Phyllanthus Niruri Standardized Extract Alleviates the Progression of Non-Alcoholic Fatty Liver Disease and Decreases Atherosclerotic Risk in Sprague–Dawley Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. High-Fat Diet Preparation
2.2. Extract Preparation
2.3. In Vivo Model
2.4. Determination of Blood and Serum Biochemical Parameters
2.5. Determination of Insulin and Free Fatty Acids Concentrations
2.6. Assessment of Atherosclerosis Risk Indexes
2.7. Assessment of Lipid Peroxidation, TG and TC Contents in Liver Tissues
2.8. Histopathological Examinations
2.9. Antioxidant Activity Tests
2.10. In Vitro Assay to Determine the Inhibitory Effects of the Most Active Extract on α-Glucosidase, Pancreatic Lipase and Cholesterol Micellization
2.11. Phytochemical Analysis by High-Performance Liquid Chromatography (HPLC)
2.12. Statistical Analysis
3. Results
3.1. P. niruri Reduced Hepatomegaly and Visceral Adiposity
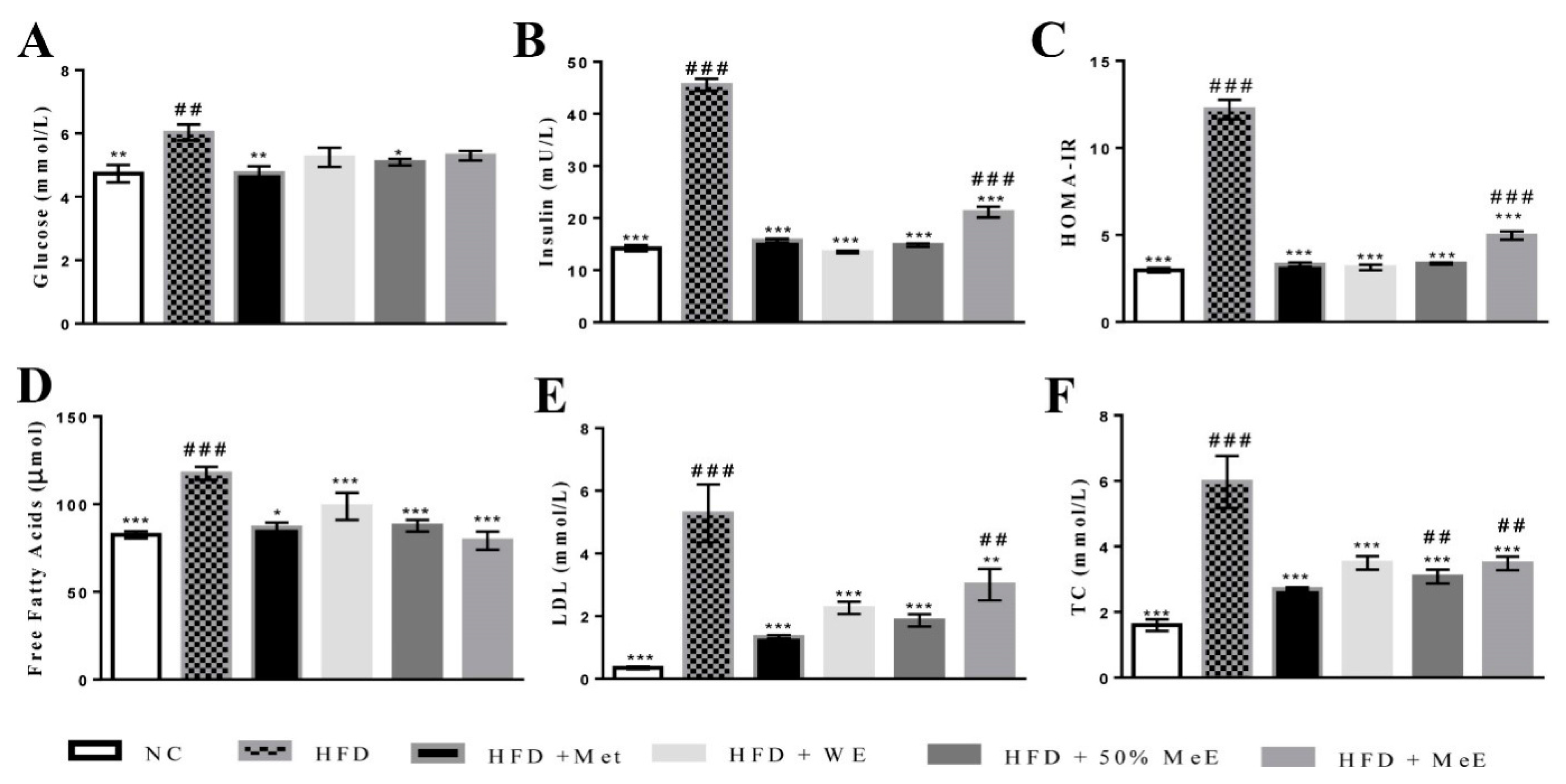
3.2. P. niruri Improved Abnormalities in Serum Indicators of NAFLD Rats
3.3. P. niruri Decreased FFAs and Insulin Resistance
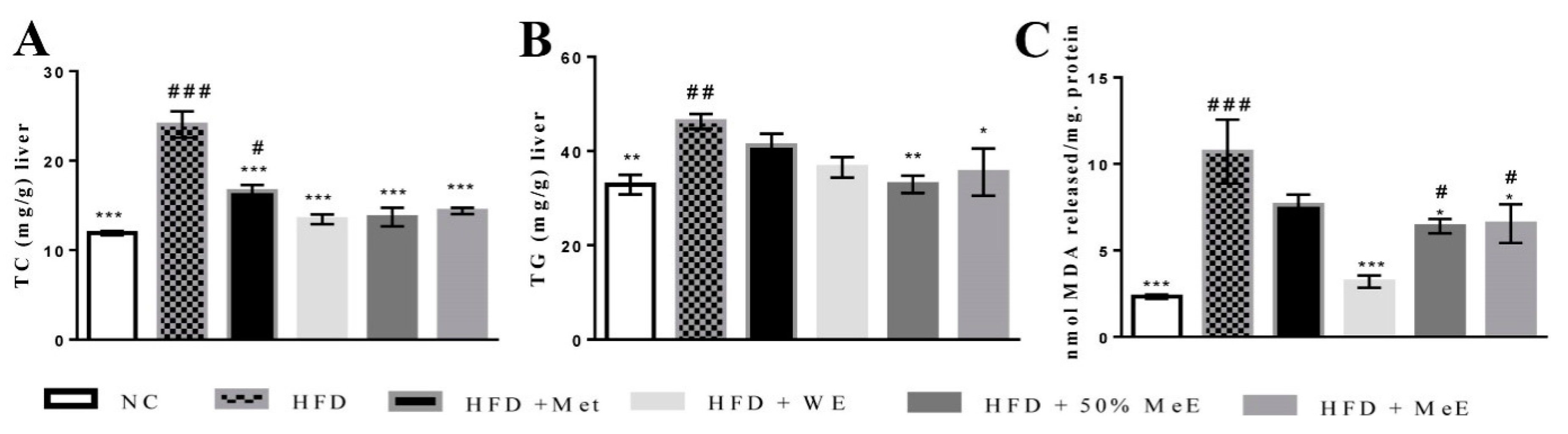
3.4. P. niruri Reduced the Liver Content of TC, TG and Oxidative Stress Indicator
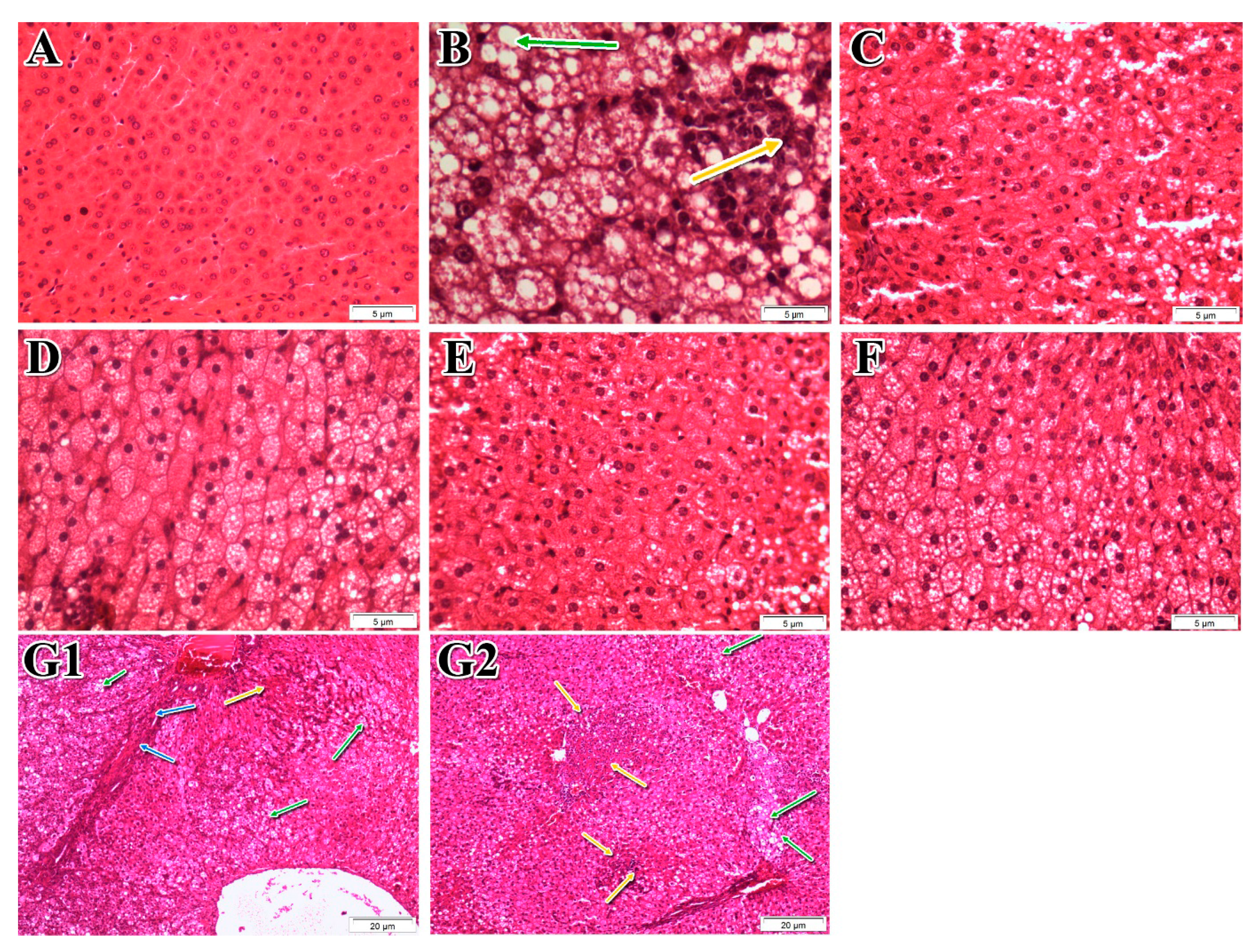
3.5. Effects of P. niruri on the Liver Histopathology
3.6. In Vitro Tests for the 50% ME of P. niruri
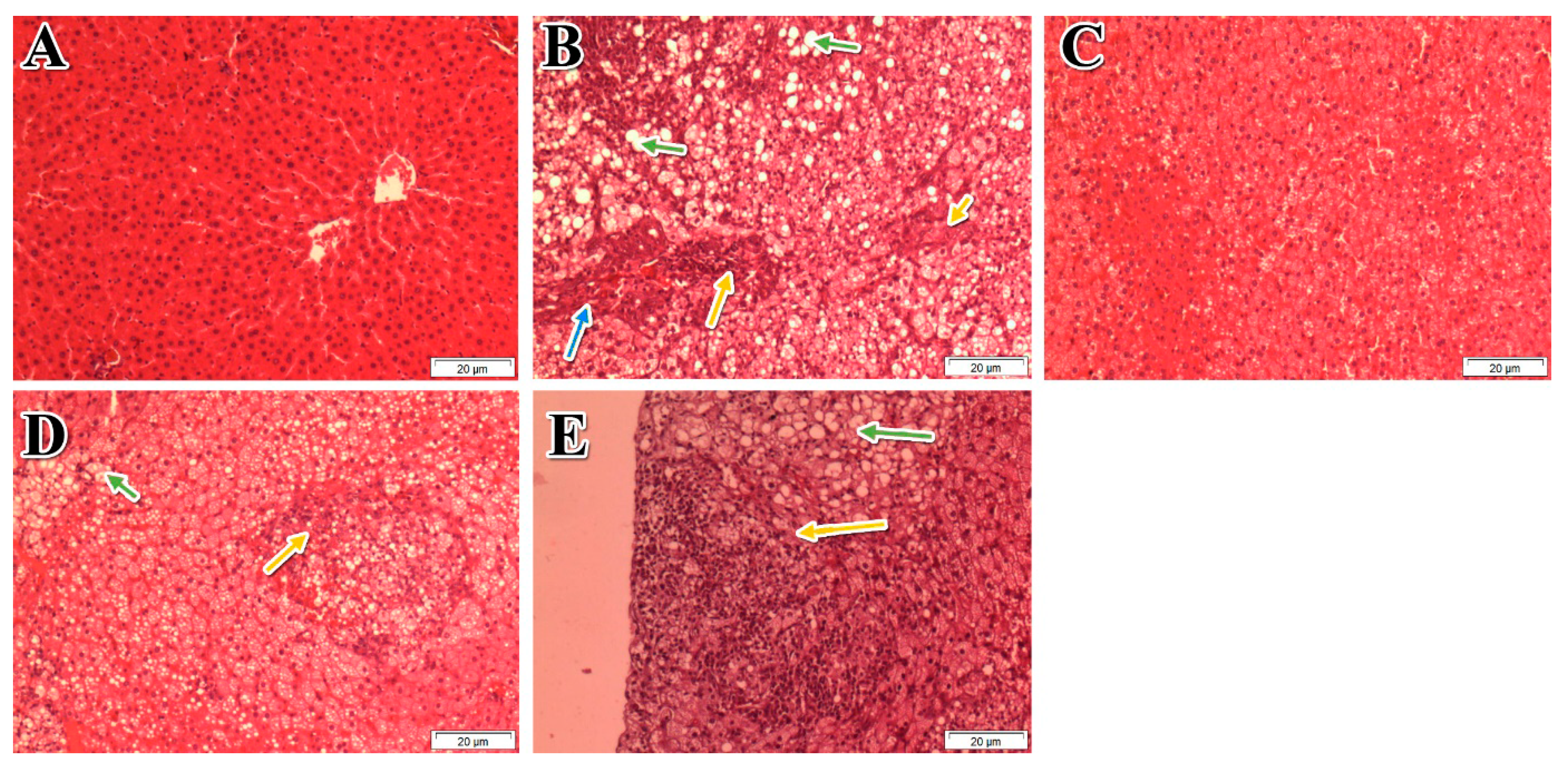
3.7. The Effect of Different Doses of 50% ME of P. niruri on NAFLD
3.8. Total Phenolic Content (TPC), Total Flavonoid Content (TFC) and Anti-Oxidative Activities of P. niruri
3.9. Extract Standardization by HPLC Analysis
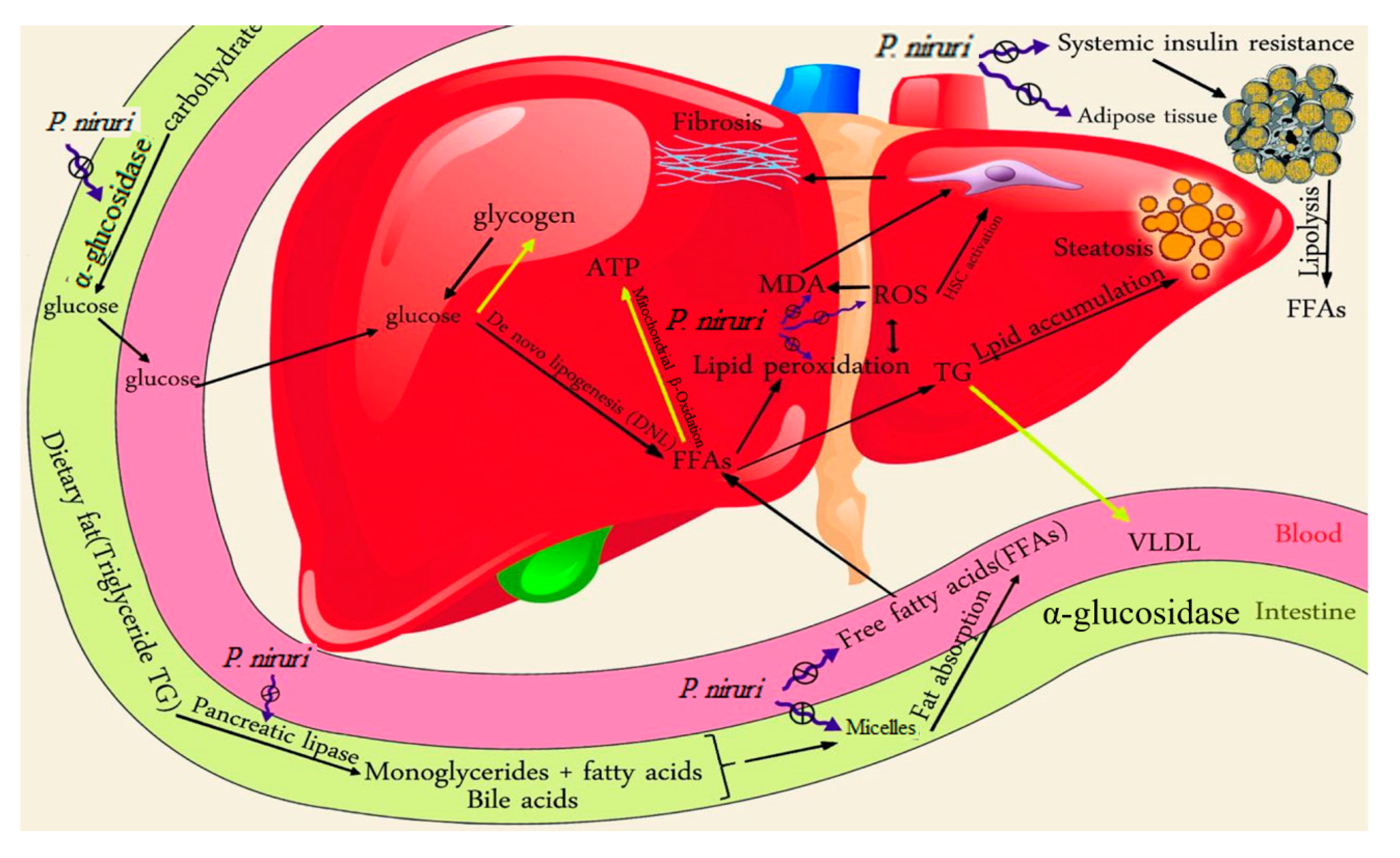
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abenavoli, L.; Milic, N.; di Renzo, L.; Preveden, T.; Medić-Stojanoska, M.; de Lorenzo, A. Metabolic aspects of adult patients with nonalcoholic fatty liver disease. World J. Gastroenterol. 2016, 22, 7006. [Google Scholar] [CrossRef] [PubMed]
- Tzanetakou, I.P.; Doulamis, I.P.; Korou, L.-M.; Agrogiannis, G.; Vlachos, I.S.; Pantopoulou, A.; Mikhailidis, D.P.; Patsouris, E.; Vlachos, I.; Perrea, D.N. Water Soluble Vitamin E Administration in Wistar Rats with Non-alcoholic Fatty Liver Disease. Open Cardiovasc. Med. J. 2012, 6, 88. [Google Scholar] [CrossRef] [PubMed]
- Videla, L.A.; Rodrigo, R.; Araya, J.; Poniachik, J. Insulin resistance and oxidative stress interdependency in non-alcoholic fatty liver disease. Trends Mol. Med. 2006, 12, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Comar, K.; Sterling, R. Review article: Drug therapy for non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2006, 23, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Vernon, G.; Baranova, A.; Younossi, Z. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 2011, 34, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Milic, S.; Stimac, D. Nonalcoholic fatty liver disease/steatohepatitis: Epidemiology, pathogenesis, clinical presentation and treatment. Dig. Dis. 2012, 30, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Charlton, M.; Kasparova, P.; Weston, S.; Lindor, K.D.; Maor-Kendler, Y.; Wiesner, R.H.; Rosen, C.B.; Batts, K.P. Frequency of nonalcoholic steatohepatitis as a cause of advanced liver disease. Liver Transplant. 