Different Effects of Maternal Low-Isoflavone Soy Protein and Genistein Consumption on Hepatic Lipid Metabolism of 21-Day-Old Male Rat Offspring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Serum Biochemical Analyses
2.3. Serum Free Amino Acid Analysis
2.4. Oral Glucose Tolerance Test
2.5. Hepatic Lipid Analyses
2.6. Total RNA Extraction, Microarray Analysis and Real-Time PCR
2.7. Tissue Extraction and Immunoblotting
2.8. Statistical Analysis
3. Results
3.1. Effects of Maternal Diet on Maternal Body Weight Changes and Serum Biochemical Parameters
3.2. Effect of Maternal Diet on Serum Amino Acid Levels in Dams
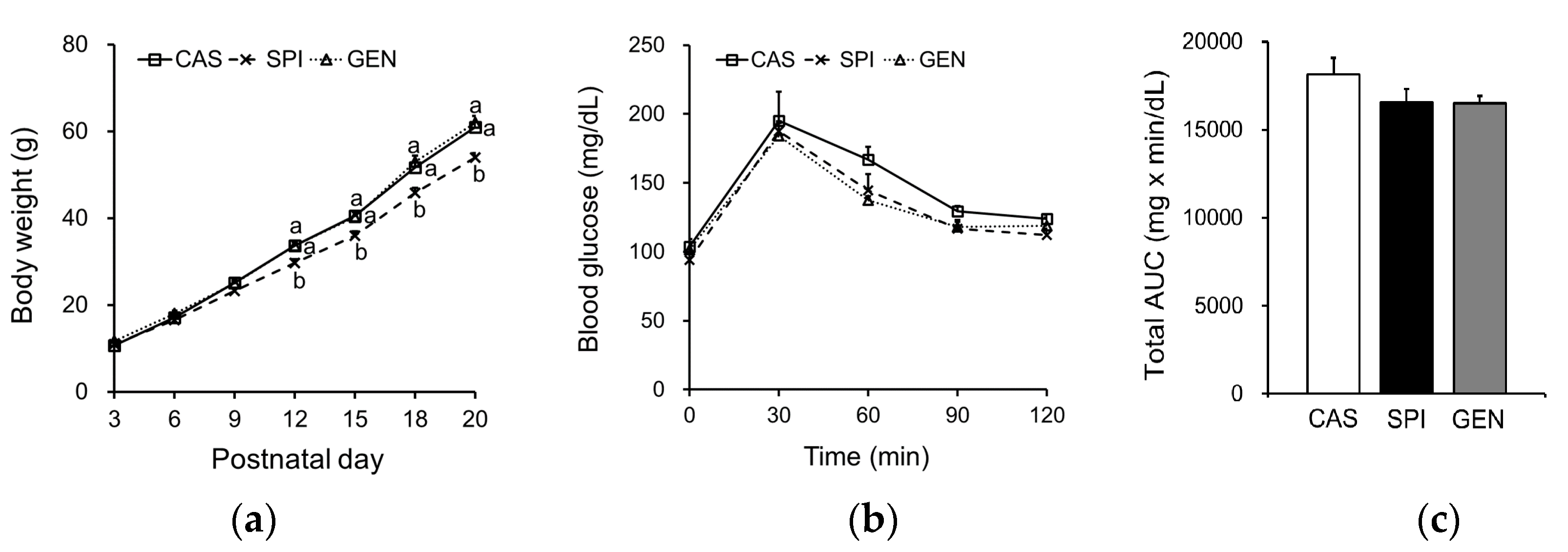
3.3. Effects of Maternal Diet on Growth and Glucose Metabolism of Offspring
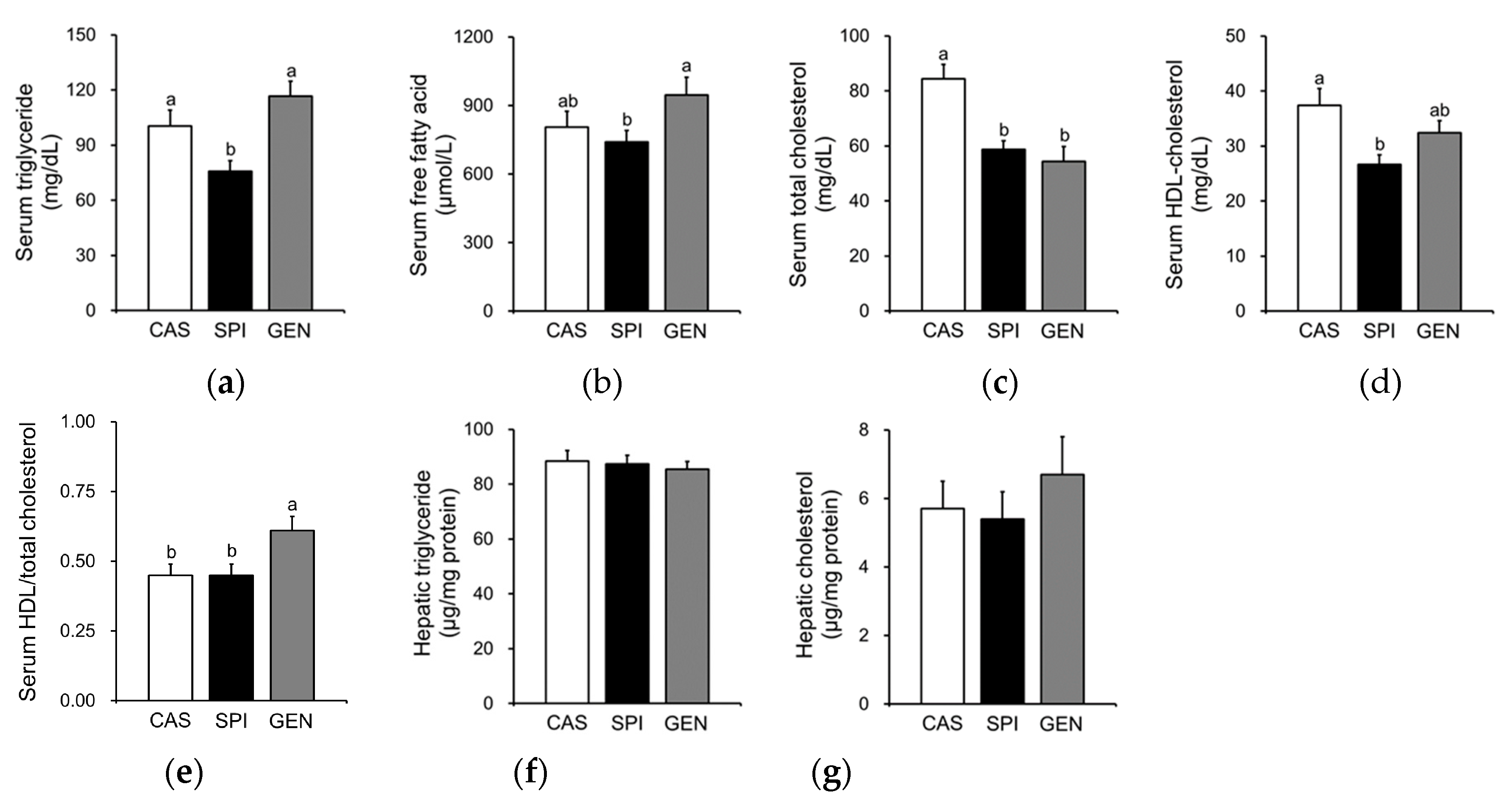
3.4. Effects of Maternal Diet on Lipid Metabolism of Offspring
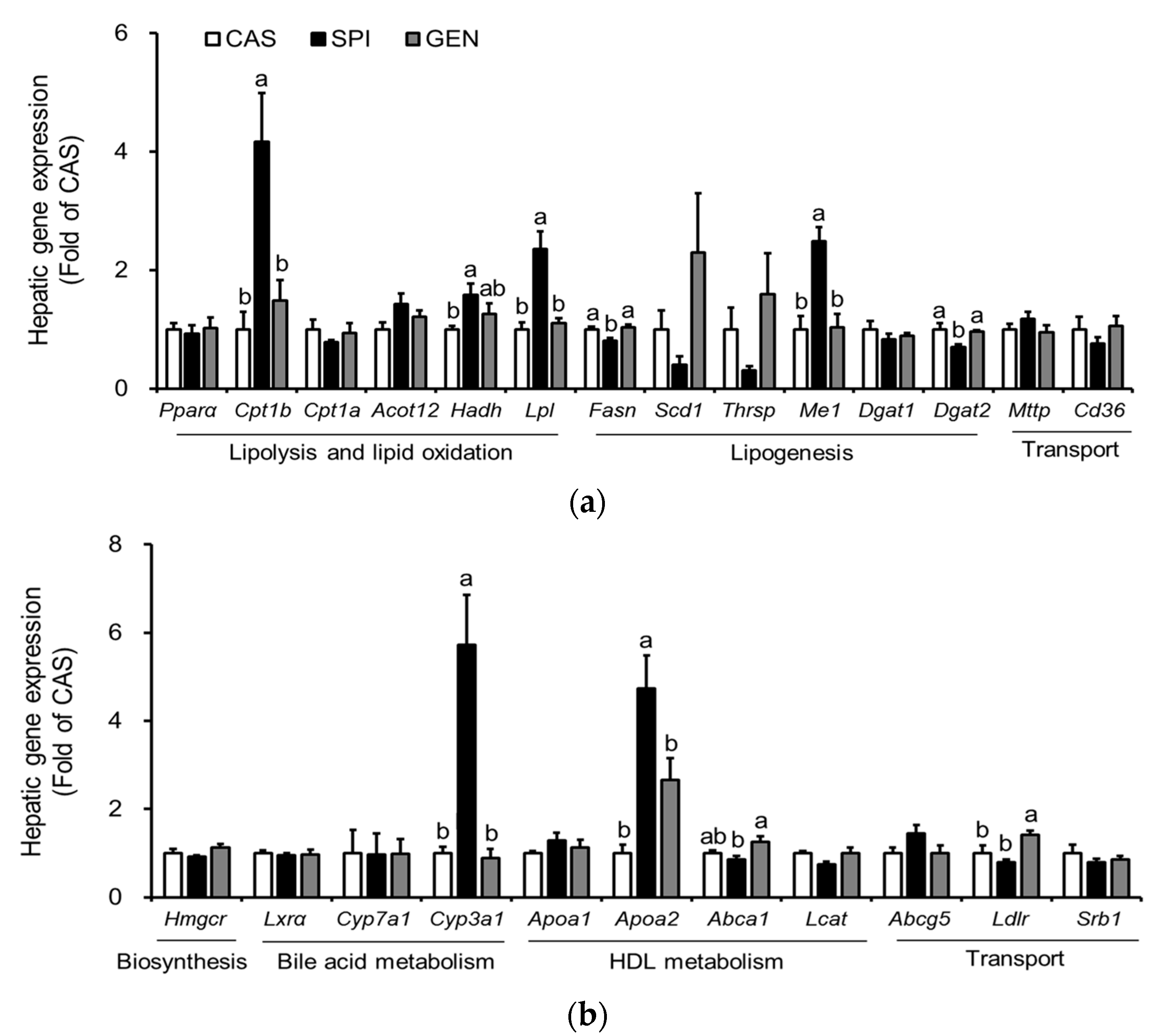
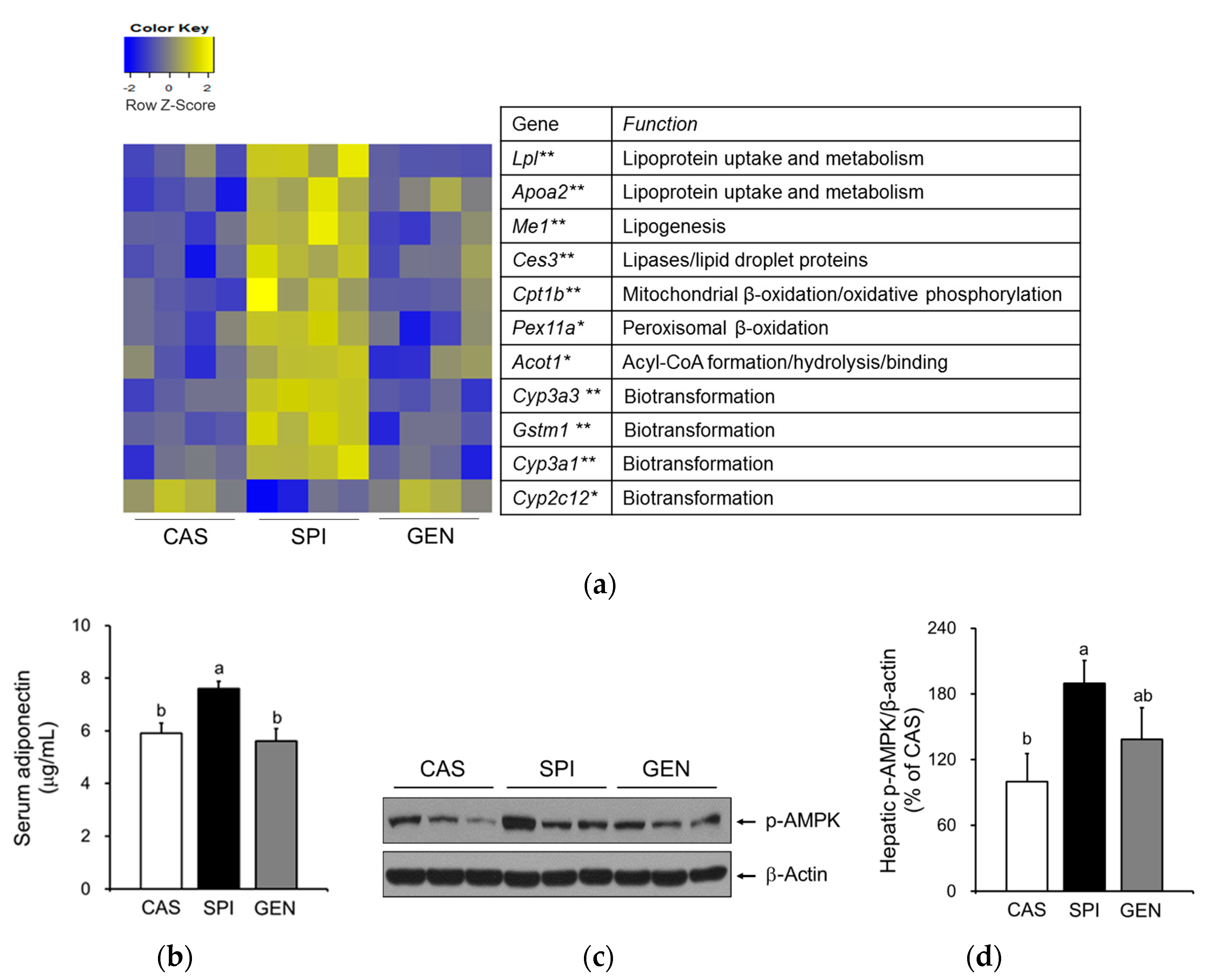
3.5. Effects of Maternal Diet on Hepatic Gene Expressions Involved in Lipid Metabolism of Offspring
3.6. Effects of Maternal Diet on the Activation of Peroxisome Proliferator-Activated Receptor Alpha (PPARα) Signaling Pathway in the Liver of Offspring
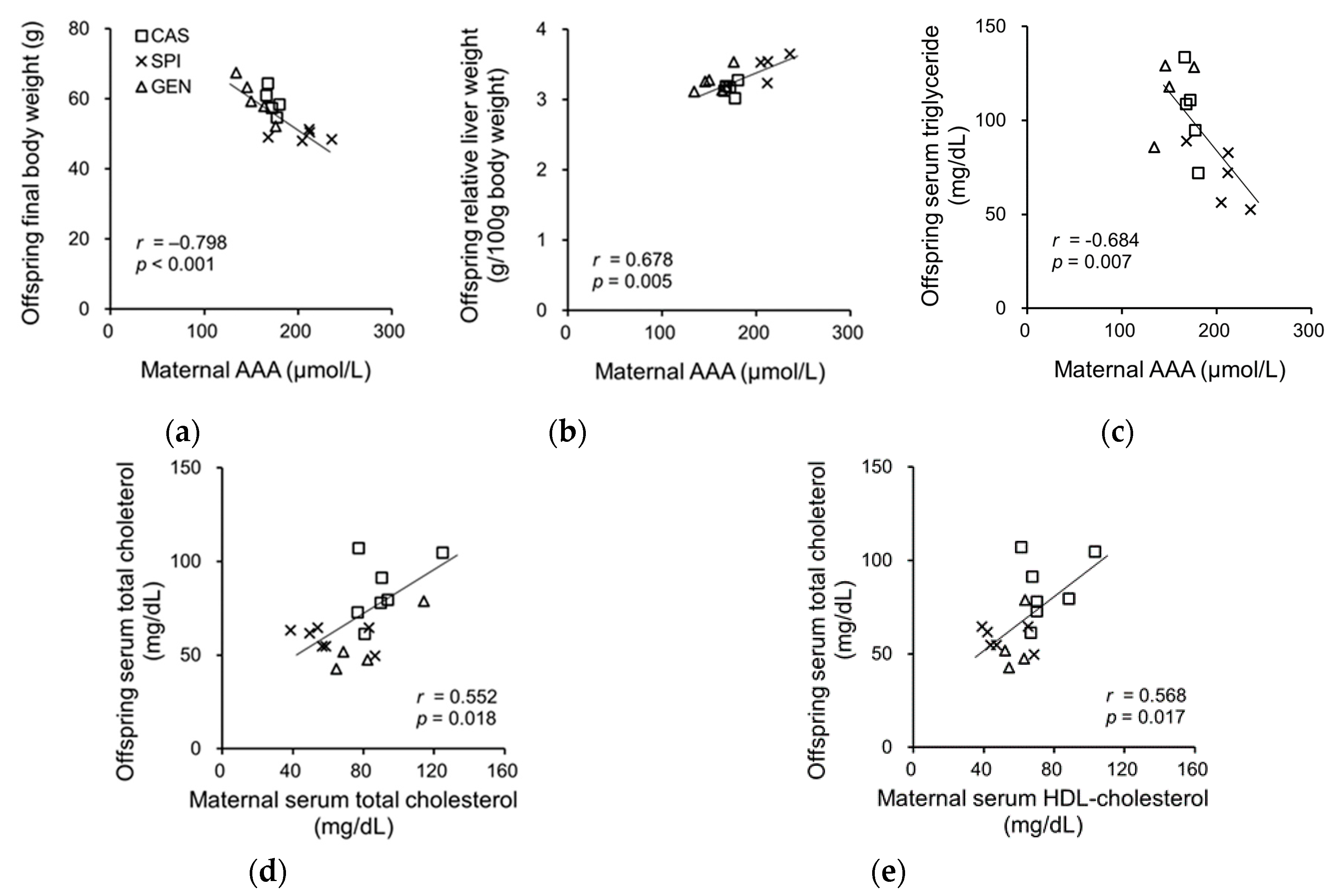
3.7. Effects of Maternal Biochemical Parameters on the Regulation of Lipid Metabolism of Offspring
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gluckman, P.D.; Hanson, M.A.; Cooper, C.; Thornburg, K.L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Cudd, T.A.; Meininger, C.J.; Spencer, T.E. Maternal nutrition and fetal development. J. Nutr. 2004, 134, 2169–2172. [Google Scholar] [PubMed]
- Chango, A.; Pogribny, I.P. Considering maternal dietary modulators for epigenetic regulation and programming of the fetal epigenome. Nutrients 2015, 7, 2748–2770. [Google Scholar] [CrossRef] [PubMed]
