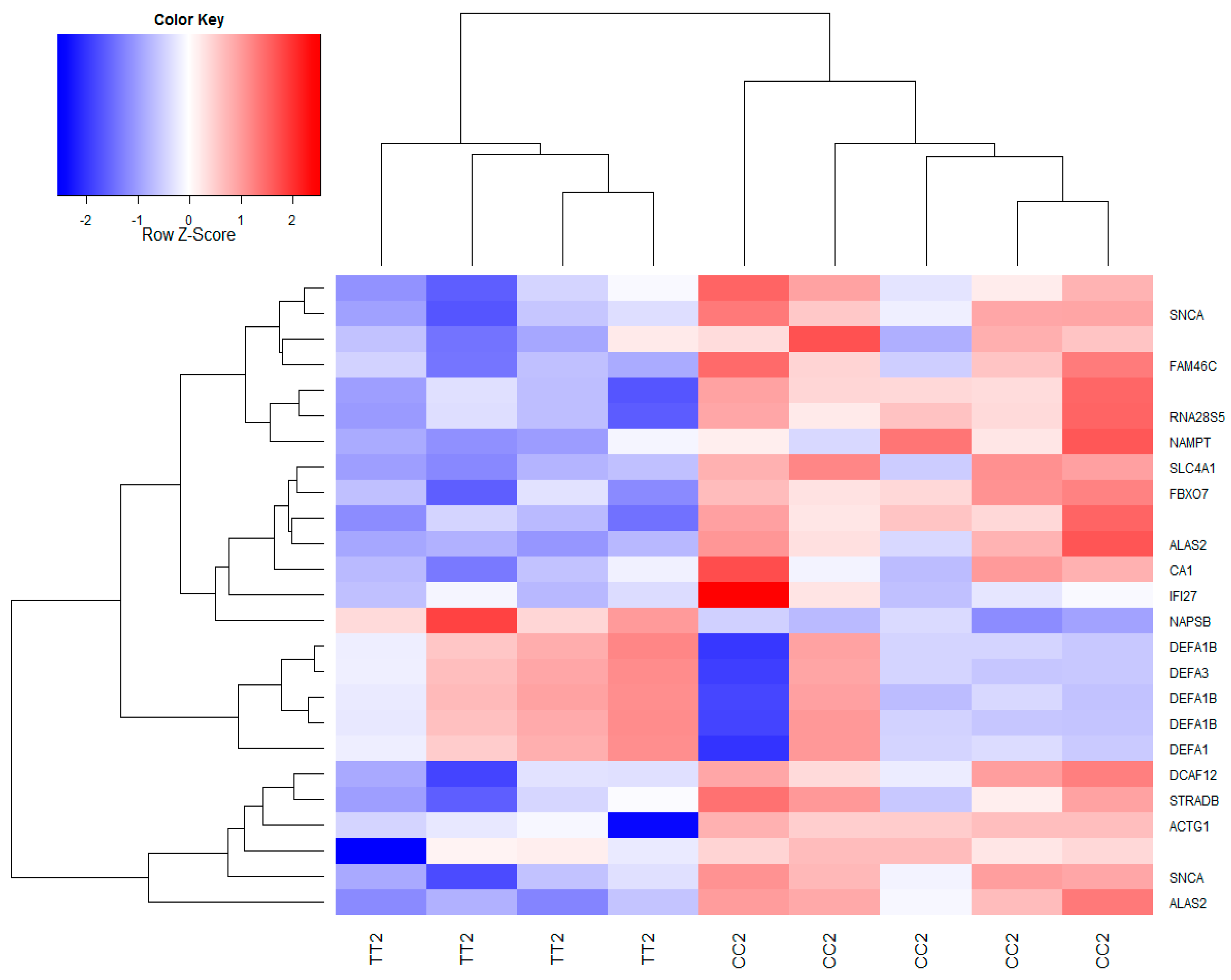
3.2. Global Gene Expression
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bolling, B.W.; Chen, C.-Y.O.; McKay, D.L.; Blumberg, J.B. Tree nut phytochemicals: Composition, antioxidant capacity, bioactivity, impact factors. A systematic review of almonds, Brazils, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts. Nutr. Res. Rev. 2011, 24, 244–275. [Google Scholar] [CrossRef] [PubMed]
- Alasalvar, C.; Bolling, B.W. Review of nut phytochemicals, fat-soluble bioactives, antioxidant components and health effects. Br. J. Nutr. 2015, 113, S68–S78. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, C.E.; Fulgoni, V.L.; Nicklas, T.A. Tree Nut consumption is associated with better adiposity measures and cardiovascular and metabolic syndrome health risk factors in U.S. adults: NHANES 2005–2010. Nutr. J. 2015, 14, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Dumont, E.; De Pauw, L.; Vanhaecke, F.; Cornelis, R. Speciation of Se in Bertholletia excelsa (Brazil nut): A hard nut to crack? Food Chem. 2006, 95, 684–692. [Google Scholar] [CrossRef]
- Thomson, C.D.; Chisholm, A.; Mclachlan, S.K.; Campbell, J.M. Brazil nuts: An effective way to improve selenium status. Am. J. Clin. Nutr. 2008, 87, 379–384. [Google Scholar] [PubMed]
- Cardoso, B.R.; Apolinário, D.; Bandeira, V.S.; Busse, A.L.; Magaldi, R.M.; Jacob-Filho, W.; Cozzolino, S.M.F. Effects of Brazil nut consumption on selenium status and cognitive performance in older adults with mild-cognitive impairment: A randomized controlled pilot trial. Eur. J. Nutr. 2016, 55, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Cominetti, C.; de Bortoli, M.C.; Garrido, A.B.; Cozzolino, S.M.F. Brazilian nut consumption improves selenium status and glutathione peroxidase activity and reduces atherogenic risk in obese women. Nutr. Res. 2012, 32, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Kryukov, G.V.; Castellano, S.; Novoselov, S.V.; Lobanov, A.V.; Zehtab, O.; Guigó, R.; Gladyshev, V.N. Characterization of mammalian selenoproteomes. Science 2003, 300, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular Pathways and Physiological Roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, L.; Schweizer, U. Hierarchical regulation of selenoprotein expression and sex-specific effects of selenium. Biochim. Biophys. Acta 2009, 1790, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Sunde, R.A.; Raines, A.M.; Barnes, K.M.; Evenson, J.K. Selenium status highly regulates selenoprotein mRNA levels for only a subset of the selenoproteins in the selenoproteome. Biosci. Rep. 2009, 29, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Ravn-Haren, G.; Bügel, S.; Krath, B.N.; Hoac, T.; Stagsted, J.; Jørgensen, K.; Bresson, J.R.; Larsen, E.H.; Dragsted, L.O. A short-term intervention trial with selenate, selenium-enriched yeast and selenium-enriched milk: Effects on oxidative defence regulation. Br. J. Nutr. 2008, 99, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Ravn-Haren, G.; Krath, B.N.; Overvad, K.; Cold, S.; Moesgaard, S.; Larsen, E.H.; Dragsted, L.O. Effect of long-term selenium yeast intervention on activity and gene expression of antioxidant and xenobiotic metabolising enzymes in healthy elderly volunteers from the Danish Prevention of Cancer by Intervention by Selenium (PRECISE) pilot study. Br. J. Nutr. 2008, 99, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Sunde, R.A.; Paterson, E.; Evenson, J.K.; Barnes, K.M.; Lovegrove, J.A.; Gordon, M.H. Longitudinal selenium status in healthy British adults: Assessment using biochemical and molecular biomarkers. Br. J. Nutr. 2008, 99 (Suppl. 3), S37–S47. [Google Scholar] [CrossRef] [PubMed]
- Pagmantidis, V.; Méplan, C.; Van Schothorst, E.M.; Keijer, J.; Hesketh, J.E. Supplementation of healthy volunteers with nutritionally relevant amounts of selenium increases the expression of lymphocyte protein biosynthesis genes. Am. J. Clin. Nutr. 2008, 87, 181–189. [Google Scholar] [PubMed]
- Cardoso, B.R.; Busse, A.L.; Hare, D.J.; Cominetti, C.; Horst, M.A.; Mccoll, G.; Magaldi, R.M.; Jacob-Filho, W.; Cozzolino, S.M.F. Pro198Leu polymorphism affects the selenium status and GPx activity in response to Brazil nut intake. Food Funct. 2016, 9, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Hesketh, J.; Méplan, C. Transcriptomics and functional genetic polymorphisms as biomarkers of micronutrient function: Focus on selenium as an exemplar. Proc. Nutr. Soc. 2011, 70, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Méplan, C.; Crosley, L.K.; Nicol, F.; Beckett, G.J.; Howie, A.F.; Hill, K.E.; Horgan, G.; Mathers, J.C.; Arthur, J.R.; Hesketh, J.E. Genetic polymorphisms in the human selenoprotein P gene determine the response of selenoprotein markers to selenium supplementation in a gender-specific manner (the SELGEN study). FASEB J. 2007, 21, 3063–3074. [Google Scholar] [CrossRef] [PubMed]
- Méplan, C.; Crosley, L.K.; Nicol, F.; Horgan, G.W.; Mathers, J.C.; Arthur, J.R.; Hesketh, J.E. Functional effects of a common single-nucleotide polymorphism (GPX4c718t) in the glutathione peroxidase 4 gene: Interaction with sex. Am. J. Clin. Nutr. 2008, 87, 1019–1027. [Google Scholar] [PubMed]