2001, 7, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Staprans, I.; Pan, X.M.; Rapp, J.H.; Feingold, K.R. The role of dietary oxidized cholesterol and oxidized fatty acids in the development of atherosclerosis. Mol. Nutr. Food Res. 2005, 49, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Calzadilla Bertot, L.; Adams, L.A. The natural course of non-alcoholic fatty liver disease. Int. J. Mol. Sci. 2016, 17, 774. [Google Scholar] [CrossRef] [PubMed]
- Boursier, J.; Mueller, O.; Barret, M.; Machado, M.; Fizanne, L.; Araujo-Perez, F.; Guy, C.D.; Seed, P.C.; Rawls, J.F.; David, L.A. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 2016, 63, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Hamad, E.M.; Taha, S.H.; Abou Dawood, A.; Sitohy, M.Z.; Abdel-Hamid, M. Protective effect of whey proteins against nonalcoholic fatty liver in rats. Lipids Health Dis. 2011, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Hanhineva, K.; Törrönen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, H.J.; Lee, K.E.; Kim, D.J.; Kim, S.K.; Ahn, C.W.; Lim, S.; Kim, K.R.; Lee, H.C.; Huh, K.B. Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch. Int. Med. 2004, 164, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Gaggini, M.; Morelli, M.; Buzzigoli, E.; de Fronzo, R.A.; Bugianesi, E.; Gastaldelli, A. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients 2013, 5, 1544–1560. [Google Scholar] [CrossRef] [PubMed]
- Kashi, M.R.; Torres, D.M.; Harrison, S.A. Current and Emerging Therapies in Nonalcoholic Fatty Liver Disease; Seminars in liver disease; Thieme Medical Publishers, Inc.: New York, NY, USA, 2008; pp. 396–406. [Google Scholar]
- Filippatos, T.D.; Elisaf, M.S. Role of ezetimibe in non-alcoholic fatty liver disease. World J. Hepatol. 2011, 3, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, Y.; Fujita, K.; Yoneda, M.; Wada, K.; Shinohara, Y.; Takahashi, H.; Kirikoshi, H.; Inamori, M.; Kubota, K.; Saito, S. Long-term combination therapy of ezetimibe and acarbose for non-alcoholic fatty liver disease. J. Hepatol. 2009, 51, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Paithankar, V.; Raut, K.; Charde, R.; Vyas, J. Phyllanthus niruri: A magic herb. Res. Pharm. 2015, 1, 1–9. [Google Scholar]
- Milosevic, N.; Milanovic, M.; Abenavoli, L.; Milic, N. Phytotherapy and NAFLD-from goals and challenges to clinical practice. Rev. Recent Clin. Trials 2014, 9, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, K.; Mohan, C.V.R.; Gobianand, K.; Karthikeyan, S. Silymarin modulates the oxidant–antioxidant imbalance during diethylnitrosamine induced oxidative stress in rats. Eur. J. Pharmacol. 2007, 560, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Lu, F.-E.; Gao, Z.-Q.; Xu, L.-J.; Wang, K.-F.; Zou, X. Effects of emodin on treating murine nonalcoholic fatty liver induced by high caloric laboratory chaw. World J. Gastroenterol. 2005, 11, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Bagalkotkar, G.; Sagineedu, S.R.; Saad, M.S.; Stanslas, J. Phytochemicals from Phyllanthus niruri Linn. and their pharmacological properties: A review. J. Pharm. Pharmacol. 