- Ascencio, C.; Torres, N.; Isoard-Acosta, F.; Gomez-Perez, F.J.; Hernandez-Pando, R.; Tovar, A.R. Soy protein affects serum insulin and hepatic SREBP-1 mRNA and reduces fatty liver in rats. J. Nutr. 2004, 134, 522–529. [Google Scholar] [PubMed]
- Tovar, A.R.; Torre-Villalvazo, I.; Ochoa, M.; Elias, A.L.; Ortiz, V.; Aguilar-Salinas, C.A.; Torres, N. Soy protein reduces hepatic lipotoxicity in hyperinsulinemic obese Zucker fa/fa rats. J. Lipid. Res. 2005, 46, 1823–1832. [Google Scholar] [CrossRef] [PubMed]
- Gudbrandsen, O.A.; Wergedahl, H.; Liaset, B.; Espe, M.; Berge, R.K. Dietary proteins with high isoflavone content or low methionine-glycine and lysine-arginine ratios are hypocholesterolaemic and lower the plasma homocysteine level in male Zucker fa/fa rats. Br. J. Nutr. 2005, 94, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Badger, T.M.; Ronis, M.J.; Wolff, G.; Stanley, S.; Ferguson, M.; Shankar, K.; Simpson, P.; Jo, C.H. Soy protein isolate reduces hepatosteatosis in yellow Avy/a mice without altering coat color phenotype. Exp. Biol. Med. 2008, 233, 1242–1254. [Google Scholar] [CrossRef] [PubMed]
- Souzeau, E.; Belanger, S.; Picard, S.; Deschepper, C.F. Dietary isoflavones during pregnancy and lactation provide cardioprotection to offspring rats in adulthood. Am. J. Physiol.-Heart Circ. Physiol. 2005, 289, H715–H721. [Google Scholar] [CrossRef] [PubMed]
- Simmen, F.A.; Mercado, C.P.; Zavacki, A.M.; Huang, S.A.; Greenway, A.D.; Kang, P.; Bowman, M.T.; Prior, R.L. Soy protein diet alters expression of hepatic genes regulating fatty acid and thyroid hormone metabolism in the male rat. J. Nutr. Biochem. 2010, 21, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Fukui, K.; Tachibana, N.; Wanezaki, S.; Tsuzaki, S.; Takamatsu, K.; Yamamoto, T.; Hashimoto, Y.; Shimoda, T. Isoflavone-free soy protein prepared by column chromatography reduces plasma cholesterol in rats. J. Agric. Food Chem. 2002, 50, 5717–5721. