In Vitro Cytotoxicity Induced by the Bufadienolides 1α,2α-Epoxyscillirosidine and Lanceotoxin B on Rat Myocardial and Mouse Neuroblastoma Cell Lines
Abstract
:1. Introduction
2. Results
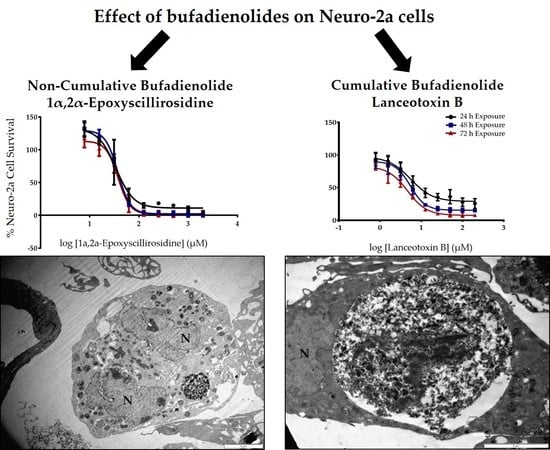
2.1. The Cytotoxicity of 1α,2α-Epoxyscillirosidine and Lanceotoxin B on H9c2 and Neuro-2a Cell Lines
2.2. The Effect of 1α,2α-Epoxyscillirosidine and Lanceotoxin B on H9c2 and Neuro-2a Cell Line Ultrastructure
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Toxins
4.3. Cell Survival Assays
4.4. Statistical Analysis
4.5. Transmission Electron Microscopy
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kellerman, T.; Naudé, T.; Fourie, N. The distribution, diagnoses and estimated economic impact of plant poisonings and mycotoxicoses in South Africa. Onderstepoort J. Vet. Res. 1996, 63, 65–90. [Google Scholar] [PubMed]
- Kellerman, T.; Coetzer, J.; Naudé, T.; Botha, C. Plant Poisonings and Mycotoxicoses of Livestock in Southern Africa; Oxford University Press Southern Africa: Cape Town, South Africa, 2005. [Google Scholar]
- Enslin, P.; Naudé, T.; Potgieter, D.; Van Wyk, A. 1α, 2α-epoxyscillirosidine, the main toxic principle of Homeria glauca (Wood and Evans) NE Br. Tetrahedron 1966, 22, 3213–3220. [Google Scholar] [CrossRef]
- Anderson, L.; Schultz, R.; Joubert, J.; Prozesky, L.; Kellerman, T.; Erasmus, G.; Procos, J. Krimpsiekte and acute cardiac glycoside poisoning in sheep caused by bufadienolides from the plant Kalanchoe lanceolata Forsk. Onderstepoort J. Vet. Res. 1983, 50, 295–300. [Google Scholar] [PubMed]
- Riganti, C.; Campia, I.; Kopecka, J.; Gazzano, E.; Doublier, S.; Aldieri, E.; Bosia, A.; Ghigo, D. Pleiotropic effects of cardioactive glycosides. Curr. Med. Chem. 2011, 18, 872–885. [Google Scholar] [CrossRef] [PubMed]
- Contreras, R.; Flores-Maldonado, C.; Lazaro, A.; Shoshani, L.; Flores-Benitez, D.; Larre, I.; Cereijido, M. Ouabain Binding to Na+, K+-ATPase Relaxes Cell Attachment and Sends a SpecificSignal (NACos) to the Nucleus. J. Membr. Biol. 2004, 198, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Contreras, R.; Shoshani, L.; Flores-Maldonado, C.; Lazaro, A.; Cereijido, M. Relationship between Na (+), K (+)-ATPase and cell attachment. J. Cell Sci. 1999, 112, 4223–4232. [Google Scholar] [PubMed]
- Botha, C.; Gehring, R.; Van Rooyen, J.; Venter, D. The effect of three bufadienolide cardiac glycosides on contraction of isolated rat jejunum. Onderstepoort J. Vet. Res. 2002, 69, 243–246. [Google Scholar] [PubMed]
- Botha, C.J.; Van der Lugt, J.J.; Erasmus, G.; Kellerman, T.S.; Schultz, R.A.; Vleggaar, R. Krimpsiekte, associated with thalamic lesions, induced by the neurotoxic cardiac glycoside, cotyledoside, isolated from Tylecodon wallichii (Harv.) Toelken subsp. wallichii. Onderstepoort J. Vet. Res. 1997, 64, 189–194. [Google Scholar] [PubMed]
- Botha, C. Potential health risks posed by plant-derived cumulative neurotoxic bufadienolides in South Africa. Molecules 2016, 21, 348. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zelenin, S.; Aperia, A.; Aizman, O. Low doses of ouabain protect from serum deprivation–triggered apoptosis and stimulate kidney cell proliferation via activation of NF-κB. J. Am. Soc. Nephrol. 2006, 17, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Huang, L.; Xie, Z.; Huang, W.-H.; Askari, A. Partial inhibition of Na/K-ATPase by ouabain induces the Ca-dependent expressions of early-response genes in cardiac myocytes. J. Biol. Chem. 1996, 271, 10372–10378. [Google Scholar] [CrossRef] [PubMed]