2006, 58, 1559–1570. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.; Schor, N.; Boim, M.A. The effect of Phyllanthus niruri on urinary inhibitors of calcium oxalate crystallization and other factors associated with renal stone formation. BJU Int. 2002, 89, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, B.; Shanmugam, K.R.; Ravi, S.; Subbaiah, G.V.; Ramakrishana, C.; Mallikarjuna, K.; Reddy, K.S. Exploratory studies of (−)-Epicatechin, a bioactive compound of Phyllanthus niruri, on the antioxidant enzymes and oxidative stress markers in d-galactosamine-induced hepatitis in rats: A study with reference to clinical prospective. Pharmacogn. Mag. 2017, 13, S56. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; Kumar, B. Efficacy of Phyllanthus niruri Linn. Extract in the management of type-2 diabetes mellitus associated hypercholesterolemia in mice diabetic model. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 507–513. [Google Scholar]
- Shajib, M.S.; Akter, S.; Ahmed, T.; Imam, M.Z. Antinociceptive and neuropharmacological activities of methanol extract of Phoenix sylvestris fruit pulp. Front. Pharm. 2015, 6, 212. [Google Scholar] [CrossRef] [PubMed]
- Pramyothin, P.; Ngamtin, C.; Poungshompoo, S.; Chaichantipyuth, C. Hepatoprotective activity of Phyllanthus amarus Schum. et. Thonn. extract in ethanol treated rats: In vitro and in vivo studies. J. Ethnopharmacol. 2007, 114, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Manjrekar, A.; Jisha, V.; Bag, P.; Adhikary, B.; Pai, M.; Hegde, A.; Nandini, M. Effect of Phyllanthus niruri Linn. treatment on liver, kidney and tests in CCl4 induced hepatotoxic rats. Indian J. Exp. Biol. 2008, 46, 514. [Google Scholar] [PubMed]
- Bhardwaj, S.; Bhattacharjee, J.; Bhatnagar, M.; Tyagi, S. Atherogenic index of plasma, castelli risk index and atherogenic coefficient-new parameters in assessing cardiovascular risk. Int. J. Pharm. Bio Sci. 2013, 3, 359–364. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane-Stanley, G. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Gurau, G.; Coman, M.; Dinu, C.A.; Busila, C.; Voicu, D.C.; Macovei, L.A.; Calin, A.M. The Electrophoretic Patterns of Serum Proteins in Children. Rev. Chim. 2016, 67, 190–194. [Google Scholar]
- Kumaran, A.; Joel Karunakaran, R. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT-Food Sci. Technol. 2007, 40, 344–352. [Google Scholar] [CrossRef]
- Orhan, N.; Orhan, I.E.; Ergun, F. Insights into cholinesterase inhibitory and antioxidant activities of five Juniperus species. Food Chem. Toxicol. 2011, 49, 2305–2312. [Google Scholar] [CrossRef] [PubMed]
