Role of Zucchini and Its Distinctive Components in the Modulation of Degenerative Processes: Genotoxicity, Anti-Genotoxicity, Cytotoxicity and Apoptotic Effects
Abstract
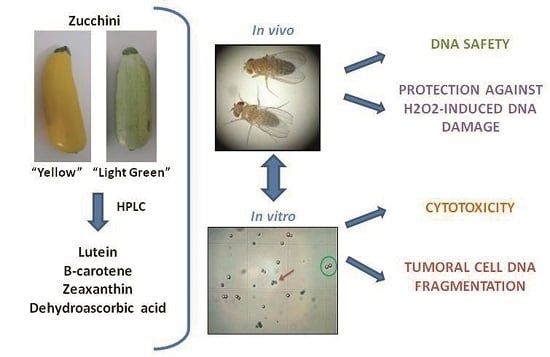
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Antioxidant Compounds
2.3. Determination of the Carotenoid Content
2.4. Extraction and Analysis of Vitamin C
2.5. Genotoxicity and Anti-Genotoxicity Tests
2.6. Data Evaluation and Statistical Analysis
2.7. Cell Culture and Cytotoxicity Assay
2.8. Assessment of Pro-Apoptosis by DNA Fragmentation and Annexin V-PI
3. Results
3.1. Quantitation of Antioxidant Compounds
3.2. Genotoxicity and Anti-Genotoxicity Analysis of C. pepo and Their Components
3.3. Effects on Tumoral Growth of HL60 Cells and Apoptosis
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Toshiya, K.; Kuno, T.; Tsukamoto, T.; Hara, A.; Tanaka, T. Cancer chemoprevention through the induction of apoptosis by natural compounds. J. Biophys. Chem. 2012. [Google Scholar] [CrossRef]
- Fernández-Bedmar, Z.; Anter, J.; de La Cruz-Ares, S.; Muñoz-Serrano, A.; Alonso-Moraga, Á.; Pérez-Guisado, J. Role of citrus juices and distinctive components in the modulation of degenerative processes: Genotoxicity, antigenotoxicity, cytotoxicity, and longevity in Drosophila. J. Toxicol. Environ. Health A 2011, 74, 1052–1066. [Google Scholar] [CrossRef] [PubMed]
- Hmamouchi, M. Medicinal plants in Morocco: Traditional use marketing and strategies for conservation and increasing value. Espérance Med. 2002, 9, 454–458. [Google Scholar]
- Mazumder, U.K. Anticancer activity of methanol extract of Cucurbita maxima against Ehrlich as-cites carcinoma. Int. J. Res. Pharm. Sci. 2011, 2, 52–59. [Google Scholar]
- Mayne, S.T.; Playdon, M.C.; Rock, C.L. Diet, nutrition, and cancer: Past, present and future. Nat. Rev. Clin. Oncol. 2016, 13, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, M.S. Nutrition and cancer: A review of the evidence for an anti-cancer diet. Nutr. J. 2004, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Díaz, M.T.; Del Río-Celestino, M.; Martínez-Valdivieso, D.; Font, R. Use of visible and near-infrared spectroscopy for predicting antioxidant compounds in summer squash (Cucurbita pepo ssp pepo). Food Chem. 2014, 164, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Valdivieso, D.; Gómez, P.; Font, R.; Del Río-Celestino, M. Mineral composition and potential nutritional contribution of 34 genotypes from different summer squash morphotypes. Eur. Food Res. Technol. 2015, 240, 71–81. [Google Scholar] [CrossRef]
- Martínez-Valdivieso, D.; Font, R.; Gómez, P.; Blanco-Díaz, T.; Río-Celestino, D. Determining the mineral composition in Cucurbita pepo fruit using near infrared reflectance spectroscopy. J. Sci. Food Agric. 2014, 94, 3171–3180. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Valdivieso, D.; Font, R.; Blanco-Díaz, M.T.; Moreno-Rojas, J.M.; Gómez, P.; Alonso-Moraga, Á.; del Río-Celestino, M. Application of near-infrared reflectance spectroscopy for predicting carotenoid content in summer squash fruit. Comput. Electron. Agric. 2014, 108, 71–79. [Google Scholar] [CrossRef]