- Combs, G.F.; Jackson, M.I.; Watts, J.C.; Johnson, L.K.; Zeng, H.; Idso, J.; Schomburg, L.; Hoeg, A.; Hoefig, C.S.; Chiang, E.C.; et al. Differential responses to selenomethionine supplementation by sex and genotype in healthy adults. Br. J. Nutr. 2012, 107, 1514–1525. [Google Scholar] [CrossRef] [PubMed]
- Jablonska, E.; Gromadzinska, J.; Reszka, E.; Wasowicz, W.; Sobala, W.; Szeszenia-Dabrowska, N.; Boffetta, P. Association between GPx1 Pro198Leu polymorphism, GPx1 activity and plasma selenium concentration in humans. Eur. J. Nutr. 2009, 48, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Bermano, G.; Pagmantidis, V.; Holloway, N.; Kadri, S.; Mowat, N.A.G.; Shiel, R.S.; Arthur, J.R.; Mathers, J.C.; Daly, A.K.; Broom, J.; et al. Evidence that a polymorphism within the 3′UTR of glutathione peroxidase 4 is functional and is associated with susceptibility to colorectal cancer. Genes Nutr. 2007, 2, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Donadio, J.L.S.; Rogero, M.M.; Guerra‑shinohara, E.M.; Desmarchelier, C.; Borel, P.; Cozzolino, S.M.F. SEPP1 polymorphisms modulate serum glucose and lipid response to Brazil nut supplementation. Eur. J. Nutr. 2017, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Dunning, M.J.; Smith, M.L.; Shi, W.; Lynch, A.G. BeadArray Expression Analysis Using Bioconductor. PLoS Comput. Biol. 2011, 7, e1002276. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Cole-Ezea, P.; Swan, D.; Shanley, D.; Hesketh, J. Glutathione peroxidase 4 has a major role in protecting mitochondria from oxidative damage and maintaining oxidative phosphorylation complexes in gut epithelial cells. Free Radic. Biol. Med. 2012, 53, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Hamanishi, T.; Furuta, H.; Kato, H.; Doi, A.; Tamai, M.; Shimomura, H.; Sakagashira, S.; Nishi, M.; Sasaki, H.; Sanke, T.; et al. Functional variants in the glutathione peroxidase-1 (GPx-1) gene are associated with increased intima-media thickness of carotid arteries and risk of macrovascular diseases in Japanese type 2 diabetic patients. Diabetes 2004, 53, 2455–2460. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.D.; Krath, B.N.; Frederiksen, K.; Tjønneland, A.; Overvad, K.; Roswall, N.; Loft, S.; Dragsted, L.O.; Vogel, U.; Raaschou-Nielsen, O. GPX1 Pro198Leu polymorphism, erythrocyte GPX activity, interaction with alcohol consumption and smoking, and risk of colorectal cancer. Mutat. Res. 2009, 664, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Méplan, C.; Nicol, F.; Burtle, B.T.; Crosley, L.K.; Arthur, J.R.; Mathers, J.C.; Hesketh, J.E. Relative abundance of selenoprotein P isoforms in human plasma depends on genotype, se intake, and cancer status. Antioxid. Redox Signal. 2009, 11, 2631–2640. [Google Scholar] [CrossRef] [PubMed]