- Al-Mansoub, M.A.; Asmawi, M.; Murugaiyah, V. Effect of extraction solvents and plant parts used on the antihyperlipidemic and antioxidant effects of Garcinia atroviridis: A comparative study. J. Sci. Food Agric. 2014, 94, 1552–1558. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Yusoff, N.A.; Ahmad, M.; al Hindi, B.; Widyawati, T.; Yam, M.F.; Mahmud, R.; Razak, K.N.A.; Asmawi, M.Z. Aqueous Extract of Nypa fruticans Wurmb. Vinegar Alleviates Postprandial Hyperglycemia in Normoglycemic Rats. Nutrients 2015, 7, 7012–7026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kang, M.; Kim, M.; Kim, M.; Song, J.; Lee, Y.; Kim, J. Pancreatic lipase inhibitory activity of taraxacum officinale in vitro and in vivo. Nutr. Res. Pract. 2008, 2, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, H.; Akazome, Y.; Shoji, T.; Yamaguchi, A.; Yasue, M.; Kanda, T.; Ohtake, Y. Oligomeric procyanidins in apple polyphenol are main active components for inhibition of pancreatic lipase and triglyceride absorption. J. Agric. Food Chem. 2007, 55, 4604–4609. [Google Scholar] [CrossRef] [PubMed]
- Kirana, C.; Rogers, P.F.; Bennett, L.E.; Abeywardena, M.Y.; Patten, G.S. Naturally derived micelles for rapid in vitro screening of potential cholesterol-lowering bioactives. J. Agric. Food Chem. 2005, 53, 4623–4627. [Google Scholar] [CrossRef] [PubMed]
- Murugaiyah, V.; Chan, K.-L. Determination of four lignans in Phyllanthus niruri L. by a simple high-performance liquid chromatography method with fluorescence detection. J. Chromatogr. A 2007, 1154, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Khalid, H.; Zhari, I.; Amirin, S.; Pazilah, I. Accelerated stability and chemical kinetics of ethanol extracts of fruit of piper sarmentosum using high performance liquid chromatography. Iran. J. Pharm. Res. 2011, 10, 403–413. [Google Scholar] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 2012, 142, 1592–1609. [Google Scholar] [PubMed]
- Brunt, E.M. Nonalcoholic Steatohepatitis: Definition and Pathology; Seminars in liver disease; Thieme Medical Publishers: New York, NY, USA, 2001; pp. 003–016. [Google Scholar] [CrossRef]
- Kirsch, R.; Clarkson, V.; Shephard, E.G.; Marais, D.A.; Jaffer, M.A.; Woodburne, V.E.; Kirsch, R.E.; Hall, P.D.L.M. Rodent nutritional model of non-alcoholic steatohepatitis: Species, strain and sex difference studies. J. Gastroenterol. Hepatol. 2003, 18, 1272–1282. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.; Torbenson, M.S.; Unalp-Arida, A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Sanyal, A.J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Dhibi, M.; Brahmi, F.; Mnari, A.; Houas, Z.; Chargui, I.; Bchir, L.; Gazzah, N.; Alsaif, M.A.; Hammami, M. The intake of high fat diet with different trans fatty acid levels differentially induces oxidative stress and non alcoholic fatty liver disease (NAFLD) in rats. Nutr. Metab. 2011, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, D.; Topczewski, F.; Pagliassotti, M.J. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am. J. Phys.-Endocrinol. Metab. 2006, 291, E275–E281. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, H.; Zheng, H.; Jiang, Y. Hepatic inflammation scores correlate with common carotid intima-media thickness in rats with NAFLD induced by a high-fat diet. BMC Vet. Res. 2014, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.C.; Watts, G.F.; Ng, T.W.K.; Hua, J.; Song, S.; Barrett, P.H.R. Measurement of liver fat by magnetic resonance imaging: Relationships with body fat distribution, insulin sensitivity and plasma lipids in healthy men. Diabetes Obes. Metab. 