- Menéndez, A.; Capó, J.T.; Menéndez Castillo, R.A.; González, O.L.; Domínguez, C.C.; Sanabria, M.L.G. Evaluation of Cucurbita pepo L. lipophilic extract on androgen-induced prostatic hyperplasia. Rev. Cuba. Plantas Med. 2006, 11, 1–6. [Google Scholar]
- Møller, P.; Loft, S. Interventions with antioxidants and nutrients in relation to oxidative DNA damage and repair. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2004, 551, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Oloyede, F.; Agbaje, G.O.; Obuotor, E.M.; Obisesan, I.O. Nutritional and antioxidant profiles of pumpkin (Cucurbita pepo Linn.) immature and mature fruits as influenced by NPK fertilizer. Food Chem. 2012, 135, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Shokrzadeh, M.; Azadbakht, M.; Ahangar, N.; Hashemi, A.; Saravi, S.S. Cytotoxicity of hydro-alcoholic extracts of Cucurbita pepo and Solanum nigrum on HepG2 and CT26 cancer cell lines. Pharmacogn. Mag. 2010, 6, 176. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.C.; Pan, H.Y.; Deng, X.M.; Xiang, H.; Gao, H.Y.; Cai, H.; Wu, L.J. Cucurbitane and hexanorcucurbitane glycosides from the fruits of Cucurbita pepo cv dayangua. J. Asian Nat. Prod. Res. 2007, 9, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bedmar, Z.; Alonso-Moraga, A. In vivo and in vitro evaluation for nutraceutical purposes of capsaicin, capsanthin, lutein and four pepper varieties. Food Chem. Toxicol. 2016, 98, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Anter, J.; Romero-Jiménez, M.; Fernández-Bedmar, Z.; Villatoro-Pulido, M.; Analla, M.; Alonso-Moraga, A.; Muñoz-Serrano, A. Antigenotoxicity, cytotoxicity, and apoptosis induction by apigenin, bisabolol, and protocatechuic acid. J. Med. Food 2011, 14, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Tasset-Cuevas, I.; Fernández-Bedmar, Z.; Lozano-Baena, M.D.; Campos-Sánchez, J.; de Haro-Bailón, A.; Muñoz-Serrano, A.; Alonso-Moraga, Á. Protective effect of borage seed oil and gamma linolenic acid on DNA: In vivo and in vitro studies. PLoS ONE 2013, 8, e56986. [Google Scholar] [CrossRef] [PubMed]
- Graf, U.; Würgler, F. E.; Katz, A. J.; Frei, H.; Juon, H.; Hall, C. B.; Kale, P. G. Somatic mutation and recombination test in Drosophila melanogaster. Environ. Mutagen. 1984, 6, 153–188. [Google Scholar] [CrossRef] [PubMed]
- Villatoro-Pulido, M.; Font, R.; Saha, S.; Obregón-Cano, S.; Anter, J.; Muñoz-Serrano, A.; de Haro-Bailón, A.; Del Río-Celestino, M. In vivo biological activity of rocket extracts (Eruca vesicaria subsp. sativa (Miller) Thell) and sulforaphane. Food Chem. Toxicol. 2012, 50, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.J.; Gallo, R.C.; Gallagher, R.E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature 1977, 270, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Hartman, P.E.; Shankel, D.M. Antimutagens and anticarcinogens: A survey of putative interceptor molecules. Environ. Mol. Mutagen. 1990, 15, 145–182. [Google Scholar] [CrossRef] [PubMed]
- Odin, A.P. Vitamins as antimutagens: Advantages and some possible mechanisms of antimutagenic action. Mutat. Res. Rev. Mutat. Res. 1997, 386, 39–67. [Google Scholar] [CrossRef]