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Brandsch, C.; Bettzieche, A.; Hirche, F.; Stangl, G.I.; Eder, K. Isoflavone-poor soy protein alters the lipid metabolism of rats by SREBP-mediated down-regulation of hepatic genes. J. Nutr. Biochem. 2007, 18, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, K.; Ohkawa, S.; Muramatsu, K. Relationship between amino acid composition of diet and plasma cholesterol level in growing rats fed a high cholesterol diet. J. Nutr. Sci. Vitaminol. 1986, 32, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Gannon, M.C.; Nuttall, J.A.; Nuttall, F.Q. The metabolic response to ingested glycine. Am. J. Clin. Nutr. 2002, 76, 1302–1307. [Google Scholar] [PubMed]
- Ramdath, D.D.; Padhi, E.M.; Sarfaraz, S.; Renwick, S.; Duncan, A.M. Beyond the Cholesterol-Lowering Effect of Soy Protein: A Review of the Effects of Dietary Soy and Its Constituents on Risk Factors for Cardiovascular Disease. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Won, S.B.; Han, A.; Kwon, Y.H. Maternal consumption of low-isoflavone soy protein isolate alters hepatic gene expression and liver development in rat offspring. J. Nutr. Biochem. 2017, 42, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Minniti, G.; Piana, A.; Armani, U.; Cerone, R. Determination of plasma and serum homocysteine by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. A 1998, 828, 401–405. [Google Scholar] [CrossRef]
- Yoon, M.; Won, S.B.; Kwon, Y.H. Altered lipid metabolism in rat offspring of dams fed a low-protein diet containing soy protein isolate. Life Sci. 2017, 174, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Forhead, A.J.; Fowden, A.L. Thyroid hormones in fetal growth and prepartum maturation. J. Endocrinol. 2014, 221, R87–R103. [Google Scholar] [CrossRef] [PubMed]
- Aye, I.L.; Powell, T.L.; Jansson, T. Review: Adiponectin—The missing link between maternal adiposity, placental transport and fetal growth? Placenta 2013, 34, S40–S45. [Google Scholar] [CrossRef] [PubMed]