- Price, E.M.; Lingrel, J.B. Structure-function relationships in the sodium-potassium ATPase. alpha. subunit: Site-directed mutagenesis of glutamine-111 to arginine and asparagine-122 to aspartic acid generates a ouabain-resistant enzyme. Biochemistry 1988, 27, 8400–8408. [Google Scholar] [CrossRef] [PubMed]
- Weinhouse, E.; Kaplanski, J.; Posner, J. Comparison of digoxin-induced cardiac toxicity in resistant and sensitive species. J. Pharm. Pharmacol. 1983, 35, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Saikumar, P.; Weinberg, J.M.; Venkatachalam, M.A. Calcium in cell injury and death. Annu. Rev. Pathol. Mech. Dis. 2006, 1, 405–434. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Levine, B. Autophagic cell death: The story of a misnomer. Nat. Rev. Mol. Cell Biol. 2008, 9, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Botella, J.; Kretzschmar, D.; Kiermayer, C.; Feldmann, P.; Hughes, D.; Schneuwly, S. Deregulation of the Egfr/Ras signaling pathway induces age-related brain degeneration in the Drosophila mutant vap. Mol. Biol. Cell 2003, 14, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Kitanaka, C.; Kato, K.; Ijiri, R.; Sakurada, K.; Tomiyama, A.; Noguchi, K.; Nagashima, Y.; Nakagawara, A.; Momoi, T.; Toyoda, Y. Increased Ras expression and caspase-independent neuroblastoma cell death: Possible mechanism of spontaneous neuroblastoma regression. Nat. Cancer Inst. 2002, 94, 358–368. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Luft, J. Improvements in epoxy resin embedding methods. J. Cell Biol. 1961, 9, 409–414. [Google Scholar] [CrossRef]
- Reynolds, E. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef] [PubMed]






| Cell Lines | Bufadienolide | 24 h | 48 h | 72 h |
|---|---|---|---|---|
| H9c2 | 1α,2α-Epoxyscillirosidine | 41.39 ± 4.37 µM n = 3 | 25.42 ± 3.73 µM n = 3 | 12.65 ± 2.75 µM n = 3 |
| Lanceotoxin B | >200 µM (95% CI: 64–92) * n = 4 | ~200 µM (95% CI: 49–68) * n = 4 | <200 µM (95% CI: 36–48) * n = 4 | |
| Neuro-2a | 1α,2α-Epoxyscillirosidine | 35.73 ± 10.59 µM n = 3 | 37.56 ± 3.18 µM n = 3 | 37.35 ± 2.30 µM n = 3 |
| Lanceotoxin B | 5.46 ± 0.37 µM n = 4 | 5.27 ± 0.59 µM n = 4 | 4.43 ± 0.67 µM n = 4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henn, D.; Venter, A.; Botha, C. In Vitro Cytotoxicity Induced by the Bufadienolides 1α,2α-Epoxyscillirosidine and Lanceotoxin B on Rat Myocardial and Mouse Neuroblastoma Cell Lines. Toxins 2019, 11, 14. https://doi.org/10.3390/toxins11010014
Henn D, Venter A, Botha C. In Vitro Cytotoxicity Induced by the Bufadienolides 1α,2α-Epoxyscillirosidine and Lanceotoxin B on Rat Myocardial and Mouse Neuroblastoma Cell Lines. Toxins. 2019; 11(1):14. https://doi.org/10.3390/toxins11010014
Chicago/Turabian StyleHenn, Danielle, Annette Venter, and Christo Botha. 2019. "In Vitro Cytotoxicity Induced by the Bufadienolides 1α,2α-Epoxyscillirosidine and Lanceotoxin B on Rat Myocardial and Mouse Neuroblastoma Cell Lines" Toxins 11, no. 1: 14. https://doi.org/10.3390/toxins11010014
APA StyleHenn, D., Venter, A., & Botha, C. (2019). In Vitro Cytotoxicity Induced by the Bufadienolides 1α,2α-Epoxyscillirosidine and Lanceotoxin B on Rat Myocardial and Mouse Neuroblastoma Cell Lines. Toxins, 11(1), 14. https://doi.org/10.3390/toxins11010014