- Kaya, B.; Creus, A.; Velázquez, A.; Yanikoğlu, A.; Marcos, R. Genotoxicity is modulated by ascorbic acid: Studies using the wing spot test in Drosophila. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2002, 520, 93–101. [Google Scholar] [CrossRef]
- Moreno, F.S.; Wu, T.S.; Penteado, M.V.; Rizzi, M.B.; Jordão, J.A.; Almeida-Muradian, L.B.; Dagli, M.L. A comparison of beta-carotene and vitamin A effects on a hepatocarcinogenesis model. International journal for vitamin and nutrition research. J. Int. Vitaminol. Nutr. 1994, 65, 87–94. [Google Scholar]
- He, Y.; Root, M.M.; Parker, R.S.; Campbell, T.C. Effects of carotenoid-rich food extracts on the development of preneoplastic lesions in rat liver and on in vivo and in vitro antioxidant status. Nutr. Cancer 1997. [Google Scholar] [CrossRef] [PubMed]
- Gradelet, S.; Astorg, P.; Le Bon, A.M.; Bergès, R.; Suschetet, M. Modulation of aflatoxin B1 carcinogenicity, genotoxicity and metabolism in rat liver by dietary carotenoids: Evidence for a protective effect of CYP1A inducers. Cancer Lett. 1997, 114, 221–223. [Google Scholar] [CrossRef]
- Ravikrishnan, R.; Rusia, S.; Ilamurugan, G.; Salunkhe, U.; Deshpande, J.; Shankaranarayanan, J.; Shankaranarayanan, M.L.; Soni, M.G. Safety assessment of lutein and zeaxanthin (Lutemax™ 2020): Subchronic toxicity and mutagenicity studies. Food Chem. Toxicol. 2011, 49, 2841–2848. [Google Scholar] [CrossRef] [PubMed]
- Bi, M.C.; Rosen, R.; Zha, R.Y.; McCormick, S.A.; Song, E.; Hu, D.N. Zeaxanthin induces apoptosis in human uveal melanoma cells through Bcl-2 family proteins and intrinsic apoptosis pathway. Evid. Based Complement. Altern. Med. 2013. [Google Scholar] [CrossRef] [PubMed]
- Kalariya, N.M.; Ramana, K.V.; Srivastava, S.K.; van Kuijk, F. Genotoxic effects of carotenoid breakdown products in human retinal pigment epithelial cells. Curr. Eye Res. 2009, 34, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Khachik, F.; Beecher, G.R. Separation of carotenol fatty acid esters by high-performance liquid chromatography. J. Chromatogr. A 1988, 449, 119–133. [Google Scholar] [CrossRef]
- Peña-Estévez, M.E.; Gómez, P.A.; Artés, F.; Aguayo, E.; Martínez-Hernández, G.B.; Otón, M.; Galindo, A.; Artés-Hernández, F. Quality changes of fresh-cut pomegranate arils during shelf life as affected by deficit irrigation and postharvest vapour treatments. J. Sci. Food Agric. 2015, 95, 2325–2336. [Google Scholar] [CrossRef] [PubMed]
- Zapata, S.; Dufour, J.P. Ascorbic, dehydroascorbic and isoascorbic acid simultaneous determinations by reverse phase ion interaction HPLC. J. Food Sci. 1992, 57, 506–511. [Google Scholar] [CrossRef]
- Gil, M.I.; Ferreres, F.; Tomás-Barberán, F.A. Effect of postharvest storage and processing on the antioxidant constituents (flavonoids and vitamin C) of fresh-cut spinach. J. Agric. Food Chem. 1999, 47, 2213–2217. [Google Scholar] [CrossRef] [PubMed]
- Frei, H.; Würgler, F.E. Optimal experimental design and sample size for the statistical evaluation of data from somatic mutation and recombination tests (SMART) in Drosophila. Mutat. Res. Environ. Mutagen. Relat. Subj. 1995, 334, 247–258. [Google Scholar] [CrossRef]