- Grinberg, D.R.; Ramirez, I.; Vilaro, S.; Reina, M.; Llobera, M.; Herrera, E. Starvation enhances lipoprotein lipase activity in the liver of the newborn rat. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1985, 833, 217–222. [Google Scholar] [CrossRef]
- Panadero, M.; Bocos, C.; Herrera, E. Relationship between lipoprotein lipase and peroxisome proliferator-activated receptor-α expression in rat liver during development. J. Physiol. Biochem. 2006, 62, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Saggerson, E.D.; Carpenter, C.A. Carnitine palmitoyltransferase in liver and five extrahepatic tissues in the rat. Inhibition by DL-2-bromopalmitoyl-CoA and effect of hypothyroidism. Biochem. J. 1986, 236, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Radominska-Pandya, A.; Shi, Y.; Simon, C.M.; Nelson, M.C.; Ong, E.S.; Waxman, D.J.; Evans, R.M. An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc. Natl. Acad. Sci. USA 2001, 98, 3375–3380. [Google Scholar] [CrossRef] [PubMed]
- Rakhshandehroo, M.; Knoch, B.; Muller, M.; Kersten, S. Peroxisome proliferator-activated receptor alpha target genes. PPAR Res. 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.Q.; Brown-Borg, H.; Brown, S.; Westin, S.; Mode, A.; Corton, J.C. PPARalpha activators down-regulate CYP2C7, a retinoic acid and testosterone hydroxylase. Toxicology 2004, 203, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Nio, Y.; Maki, T.; Kobayashi, M.; Takazawa, T.; Iwabu, M.; Okada-Iwabu, M.; Kawamoto, S.; Kubota, N.; Kubota, T.; et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat. Med. 2007, 13, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Messina, M. Soy and health update: Evaluation of the clinical and epidemiologic literature. Nutrients 2016. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Huang, J.; Jiang, Y.; Zeng, Y.; He, F.; Zhang, M.Q.; Han, Z.; Zhang, X. Multi-stage analysis of gene expression and transcription regulation in C57/B6 mouse liver development. Genomics 2009, 93, 235–242. [Google Scholar] [CrossRef] [PubMed]