- Crosley, L.K.; Bashir, S.; Nicol, F.; Arthur, J.R.; Hesketh, J.E.; Sneddon, A.A. The single-nucleotide polymorphism (GPX4c718t) in the glutathione peroxidase 4 gene influences endothelial cell function: Interaction with selenium and fatty acids. Mol. Nutr. Food Res. 2013, 57, 2185–2194. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, W.C.; Richter, D.; Alkan, Z. Dietary selenium supplementation and whole blood gene expression in healthy North American men. Biol. Trace Elem. Res. 2013, 155, 201–208. [Google Scholar] [CrossRef] [PubMed]
| Name | Size | ES | NES | NOM p-Value | FDR q-Value |
|---|---|---|---|---|---|
| Cellular component | |||||
| Organellar ribosome | 22 | −0.69 | −1.56 | 0.035 | 1.000 |
| Mitochondrial ribosome | 22 | −0.69 | −1.56 | 0.035 | 0.571 |
| Early endosome | 15 | −0.74 | −1.53 | 0.012 | 0.582 |
| ER Golgi Intermediate compartment | 20 | −0.52 | −1.52 | 0.004 | 0.496 |
| Microtubule cytoskeleton | 101 | −0.41 | −1.51 | 0.025 | 0.397 |
| Ribosomal subunit | 20 | −0.66 | −1.51 | 0.076 | 0.332 |
| Intrinsic to endoplasmic reticulum membrane | 23 | −0.58 | −1.50 | 0.035 | 0.320 |
| Integral to endoplasmic reticulum membrane | 23 | −0.58 | −1.50 | 0.035 | 0.280 |
| Mitochondrial matrix | 44 | −0.55 | −1.49 | 0.066 | 0.289 |
| Mitochondrial lumen | 44 | −0.55 | −1.49 | 0.066 | 0.260 |
| Replication fork | 16 | −0.61 | −1.48 | 0.014 | 0.242 |
| Nuclear chromosome part | 25 | −0.55 | −1.48 | 0.036 | 0.223 |
| Golgi apparatus | 166 | −0.37 | −1.45 | 0.008 | 0.280 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donadio, J.L.S.; Rogero, M.M.; Cockell, S.; Hesketh, J.; Cozzolino, S.M.F. Influence of Genetic Variations in Selenoprotein Genes on the Pattern of Gene Expression after Supplementation with Brazil Nuts. Nutrients 2017, 9, 739. https://doi.org/10.3390/nu9070739
Donadio JLS, Rogero MM, Cockell S, Hesketh J, Cozzolino SMF. Influence of Genetic Variations in Selenoprotein Genes on the Pattern of Gene Expression after Supplementation with Brazil Nuts. Nutrients. 2017; 9(7):739. https://doi.org/10.3390/nu9070739
Chicago/Turabian StyleDonadio, Janaina L. S., Marcelo M. Rogero, Simon Cockell, John Hesketh, and Silvia M. F. Cozzolino. 2017. "Influence of Genetic Variations in Selenoprotein Genes on the Pattern of Gene Expression after Supplementation with Brazil Nuts" Nutrients 9, no. 7: 739. https://doi.org/10.3390/nu9070739
APA StyleDonadio, J. L. S., Rogero, M. M., Cockell, S., Hesketh, J., & Cozzolino, S. M. F. (2017). Influence of Genetic Variations in Selenoprotein Genes on the Pattern of Gene Expression after Supplementation with Brazil Nuts. Nutrients, 9(7), 739. https://doi.org/10.3390/nu9070739