- Abraham, S.K. Antigenotoxicity of coffee in the Drosophila assay for somatic mutation and recombination. Mutagenesis 1994, 9, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Romero-Jimenez, M.; Campos-Sánchez, J.; Analla, M.; Muñoz-Serrano, A.; Alonso-Moraga, Á. Genotoxicity and anti-genotoxicity of some traditional medicinal herbs. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2005, 585, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Frei, H.; Würgler, F. Statistical methods to decide whether mutagenicity test data from Drosophila assays indicate a positive, negative, or inconclusive result. Mutat. Res. Environ. Mutagen. Relat. Subj. 1988, 203, 297–308. [Google Scholar] [CrossRef]
- Villatoro-Pulido, M.; Font, R.; De Haro-Bravo, M.I.; Romero-Jiménez, M.; Anter, J.; De Haro Bailón, A.; Alonso-Moraga, A.; Del Río-Celestino, M. Modulation of genotoxicity and cytotoxicity by radish grown in metal-contaminated soils. Mutagenesis 2009, 24, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Melo, P.c.S.; Justo, G.Z.; de Azevedo, M.B.; Durán, N.; Haun, M. Violacein and its β-cyclodextrin complexes induce apoptosis and differentiation in HL60 cells. Toxicology 2003, 186, 217–225. [Google Scholar] [CrossRef]
- Surh, Y.-J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Fimognari, C.; Hrelia, P. Sulforaphane as a promising molecule for fighting cancer. Mutat. Res. Rev. Mutat. Res. 2007, 635, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Bode, A.M.; Dong, Z. Molecular targets of phytochemicals for cancer prevention. Nat. Rev. Cancer 2011, 11, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Tadmor, Y.; Paris, H.S.; Meir, A.; Schaffer, A.A.; Lewinsohn, E. Dual role of the pigmentation gene B in affecting carotenoid and vitamin E content in squash (Cucurbita pepo) mesocarp. J. Agric. Food Chem. 2005, 53, 9759–9763. [Google Scholar] [CrossRef] [PubMed]
- EL-Qudah, J.M. Identification and quantification of major carotenoids in some vegetables. Am. J. Appl. Sci. 2009, 6, 492. [Google Scholar] [CrossRef]
- Retsky, K.; Freeman, M.; Frei, B. Ascorbic acid oxidation product(s) protect human low density lipoprotein against atherogenic modification. Anti-rather than prooxidant activity of vitamin C in the presence of transition metal ions. J. Biol. Chem. 1993, 268, 1304–1309. [Google Scholar] [PubMed]
- Moser, U.; Bendich, A.; Machlin, L. Handbook of Vitamins; FAO: New York, NY, USA, 1991. [Google Scholar]
- De Ancos, B.; Sánchez-Moreno, C.; Plaza, L.; Cano, M.P. Nutritional and Health Aspects of Fresh-Cut Vegetables. In Advances in Fresh-Cut Fruits and Vegetables Processing; CRC Press: New York, NY, USA, 2011; pp. 145–184. [Google Scholar]