- McMullin, T.S.; Lowe, E.R.; Bartels, M.J.; Marty, M.S. Dynamic changes in lipids and proteins of maternal, fetal, and pup blood and milk during perinatal development in CD and Wistar rats. Toxicol. Sci. 2008, 105, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.F.; Jian, M.R. Effects of soy protein and casein on lipid metabolism in mature and suckling rats. Nutr. Res. 1997, 17, 1341–1350. [Google Scholar] [CrossRef]
- Troina, A.A.; Figueiredo, M.S.; Moura, E.G.; Boaventura, G.T.; Soares, L.L.; Cardozo, L.F.; Oliveira, E.; Lisboa, P.C.; Passos, M.A.; Passos, M.C. Maternal flaxseed diet during lactation alters milk composition and programs the offspring body composition, lipid profile and sexual function. Food Chem. Toxicol. 2010, 48, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Kersten, S. Integrated physiology and systems biology of PPARalpha. Mol. Metab. 2014, 3, 354–371. [Google Scholar] [CrossRef] [PubMed]
- Lillycrop, K.A.; Phillips, E.S.; Jackson, A.A.; Hanson, M.A.; Burdge, G.C. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J. Nutr. 2005, 135, 1382–1386. [Google Scholar] [PubMed]
- Zheng, J.; Xiao, X.; Zhang, Q.; Yu, M.; Xu, J.; Wang, Z. Maternal protein restriction induces early-onset glucose intolerance and alters hepatic genes expression in the peroxisome proliferator-activated receptor pathway in offspring. J. Diabetes Investig. 2015, 6, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Ringseis, R.; Gutgesell, A.; Dathe, C.; Brandsch, C.; Eder, K. Feeding oxidized fat during pregnancy up-regulates expression of PPARalpha-responsive genes in the liver of rat fetuses. Lipids Health Dis. 2007, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.P.; Barnes, S. Isoflavones and PPAR Signaling: A Critical Target in Cardiovascular, Metastatic, and Metabolic Disease. PPAR Res. 2010, 2010, 153252. [Google Scholar] [CrossRef] [PubMed]