2006, 8, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.R.D.A.; Diniz, M.D.F.F.D.; Medeiros-Filho, J.M.D.; Araújo, M.S.T.D. Metabolic syndrome and risk factors for non-alcoholic fatty liver disease. Arq. Gastroenterol. 2012, 49, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Nehra, V.; Angulo, P.; Buchman, A.L.; Lindor, K.D. Nutritional and metabolic considerations in the etiology of nonalcoholic steatohepatitis. Dig. Dis. Sci. 2001, 46, 2347–2352. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zhang, X.-G.; Li, Y.-M.; Yu, C.-H.; Xu, L.; Xu, G.-Y. Research on the protection effect of pioglitazone for non-alcoholic fatty liver disease (NAFLD) in rats. J. Zhejiang Univ. Sci. B 2006, 7, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Letteron, P.; Fromenty, B.; Benoît, T.; Degott, C.; Pessayre, D. Acute and chronic hepatic steatosis lead to in vivo lipid peroxidation in mice. J. Hepatol. 1996, 24, 200–208. [Google Scholar] [CrossRef]
- Yokozawa, T.; Cho, E.J.; Sasaki, S.; Satoh, A.; Okamoto, T.; Sei, Y. The protective role of Chinese prescription Kangen-karyu extract on diet-induced hypercholesterolemia in rats. Biol. Pharm. Bull. 2006, 29, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, P.-J.; Hsieh, T.-J.; Kuo, K.-K.; Hung, W.-W.; Tsai, K.-B.; Yang, C.-H.; Yu, M.-L.; Shin, S.-J. Pioglitazone retrieves hepatic antioxidant DNA repair in a mice model of high fat diet. BMC Mol. Biol. 2008, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Mai, T.T.; Thu, N.N.; Tien, P.G.; CHUYEN, N.V. Alpha-glucosidase inhibitory and antioxidant activities of Vietnamese edible plants and their relationships with polyphenol contents. J. Nutr. Sci. Vitaminol. 2007, 53, 267–276. [Google Scholar] [CrossRef] [PubMed]
- De Bari, O.; Neuschwander-Tetri, B.A.; Liu, M.; Portincasa, P.; Wang, D.Q.-H. Ezetimibe: Its novel effects on the prevention and the treatment of cholesterol gallstones and nonalcoholic Fatty liver disease. J. Lipids 2011, 2012, 302847. [Google Scholar] [CrossRef] [PubMed]
- Day, C.P. Non-alcoholic fatty liver disease: Current concepts and management strategies. Clin. Med. 2006, 6, 19–25. [Google Scholar] [CrossRef]
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Choi, Y.; Cicerchi, C.; Kanbay, M.; Roncal-Jimenez, C.A.; Ishimoto, T.; Li, N.; Marek, G.; Duranay, M. Uric Acid induces hepatic steatosis by generation of mitochondrial oxidative stress potential role in fructose-dependent and-independent fatty liver. J. Biol. Chem. 2012, 287, 40732–40744. [Google Scholar] [CrossRef] [PubMed]
- Athyros, V.G.; Mikhailidis, D.P.; Liberopoulos, E.N.; Kakafika, A.I.; Karagiannis, A.; Papageorgiou, A.A.; Tziomalos, K.; Ganotakis, E.S.; Elisaf, M. Effect of statin treatment on renal function and serum uric acid levels and their relation to vascular events in patients with coronary heart disease and metabolic syndrome A subgroup analysis of the GREek Atorvastatin and Coronary heart disease Evaluation (GREACE) Study. Nephrol. Dial. Transplant. 2007, 22, 118–127. [Google Scholar] [PubMed]