- Asami, D.K.; Hong, Y.J.; Barrett, D.M.; Mitchell, A.E. Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn grown using conventional, organic, and sustainable agricultural practices. J. Agric. Food Chem. 2003, 51, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Vanamala, J.; Cobb, G.; Turner, N.D.; Lupton, J.R.; Yoo, K.S.; Pike, L.M.; Patil, B.S. Bioactive compounds of grapefruit (Citrus paradisi Cv. Rio Red) respond differently to postharvest irradiation, storage, and freeze drying. J. Agric. Food Chem. 2005, 53, 3980–3985. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Lin, H.Y.; Chang, C.Y.; Liu, Y.C. Comparisons on the antioxidant properties of fresh, freeze-dried and hot-air-dried tomatoes. J. Food Eng. 2006, 77, 478–485. [Google Scholar] [CrossRef]
- Graf, U.; Abraham, S.K.; Guzmán-Rincón, J.; Würgler, F.E. Antigenotoxicity studies in Drosophila melanogaster. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1998, 402, 203–209. [Google Scholar] [CrossRef]
- Anter, J.; Fernández-Bedmar, Z.; Villatoro-Pulido, M.; Demyda-Peyras, S.; Moreno-Millán, M.; Alonso-Moraga, Á.; Muñoz-Serrano, A.; de Castro, M.D.L. A pilot study on the DNA-protective, cytotoxic, and apoptosis-inducing properties of olive-leaf extracts. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2011, 723, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Rueff, J.; Bras, A.; Cristovao, L.; Mexia, J.; Costa, M.S.; Pires, V. DNA strand breaks and chromosomal aberrations induced by H2O2 and 60Co γ-radiation. Mutat. Res. Fundament. Mol. Mech. Mutagen. 1993, 289, 197–204. [Google Scholar] [CrossRef]
- Azizah, A.; Wee, K.C.; Azizah, O.; Azizah, M. Effect of boiling and stir frying on total phenolics, carotenoids and radical scavenging activity of pumpkin (Cucurbita moschato). Int. Food Res. J. 2009, 16, 45–51. [Google Scholar]
- Gacche, R.; Kabaliye, V.N.; Dhole, N.A.; Jadhav, A.D. Antioxidant potential of selected vegetables commonly used in diet in Asian subcontinent. Indian J. Nat. Prod. Resour. 2010, 13, 306–313. [Google Scholar]
- Mi, X.; Wang, C.; Sun, C.; Chen, X.; Huo, X.; Zhang, Y.; Li, G.; Xu, B.; Zhang, J.; Xie, J.; et al. Xanthohumol induces paraptosis of leukemia cells through p38 mitogen activated protein kinase signaling pathway. Oncotarget 2017, 8, 31297. [Google Scholar] [CrossRef] [PubMed]
- Sharoni, Y.; Danilenko, M.; Walfisch, S.; Amir, H.; Nahum, A.; Ben-Dor, A.; Hirsh, K.; Khanin, M.; Steiner, M.; Agemy, L.; et al. Role of gene regulation in the anticancer activity of carotenoids. Pure Appl. Chem. 2002, 74, 1469–1477. [Google Scholar] [CrossRef]
- Dias, C.; Araújo, B.C.; Dutra, E.S.; Nepomuceno, J.C. Protective effects of beta carotene against the genotoxicity of doxorubicin in somatic cells of Drosophila melanogaster. Genet. Mol. Res. 2009, 17, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Omaye, S. Antioxidant and prooxidant roles for β-carotene, α-tocopherol and ascorbic acid in human lung cells. Toxicol. In Vitro 2001, 15, 13–24. [Google Scholar] [CrossRef]
- Burri, B.J. β-Carotene and human health: A review of current research. Nutr. Res. 1997, 17, 547–580. [Google Scholar] [CrossRef]
- Hazuka, M.B.; Edwards-Prasad, J.; Newman, F.; Kinzie, J.J.; Prasad, K.N. β-Carotene induces morphological differentiation and decreases adenylate cyclase activity in melanoma cells in culture. J. Am. Coll. Nutr. 1990, 9, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.W.; Boileau, T.W.M.; Zhou, J.R.; Clinton, S.K.; Erdman, J.W. β-Carotene modulates human prostate cancer cell growth and may undergo intracellular metabolism to retinol. J. Nutr. 2000, 130, 728–732. [Google Scholar] [PubMed]