- Gudbrandsen, O.A.; Wergedahl, H.; Berge, R.K. A casein diet added isoflavone-enriched soy protein favorably affects biomarkers of steatohepatitis in obese Zucker rats. Nutrition 2009, 25, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Ronis, M.J.; Chen, Y.; Badeaux, J.; Badger, T.M. Dietary soy protein isolate attenuates metabolic syndrome in rats via effects on PPAR, LXR, and SREBP signaling. J. Nutr. 2009, 139, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Kim, J.H.; Nam, B.; Kim, J.; Lee, J.H.; Hwang, K.Y.; Lee, S.J. The dipeptide H-Trp-Glu-OH (WE) shows agonistic activity to peroxisome proliferator-activated protein-alpha and reduces hepatic lipid accumulation in lipid-loaded H4IIE cells. Bioorg. Med. Chem. Lett. 2014, 24, 2957–2962. [Google Scholar] [CrossRef] [PubMed]
- Milke Garcia, M.P. Nutritional support in the treatment of chronic hepatic encephalopathy. Ann. Hepatol. 2011, 10 (Suppl. 2), S45–S49. [Google Scholar] [PubMed]
- Bhasin, K.K.; van Nas, A.; Martin, L.J.; Davis, R.C.; Devaskar, S.U.; Lusis, A.J. Maternal low-protein diet or hypercholesterolemia reduces circulating essential amino acids and leads to intrauterine growth restriction. Diabetes 2009, 58, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Jansson, N.; Pettersson, J.; Haafiz, A.; Ericsson, A.; Palmberg, I.; Tranberg, M.; Ganapathy, V.; Powell, T.L.; Jansson, T. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J. Physiol. 2006, 576, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Huang, C.; Cao, Y.; Wang, J.; dong, B. Branched-chain amino acids reverse the growth of intrauterine growth retardation rats in a malnutrition model. Asian-Aust. J. Anim. Sci. 2009, 22, 1495–1503. [Google Scholar] [CrossRef]