- Binder, C.J.; Hartvigsen, K.; Chang, M.-K.; Miller, M.; Broide, D.; Palinski, W.; Curtiss, L.K.; Corr, M.; Witztum, J.L. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J. Clin. Investig. 2004, 114, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Malhi, H.; Bronk, S.F.; Werneburg, N.W.; Gores, G.J. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J. Biol. Chem. 2006, 281, 12093–12101. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-C.; Yang, S.-H.; Hsia, S.-M.; Wu, C.-H.; Yen, G.-C. Inhibitory effects of Phyllanthus emblica L. on hepatic steatosis and liver fibrosis in vitro. J. Funct. Foods 2016, 20, 20–30. [Google Scholar] [CrossRef]
- Okla, M.; Kang, I.; Kim, D.M.; Gourineni, V.; Shay, N.; Gu, L.; Chung, S. Ellagic acid modulates lipid accumulation in primary human adipocytes and human hepatoma Huh7 cells via discrete mechanisms. J. Nutr. Biochem. 2015, 26, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Kang, I. Mechanisms by Which Dietary Ellagic Acid Attenuates Obesity and Obesity-Mediated Metabolic Complications. Ph.D. Thesis, University of Nebraska, Lincoln, NE, USA, November 2015. [Google Scholar]
- Jagtap, S.; Khare, P.; Mangal, P.; Kondepudi, K.K.; Bishnoi, M.; Bhutani, K.K. Protective effects of phyllanthin, a lignan from Phyllanthus amarus, against progression of high fat diet induced metabolic disturbances in mice. RSC Adv. 2016, 6, 58343–58353. [Google Scholar] [CrossRef]
- Asrih, M.; Jornayvaz, F.R. Diets and nonalcoholic fatty liver disease: The good and the bad. Clin. Nutr. 2014, 33, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Van Rooyen, D.M.; Gan, L.T.; Yeh, M.M.; Haigh, W.G.; Larter, C.Z.; Ioannou, G.; Teoh, N.C.; Farrell, G.C. Pharmacological cholesterol lowering reverses fibrotic NASH in obese, diabetic mice with metabolic syndrome. J. Hepatol. 2013, 59, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, C.; de Ledinghen, V.; Vergniol, J.; Foucher, J.; Le Bail, B.; Carlier, S.; Maury, E.; Gin, H.; Rigalleau, V. Hepatic steatosis, carbohydrate intake, and food quotient in patients with NAFLD. Int. J. Endocrinol. 2013, 2013, 428542. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ren, Q.; Wu, T.; Guo, Y.; Liang, Y.; Liu, S. Ezetimibe prevents the development of non-alcoholic fatty liver disease induced by high-fat diet in C57BL/6J mice. Mol. Med. Rep. 2014, 10, 2917–2923. [Google Scholar] [CrossRef] [PubMed]
- Adisakwattana, S.; Charoenlertkul, P.; Yibchok-anun, S. α-Glucosidase inhibitory activity of cyanidin-3-galactoside and synergistic effect with acarbose. J. Enzym. Inhib. Med. Chem. 2009, 24, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.A.; Cho, E.J.; Tanaka, T.; Yokozawa, T. Inhibitory activities of proanthocyanidins from persimmon against oxidative stress and digestive enzymes related to diabetes. J. Nutr. Sci. Vitaminol. 2007, 53, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Asare, G.; Addo, P.; Bugyei, K.; Gyan, B.; Adjei, S.; Otu-Nyarko, L.; Wiredu, E.; Nyarko, A. Acute toxicity studies of aqueous leaf extract of Phyllanthus Niruri. Interdiscip. Toxicol. 2011, 4, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Sarisetyaningtyas, P.V.; Hadinegoro, S.R.; Munasir, Z. Randomized controlled trial of Phyllanthus niruri Linn extract. Paediatr. Indones. 2016, 46, 77–81. [Google Scholar] [CrossRef]