- Palozza, P.; Serini, S.; Maggiano, N.; Angelini, M.; Boninsegna, A.; Di Nicuolo, F.; Ranelleti, F.O.; Calviello, G. Induction of cell cycle arrest and apoptosis in human colon adenocarcinoma cell lines by β-carotene through down-regulation of cyclin A and Bcl-2 family proteins. Carcinogenesis 2002, 23, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.; Shklar, G. The selective cytotoxic effect of carotenoids and α-tocopherol on human cancer cell lines in vitro. J. Oral Maxillofac. Surg. 1992, 50, 367–373. [Google Scholar] [CrossRef]
- Sacha, T.; Zawada, M.; Hartwich, J.; Lach, Z.; Polus, A.; Szostek, M.; Zdzilowska, E.; Libura, M.; Bodzioch, M.; Skotnicki, A.B.; et al. The effect of β-carotene and its derivatives on cytotoxicity, differentiation, proliferative potential and apoptosis on the three human acute leukemia cell lines: U-937, HL-60 and TF-1. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2005, 1740, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Palozza, P.; Serini, S.; Torsello, A.; Boninsegna, A.; Covacci, V.; Maggiano, N.; Ranelleti, F.O.; Wolf, F.I.; Calviello, G. Regulation of cell cycle progression and apoptosis by β-carotene in undifferentiated and differentiated HL-60 leukemia cells: Possible involvement of a redox mechanism. Int. J. Cancer 2002, 97, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Guruvayoorappan, C.; Kuttan, G. β-Carotene down-regulates inducible nitric oxide synthase gene expression and induces apoptosis by suppressing bcl-2 expression and activating caspase-3 and p53 genes in B16F-10 melanoma cells. Nutr. Res. 2007, 27, 336–342. [Google Scholar] [CrossRef]
- Cui, Y.; Lu, Z.; Lin, B.; Shi, Z.; Zhao, W.; Zhao, B. β-Carotene induces apoptosis and up-regulates peroxisome proliferator-activated receptor γ expression and reactive oxygen species production in MCF-7 cancer cells. Eur. J. Cancer 2007, 43, 2590–2601. [Google Scholar] [CrossRef] [PubMed]
- Lakshminarayana, R.; Sathish, U.V.; Dharmesh, S.M.; Baskaran, V. Antioxidant and cytotoxic effect of oxidized lutein in human cervical carcinoma cells (HeLa). Food Chem. Toxicol. 2010, 48, 1811–1816. [Google Scholar] [CrossRef] [PubMed]
- Ayyadurai, N.; Valarmathy, N.; Kannan, S.; Jansirani, D.; Alsenaidy, A. Evaluation of cytotoxic properties of Curcuma longa and Tagetes erecta on cancer cell line (Hep2). Afr. J. Pharm. Pharmacol. 2013, 7, 736–739. [Google Scholar] [CrossRef]
- Cha, K.H.; Koo, S.Y.; Lee, D.-U. Antiproliferative effects of carotenoids extracted from Chlorella ellipsoidea and Chlorella vulgaris on human colon cancer cells. J. Agric. Food Chem. 2008, 56, 10521–10526. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.E.; Wielgus, A.R.; Boyes, W.K.; Andley, U.; Chignell, C.F. Phototoxicity and cytotoxicity of fullerol in human lens epithelial cells. Toxicol. Appl. Pharmacol. 2008, 228, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Ravi, K.; Reddy, K.R.; Shankaranarayanan, J.; Deshpande, J.V.; Juturu, V.; Soni, M.G. Safety evaluation of zeaxanthin concentrate (OmniXan™): Acute, subchronic toxicity and mutagenicity studies. Food Chem. Toxicol. 