| Composition (g/kg) | Diet | ||
|---|---|---|---|
| CAS | SPI | GEN | |
| Cornstarch | 397.5 | 397.5 | 397.5 |
| Casein 1 | 200 | - | 200 |
| Soy protein isolate 2 | - | 200 | - |
| Dextrinized cornstarch | 132 | 132 | 132 |
| Sucrose | 100 | 100 | 99.75 |
| Corn oil | 70 | 70 | 70 |
| Fiber | 50 | 50 | 50 |
| Mineral mix 3 | 35 | 35 | 35 |
| Vitamin mix 4 | 10 | 10 | 10 |
| L-Cystine | 3 | 3 | 3 |
| Choline bitartrate | 2.5 | 2.5 | 2.5 |
| t-Butylhydroquinone | 0.014 | 0.014 | 0.014 |
| Genistein 5 | - | - | 0.25 |
| Group | |||
|---|---|---|---|
| CAS-D | SPI-D | GEN-D | |
| Body weight at sacrifice (g) | 299.5 ± 12.6 | 299.9 ± 3.4 | 304.0 ± 11.2 |
| Serum | |||
| Glucose (mg/dL) | 94.5 ± 6.6 | 99.3 ± 10.1 | 104.2 ± 13.3 |
| Triglyceride (mg/dL) | 81.8 ± 10.4 a | 48.7 ± 7.3 b | 80.4 ± 15.5 a |
| Total cholesterol (mg/dL) | 92.0 ± 5.7 a | 59.4 ± 6.0 b | 77.0 ± 8.2 a,b |
| HDL cholesterol (mg/dL) | 74.8 ± 4.9 a | 49.0 ± 4.8 b | 56.9 ± 4.1 b |
| Free fatty acids (μmol/L) | 722.1 ± 88.5 | 617.1 ± 32.1 | 694.9 ± 75.4 |
| Homocysteine (μmol/L) | 6.9 ± 0.4 | 7.6 ± 0.5 | 6.9 ± 0.4 |
| Insulin (ng/mL) | 0.5 ± 0.1 | 0.3 ± 0.1 | 0.9 ± 0.4 |
| Adiponectin (ng/mL) | 6.7 ± 0.4 | 8.1 ± 0.8 | 6.3 ± 0.7 |
| T3 (ng/mL) | 1.7 ± 0.1 a | 1.4 ± 0.1 a,b | 1.2 ± 0.2 b |
| Liver | |||
| Triglyceride (μg/mg protein) | 81.0 ± 2.7 a | 63.5 ± 3.9 b | 87.3 ± 5.2 a |
| Cholesterol (μg/mg protein) | 5.8 ± 1.3 a | 2.5 ± 0.5 b | 5.4 ± 0.6 a |
| Amino Acid (μmol/L) | Group | ||
|---|---|---|---|
| CAS-D | SPI-D | GEN-D | |
| Alanine | 489.2 ± 44.7 | 464.5 ± 33.7 | 490.1 ± 13.8 |
| Arginine | 170.6 ± 5.0 | 191.3 ± 13.3 | 141.0 ± 23.3 |
| Asparagine | 76.8 ± 3.6 | 74.7 ± 6.6 | 72.2 ± 1.5 |
| Aspartate | 47.8 ± 3.0 | 48.8 ± 9.4 | 40.3 ± 4.3 |
| Glutamate | 186.9 ± 9.2 | 198.2 ± 24.0 | 203.6 ± 24.0 |
| Glutamine | 610.5 ± 42.9 | 550.7 ± 34.1 | 513.7 ± 30.8 |
| Glycine | 234.7 ± 16.0 | 255.6 ± 25.8 | 225.1 ± 20.5 |