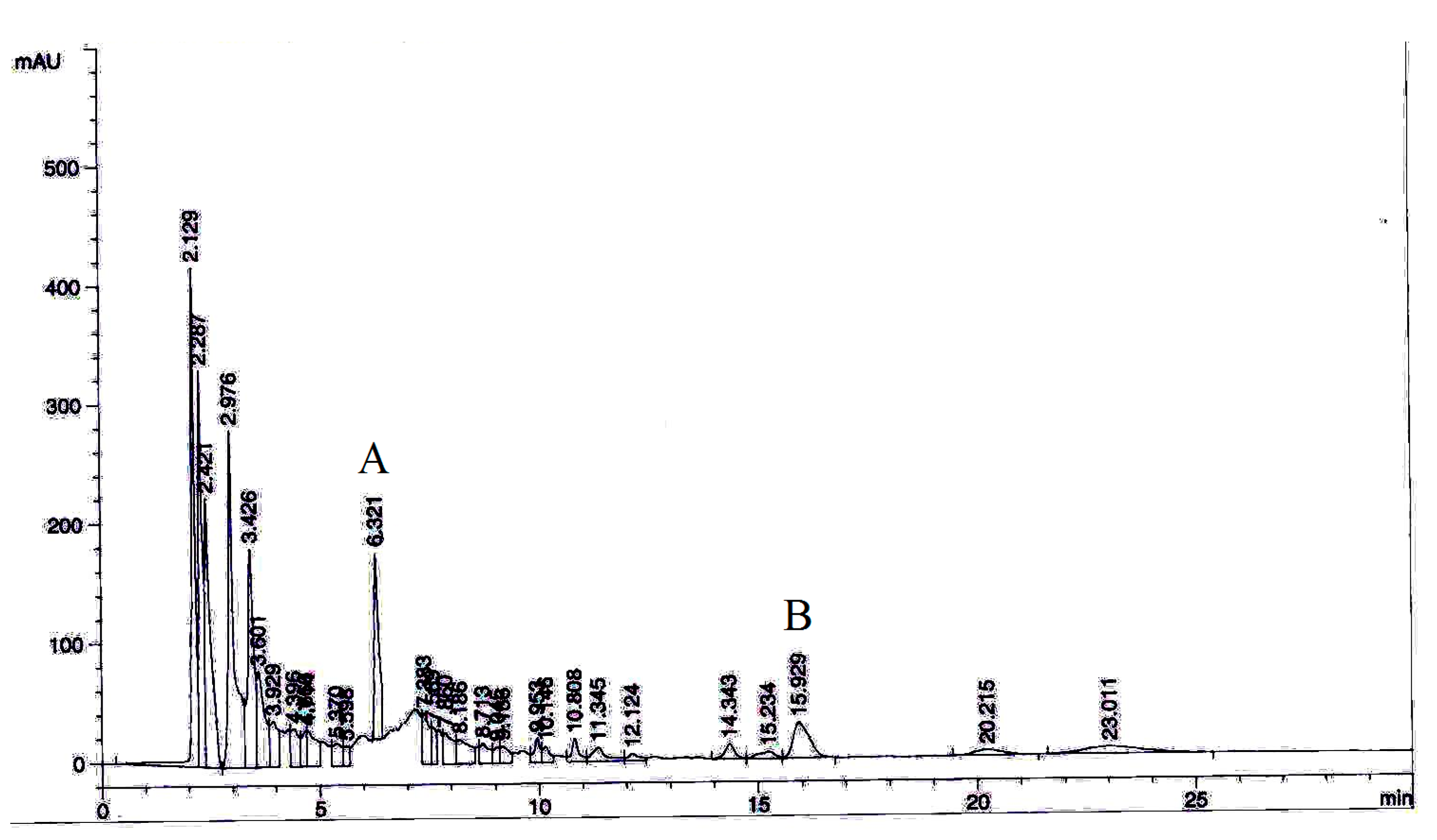
| Parameters | NC Group | HFD Group | HFD + Met | HFD + WE | HFD + 50% ME | HFD + ME |
|---|---|---|---|---|---|---|
| CRI-I = TC/HDL | 1.53 ± 0.03 b | 4.50 ± 0.57 d | 2.40 ± 0.10 b | 3.53 ± 0.26 d | 3.18 ± 0.16 a,d | 4.48 ± 0.39 d |
| CRI-II = LDL/HDL | 0.41 ± 0.05 b | 5.62 ± 1.10 d | 1.42 ± 0.19 b | 2.28 ± 0.24 b | 1.92 ± 0.17 b | 3.29 ± 0.44 a,d |
| AC = (TC–HDL)/HDL | 0.60 ± 0.06 b | 6.14 ± 1.18 d | 1.64 ± 0.21 b | 2.52 ± 0.26 b | 2.18 ± 0.16 b | 3.93 ± 0.64 a,d |
| Steatosis | Ballooning | Lobular Inflammation | NAS |
|---|---|---|---|
| <5% (0) | None (0) | None (0) | 0 |
| 5–33% (1) | Rare or few (1) | 1–2 foci per 20 × field (1) | 3 |
| 34–66% (2) | Many (2) | 2–4 foci/20 × field (2) | 6 |
| >66% (3) | Many (2) | >4 foci/20 × field (3) | 8 |
| Experimental Groups | Steatosis | Lobular Inflammation | Hepatocyte Ballooning | NAS |
|---|---|---|---|---|
| NC | 0 b | 0 b | 0 b | 0 b |
| HFD | 3 ± 0 d | 1.83 ± 0.17 d | 2 ± 0 | 6.83 ± 0.17 d |
| HFD + Met | 1.5 ± 0.34 b,d | 0.67 ± 0.21 b | 1.83 ± 0.16 d | 4 ± 0.63 b,d |
| HFD + WE | 2 ± 0.26 a,d | 1.33 ± 0.33 d | 2 ± 0 d | 5.33 ± 0.42 a,d |
| HFD + 50% ME | 1.5 ± 0.22 b,d | 1 ± 0 a,c | 2 ± 0 d | 4.5 ± 0.22 b,d |
| HFD + ME | 1.67 ± 0.21 b,d | 1 ± 0.26 a,c | 2 ± 0 d | 4.67 ± 0.42 b,d |
| Parameters | NC | HFD | HFD + 50% ME | ||
|---|---|---|---|---|---|
| 1000 mg/kg | 500 mg/kg | 250 mg/kg | |||
| Liver/body weight (%) | 2.69 ± 0.21 b | 5.80 ± 0.11d | 4.22 ± 0.37 a | 4.98 ± 0.12 | 4.79 ± 0.11 |
| Visceral fat/body weight (%) | 0.86 ± 0.10 b | 1.69 ± 0.16 d | 0.82 ± 0.08 b | 1.36 ± 0.07 | 1.32 ± 0.12 |
| Glucose (mmol/L) | 4.60 ± 0.27 a | 5.42 ± 0.26 c | 4.54 ± 0.11 b | 4.68 ± 0.10 a | 4.80 ± 0.10 |
| TC (mmol/L) | 1.64 ± 0.09 b | 7.06 ± 0.85 d | 4.18 ± 0.39 b | 4.64 ± 0.54 b,c | 4.76 ± 0.82 b,c |
| LDL (mmol/L) | 0.41 ± 0.02 b | 5.92 ± 0.81 d | 3.11 ± 0.34 b | 3.53 ± 0.49 b,c | 3.84 ± 3.84 a,d |
| LDL/HDL | 0.42 ± 0.03 b | 6.79 ± 0.57 d | 3.27 ± 0.34 a | 3.83 ± 0.45 | 4.54 ± 0.49 |
| CRI-I = TC/HDL | 1.66 ± 0.05 b | 7.40 ± 0.66 d | 4.38 ± 0.26 b | 5.26 ± 0.54 a,d | 5.76 ± 0.50 a,d |
| CRI-II = LDL/HDL | 0.42 ± 0.03 b | 6.79 ± 0.57 d | 3.27 ± 0.34 a | 3.83 ± 0.45 | 4.54 ± 0.49 |
| AC = (TC − HDL)/HDL | 0.65 ± 0.06 b | 6.99 ± 0.58 d | 3.37 ± 0.25 a | 4.27 ± 0.56 | 6.47 ± 0.73 d |
| ALP (IU/L) | 230.60 ± 20.18 a | 558.20 ± 34.75 c | 301.00 ± 9.54 a | 316.60 ± 21.76 a | 431.00 ± 35.05 |
| ALT (IU/L) | 67.20 ± 5.32 b | 107.80 ± 12.68 d | 65.60 ± 5.90 b | 70.60 ± 1.44 b | 59.40 ± 2.18 b |
| AST/ALT | 2.39 ± 0.17a | 1.31 ± 0.11 c | 2.32 ± 0.29 a | 2.17 ± 0.08 a | 2.23 ± 0.09 a |
| Insulin (mU/L) | 13.40 ± 0.74 b | 44.67 ± 1.06 d | 14.86 ± 0.84 b | 15.34 ± 0.64 b | 19.26 ± 1.23 b |
| HOMA-IR | 2.73 ± 0.19 b | 10.90 ± 0.41 d | 2.99 ± 0.16 b | 3.19 ± 0.16 b | 4.10 ± 0.23 b |
| Steatosis | 0.00 ± 0.00 b | 3.00 ± 0.00 d | 1.20 ± 0.20 b | 1.40 ± 0.24 a | 1.80 ± 0.20 |
| Lobular inflammation | 0.00 ± 0.00 b | 2.00 ± 0.00 d | 1.00 ± 0.00 a | 1.20 ± 0.20 | 1.20 ± 0.20 |