2014, 72, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Thurnham, D.I.; Howard, A.N. Studies on meso-zeaxanthin for potential toxicity and mutagenicity. Food Chem. Toxicol. 2013, 59, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Park, C.H.; Hahm, E.R.; Kim, K.; Kimler, B.F.; Lee, S.J.; Park, H.K.; Lee, S.H.; Kim, W.S.; Jung, C.W.; et al. Activation of Raf1 and the ERK pathway in response to l-ascorbic acid in acute myeloid leukemia cells. Cell Signal. 2005, 17, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.N.; Rahman, M.H.; Guo, R.; Hirashima, A. Hypoglycemic activity of methanolic leaf extract of Blumea lacera in Swiss-albino mice. Asian Pac. J. Trop. Dis. 2015, 5, 195–198. [Google Scholar] [CrossRef]
- Miro, M. Cucurbitacins and their pharmacological effects. Phytother. Res. 1995, 9, 159–168. [Google Scholar] [CrossRef]
- Wang, X.; Tanaka, M.; Peixoto, H.S.; Wink, M. Cucurbitacins: Elucidation of their interactions with the cytoskeleton. PeerJ 2017, 5, e3357. [Google Scholar] [CrossRef] [PubMed]








| Yellow | Light | |
|---|---|---|
| Epicarp | ||
| Lutein | 1036.9 a | 135 b |
| β-carotene | 99.5 a | n.d. b |
| Zeaxanthin | 18.6 a | 1.7 b |
| DHA | 369.3 b | 592 a |
| Mesocarp | ||
| Lutein | 362.7 a | 63.2 b |
| β-carotene | 31.6 a | 4.0 b |
| Zeaxanthin | 1.9 a | 1.7 a |
| DHA | 3200 a | 683.3 b |
| Clones Per Wing (Number of Spots) 1 | ||||||
|---|---|---|---|---|---|---|
| Compound | N | Small Spots (1–2 Cells) m = 2 | Large Spots (>2 Cells) m = 5 | Twin Spots m = 5 | Total Spots m = 2 | Mann-Whitney Test 3 |
| Negative Control (H2O) | 40 | 0.08 (3) 2 | 0.00 (0) | 0.05 (2) | 0.13 (5) | |
| Epicarp | ||||||
| Yellow (mg/mL) | ||||||
| 0.25 | 40 | 0.15 (6) | 0.00 (0) | 0.03 (1) | 0.18 (7) | n.s. |
| 8 | 40 | 0.00 (0) | 0.00 (0) | 0.03 (1) | 0.03 (1) | n.s. |
| Light Green (mg/mL) | ||||||
| 0.25 | 40 | 0.10 (4) | 0.03 (1) | 0.00 (0) | 0.13 (5) | n.s. |
| 8 | 40 | 0.05 (2) | 0.00 (0) | 0.00 (0) | 0.05 (2) | n.s. |
| Mesocarp | ||||||
| Yellow (mg/mL) | ||||||
| 0.25 | 40 | 0.08 (3) | 0.03 (1) | 0.00 (0) | 0.10 (4) | n.s. |
| 8 | 40 | 0.05 (2) | 0.00 (0) | 0.00 (0) | 0.05 (2) | n.s. |
| Light Green (mg/mL) | ||||||
| 0.25 | 40 | 0.03 (1) | 0.03 (1) | 0.00 (0) | 0.05 (2) | n.s. |
| 8 | 40 | 0.03 (1) | 0.03 (1) | 0.00 (0) | 0.05 (2) | n.s. |
| Single Compounds | ||||||
| Lutein (μM) | ||||||
| 0.039 | 40 | 0.15 (6) | 0.08 (3) | 0.00 (0) | 0.23 (9) | n.s. |
| 0.615 | 40 | 0.33 (13) | 0.08 (3) | 0.05 (2) | 0.45 (18) | n.s. |
| β-carotene (μM) | ||||||
| 0.0003 | 36 | 0.08 (3) | 0.00 (0) | 0.00 (0) | 0.08 (3) | n.s. |
| 0.0689 | 40 | 0.03 (1) | 0.03 (1) | 0.00 (0) | 0.05 (2) | n.s. |
| Zeaxanthin (μM) | ||||||
| 0.0001 | 40 | 0.05 (2) | 0.00 (0) | 0.00 (0) | 0.05 (2) | n.s. |
| 0.105 | 40 | 0.10 (4) | 0.03 (1) | 0.03 (1) | 0.15 (6) | n.s. |
| DHA (mM) | ||||||
| 0.003 | 40 | 0.10 (4) | 0.03 (1) | 0.00 (0) | 0.13 (5) | n.s. |
| 0.107 | 40 | 0.15 (6) | 0.03 (1) | 0.00 (0) | 0.18 (7) | n.s. |
| Clones Per Wing (Number of Spots) 1 | ||||||