| Histidine | 56.0 ± 1.2 | 52.1 ± 2.8 | 50.3 ± 1.3 |
| Isoleucine | 95.8 ± 6.9 | 80.1 ± 3.4 | 85.3 ± 8.1 |
| Leucine | 145.5 ± 9.5 | 117.6 ± 5.7 | 129.2 ± 12.6 |
| Lysine | 477.4 ± 25.0 | 477.7 ± 62.0 | 431.1 ± 47.5 |
| Methionine | 52.9 ± 1.2 a | 52.9 ± 1.6 a | 40.2 ± 6.2 b |
| Phenylalanine | 61.9 ± 1.5 a,b | 63.2 ± 3.4 a | 54.1 ± 2.6 b |
| Proline | 172.4 ± 6.5 | 148.9 ± 9.8 | 169.5 ± 11.2 |
| Serine | 416.9 ± 18.0 | 401.3 ± 24.8 | 373.7 ± 12.7 |
| Threonine | 662.0 ± 97.0 | 426.3 ± 37.3 | 608.9 ± 100.9 |
| Tryptophan | 62.9 ± 1.4 b | 78.8 ± 6.6 a | 53.5 ± 3.7 b |
| Tyrosine | 47.8 ± 1.9 b | 64.6 ± 5.8 a | 46.2 ± 3.2 b |
| Valine | 152.4 ± 8.6 a | 119.4 ± 3.5 b | 137.7 ± 10.4 a,b |
| BCAA | 393.7 ± 24.6 | 317.1 ± 12.4 | 352.1 ± 30.7 |
| AAA | 172.5 ± 2.7 b | 206.6 ± 11.0 a | 153.9 ± 7.3 b |
| BCAA/AAA | 2.3 ± 0.1 a | 1.5 ± 0.1 b | 2.3 ± 0.2 a |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, A.; Won, S.B.; Kwon, Y.H. Different Effects of Maternal Low-Isoflavone Soy Protein and Genistein Consumption on Hepatic Lipid Metabolism of 21-Day-Old Male Rat Offspring. Nutrients 2017, 9, 1039. https://doi.org/10.3390/nu9091039
Han A, Won SB, Kwon YH. Different Effects of Maternal Low-Isoflavone Soy Protein and Genistein Consumption on Hepatic Lipid Metabolism of 21-Day-Old Male Rat Offspring. Nutrients. 2017; 9(9):1039. https://doi.org/10.3390/nu9091039
Chicago/Turabian StyleHan, Anna, Sae Bom Won, and Young Hye Kwon. 2017. "Different Effects of Maternal Low-Isoflavone Soy Protein and Genistein Consumption on Hepatic Lipid Metabolism of 21-Day-Old Male Rat Offspring" Nutrients 9, no. 9: 1039. https://doi.org/10.3390/nu9091039
APA StyleHan, A., Won, S. B., & Kwon, Y. H. (2017). Different Effects of Maternal Low-Isoflavone Soy Protein and Genistein Consumption on Hepatic Lipid Metabolism of 21-Day-Old Male Rat Offspring. Nutrients, 9(9), 1039. https://doi.org/10.3390/nu9091039