| Hepatocyte ballooning | 0.00 ± 0.00 b | 2.00 ± 0.00 | 2.00 ± 0.00 | 2.00 ± 0.00 | 2.00 ± 0.00 |
| NAS | 0.00 ± 0.00 b | 7.00 ± 0.00 d | 4.20 ± 0.20 a,c | 4.60 ± 0.24 d | 5.20 ± 0.37 d |
| Total Phenolic (μg Gallic Acid Equiv./mg Extract) | Total Flavonoid (μg Quercetin Equiv./mg Extract) | DPPH IC50 (μg/mL) | ABTS IC50 (μg/mL) | FRAP (nmol Fe+2 Equiv./mg Extract) | |
|---|---|---|---|---|---|
| WE | 224.13 ± 1.14 | 39.41 ± 0.72 | 7.38 ± 0.84 | 31.94 ± 1.36 | 272.56 ± 4.39 |
| 50% ME | 323.98 ± 0.33 | 64.46 ± 1.36 | 2.72 ± 0.25 | 26.51 ± 0.63 | 220.01 ± 1.90 |
| ME | 237.87 ± 0.46 | 49.85 ± 0.62 | 8.32 ± 0.29 | 99.66 ± 0.98 | 333.95 ± 11.73 |
| Ascorbic acid (Standard) | --- | --- | 1.51 ± 0.22 | 5.73 ± 0.09 | --- |
| Compounds | Concentrations (µg/mL) | Within-Day | Between-Day | Recovery | |||
|---|---|---|---|---|---|---|---|
| Accuracy (%) | Precision (CV%) | Accuracy (%) | Precision (CV%) | Mean (%) | Mean (CV%) | ||
| Ellagic acid | 50.00 | 100.30 | 0.18 | 100.01 | 0.27 | 99.25 | 0.80 |
| 6.25 | 97.50 | 0.19 | 97.66 | 2.64 | - | - | |
| 3.12 | 102.18 | 0.26 | 100.50 | 2.09 | 104.75 | 2.18 | |
| 1.56 | 104.36 | 0.51 | 100.64 | 2.83 | - | - | |
| 0.78 | 104.62 | 0.67 | 104.89 | 4.15 | 103.62 | 3.90 | |
| Phyllanthin | 25.00 | 96.28 | 0.25 | 98.47 | 1.82 | 102.65 | 1.19 |
| 12.50 | 104.94 | 1.28 | 102.50 | 3.27 | - | - | |
| 6.25 | 104.42 | 4.78 | 100.42 | 1.45 | 100.04 | 1.49 | |
| 3.12 | 100.83 | 0.28 | 101.10 | 2.63 | - | - | |
| 1.56 | 95.90 | 2.04 | 98.35 | 4.77 | 101.31 | 3.41 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Zarzour, R.H.; Ahmad, M.; Asmawi, M.Z.; Kaur, G.; Saeed, M.A.A.; Al-Mansoub, M.A.; Saghir, S.A.M.; Usman, N.S.; Al-Dulaimi, D.W.; Yam, M.F. Phyllanthus Niruri Standardized Extract Alleviates the Progression of Non-Alcoholic Fatty Liver Disease and Decreases Atherosclerotic Risk in Sprague–Dawley Rats. Nutrients 2017, 9, 766. https://doi.org/10.3390/nu9070766
Al Zarzour RH, Ahmad M, Asmawi MZ, Kaur G, Saeed MAA, Al-Mansoub MA, Saghir SAM, Usman NS, Al-Dulaimi DW, Yam MF. Phyllanthus Niruri Standardized Extract Alleviates the Progression of Non-Alcoholic Fatty Liver Disease and Decreases Atherosclerotic Risk in Sprague–Dawley Rats. Nutrients. 2017; 9(7):766. https://doi.org/10.3390/nu9070766
Chicago/Turabian StyleAl Zarzour, Raghdaa Hamdan, Mariam Ahmad, Mohd. Zaini Asmawi, Gurjeet Kaur, Mohammed Ali Ahmed Saeed, Majed Ahmed Al-Mansoub, Sultan Ayesh Mohammed Saghir, Nasiba Salisu Usman, Dhamraa W. Al-Dulaimi, and Mun Fei Yam. 2017. "Phyllanthus Niruri Standardized Extract Alleviates the Progression of Non-Alcoholic Fatty Liver Disease and Decreases Atherosclerotic Risk in Sprague–Dawley Rats" Nutrients 9, no. 7: 766. https://doi.org/10.3390/nu9070766
APA StyleAl Zarzour, R. H., Ahmad, M., Asmawi, M. Z., Kaur, G., Saeed, M. A. A., Al-Mansoub, M. A., Saghir, S. A. M., Usman, N. S., Al-Dulaimi, D. W., & Yam, M. F. (2017). Phyllanthus Niruri Standardized Extract Alleviates the Progression of Non-Alcoholic Fatty Liver Disease and Decreases Atherosclerotic Risk in Sprague–Dawley Rats. Nutrients, 9(7), 766. https://doi.org/10.3390/nu9070766