|---|---|---|---|---|---|---|
| Compounds | N | Small Spots (1–2 Cells) m = 2 | Large Spots (>2 Cells) m = 5 | Twin Spots m = 5 | Total Spots m = 2 | Mann-Whitney Test 3 |
| Negative Control (H2O) | 40 | 0.08 (3) 2 | 0.00 (0) | 0.05 (2) | 0.13 (5) | |
| Positive Control (H2O2 120 mM) | 40 | 0.38 (15) | 0.13 (5) | 0.03 (1) | 0.52 (21) | * |
| Epicarp | ||||||
| Yellow (mg/mL) | ||||||
| 0.25 | 16 | 0.13 (5) | 0.00 (0) | 0.00 (0) | 0.13 (5) | n.s. |
| 8 | 40 | 0.15 (6) | 0.03 (1) | 0.00 (0) | 0.18 (7) | n.s. |
| Light Green (mg/mL) | ||||||
| 0.25 | 40 | 0.13 (5) | 0.05 (2) | 0.03 (1) | 0.20 (8) | n.s. |
| 8 | 40 | 0.05 (2) | 0.03 (1) | 0.03 (1) | 0.10 (4) | n.s. |
| Mesocarp | ||||||
| Yellow (mg/mL) | ||||||
| 0.25 | 18 | 0.00 (0) | 0.00 (0) | 0.00 (0) | 0.00 (0) | n.s. |
| 8 | 40 | 0.38 (15) | 0.00 (0) | 0.03 (1) | 0.40 (16) | n.s. |
| Light Green (mg/mL) | ||||||
| 0.25 | 40 | 0.05 (2) | 0.03 (1) | 0.00 (0) | 0.08 (3) | n.s. |
| 8 | 40 | 0.05 (2) | 0.00 (0) | 0.00 (0) | 0.05 (2) | n.s. |
| Single Compounds | ||||||
| Lutein (μM) | ||||||
| 0.039 | 32 | 0.06 (2) | 0.06 (2) | 0.00 (0) | 0.13 (4) | n.s. |
| 0.615 | 32 | 0.25 (8) | 0.16 (5) | 0.06 (2) | 0.47 (15) | * |
| β-carotene (μM) | ||||||
| 0.0003 | 12 | 0.00 (0) | 0.00 (0) | 0.00 (0) | 0.00 (0) | n.s. |
| 0.0689 | 38 | 0.08 (3) | 0.03 (1) | 0.00 (0) | 0.11 (4) | n.s. |
| Zeaxanthin (μM) | ||||||
| 0.0001 | 40 | 0.03 (1) | 0.00 (0) | 0.03 (1) | 0.05 (2) | n.s. |
| 0.105 | 30 | 0.07 (2) | 0.00 (0) | 0.00 (0) | 0.07 (2) | n.s. |
| DHA (mM) | ||||||
| 0.003 | 40 | 0.08 (3) | 0.00 (0) | 0.00 (0) | 0.08 (3) | n.s. |
| 0.107 | 40 | 0.10 (4) | 0.03 (1) | 0.00 (0) | 0.13 (5) | n.s. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Valdivieso, D.; Font, R.; Fernández-Bedmar, Z.; Merinas-Amo, T.; Gómez, P.; Alonso-Moraga, Á.; Del Río-Celestino, M. Role of Zucchini and Its Distinctive Components in the Modulation of Degenerative Processes: Genotoxicity, Anti-Genotoxicity, Cytotoxicity and Apoptotic Effects. Nutrients 2017, 9, 755. https://doi.org/10.3390/nu9070755
Martínez-Valdivieso D, Font R, Fernández-Bedmar Z, Merinas-Amo T, Gómez P, Alonso-Moraga Á, Del Río-Celestino M. Role of Zucchini and Its Distinctive Components in the Modulation of Degenerative Processes: Genotoxicity, Anti-Genotoxicity, Cytotoxicity and Apoptotic Effects. Nutrients. 2017; 9(7):755. https://doi.org/10.3390/nu9070755
Chicago/Turabian StyleMartínez-Valdivieso, Damián, Rafael Font, Zahira Fernández-Bedmar, Tania Merinas-Amo, Pedro Gómez, Ángeles Alonso-Moraga, and Mercedes Del Río-Celestino. 2017. "Role of Zucchini and Its Distinctive Components in the Modulation of Degenerative Processes: Genotoxicity, Anti-Genotoxicity, Cytotoxicity and Apoptotic Effects" Nutrients 9, no. 7: 755. https://doi.org/10.3390/nu9070755
APA StyleMartínez-Valdivieso, D., Font, R., Fernández-Bedmar, Z., Merinas-Amo, T., Gómez, P., Alonso-Moraga, Á., & Del Río-Celestino, M. (2017). Role of Zucchini and Its Distinctive Components in the Modulation of Degenerative Processes: Genotoxicity, Anti-Genotoxicity, Cytotoxicity and Apoptotic Effects. Nutrients, 9(7), 755. https://doi.org/10.3390/nu9070755