The Botulinum Treatment of Neurogenic Detrusor Overactivity: The Double-Face of the Neurotoxin
Abstract
:1. Introduction
2. Bladder Physiology
2.1. Bladder Innervation
- (1)
- The sympathetic thoracolumbar center (T10–L2) which, by mean the hypogastric nerve, sustains the bladder muscle relaxation (bladder compliance) and the internal sphincter contraction allowing the accommodation of the growing urine volume without increasing intra vesical pressure;
- (2)
- The parasympathetic sacral center (S2–S4) which, by means the pelvic nerve, sustains the detrusor contraction and the internal sphincter relaxation, creating the conditions for bladder emptying.
2.2. Sensory System
2.2.1. The Urothelium
2.2.2. The Lamina Propria
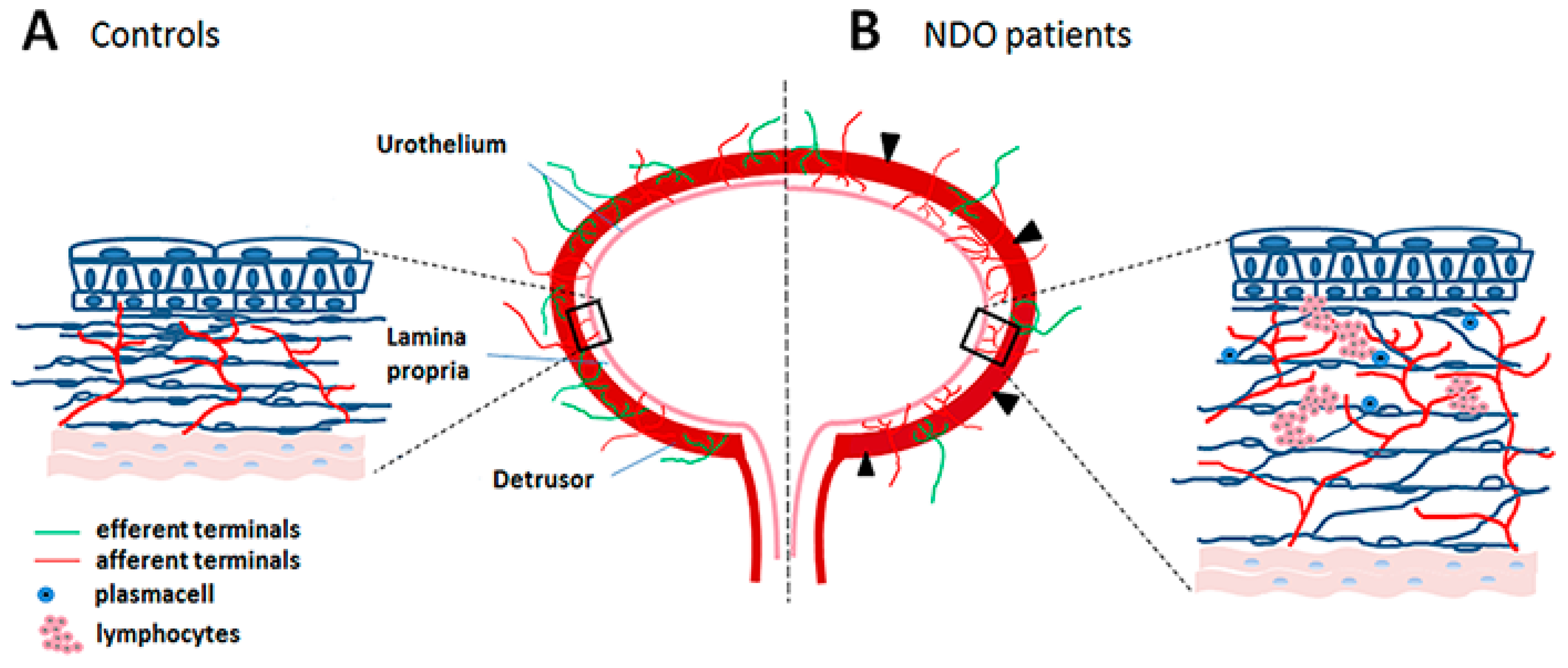
3. Neurogenic Detrusor Overactivity (NDO)
3.1. Pathophysiology
3.2. First-Choice Therapy
3.3. Second-Choice Therapy: BoNT/A Injections
4. BoNT Sites of Action in the Bladder
4.1. Efferent Nerve Terminals
4.2. Afferent Nerve Terminals
4.3. Urothelium
4.4. Lamina Propria
5. The Double-Face of the Neurotoxin
5.1. Cyclic BoNT Treatments Generate Substantial Changes in the Bladder Wall
5.2. Sprouting and Chronic Neurogenic Inflammation
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, S. Clinical Uses of Botulinum Neurotoxins: Current Indications, Limitations and Future Developments. Toxins 2012, 4, 913–939. [Google Scholar] [CrossRef] [Green Version]
- Wilkes, J. AAN Updates Guidelines on the Uses of Botulinum Neurotoxin. Am. Fam. Physician 2017, 95, 198–199. [Google Scholar]
- Davies, K.; Black, A.; Hunt, M.; Holsti, L. Long-term gait outcomes following conservative management of idiopathic toe walking. Gait Posture 2018, 62, 214–219. [Google Scholar] [CrossRef]
- Niemann, N.; Jankovic, J. Botulinum Toxin for the Treatment of Hand Tremor. Toxins 2018, 10, 299. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Chung, M.E. Botulinum Toxin for Central Neuropathic Pain. Toxins 2018, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Rieder, E.A. A Systematic Review of Patient-Reported Outcomes for Cosmetic Indications of Botulinum Toxin Treatment. Dermatol. Surg. 2019, 45, 668–688. [Google Scholar] [CrossRef] [PubMed]
- Weise, D.; Weise, C.M.; Naumann, M. Central Effects of Botulinum Neurotoxin—Evidence from Human Studies. Toxins 2019, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Hamid, R.; Averbeck, M.A.; Chiang, H.; Garcia, A.; Al Mousa, R.T.; Oh, S.-J.; Patel, A.; Plata, M.; Del Popolo, G. Epidemiology and pathophysiology of neurogenic bladder after spinal cord injury. World J. Urol. 2018, 36, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Cruz, F.; Herschorn, S.; Aliotta, P.; Brin, M.; Thompson, C.; Lam, W.; Daniell, G.; Heesakkers, J.; Haag-Molkenteller, C. Efficacy and Safety of OnabotulinumtoxinA in Patients with Urinary Incontinence Due to Neurogenic Detrusor Overactivity: A Randomised, Double-Blind, Placebo-Controlled Trial. Eur. Urol. 2011, 60, 742–750. [Google Scholar] [CrossRef]
- Ginsberg, D.; Gousse, A.; Keppenne, V.; Sievert, K.-D.; Thompson, C.; Lam, W.; Brin, M.F.; Jenkins, B.; Haag-Molkenteller, C. Phase 3 Efficacy and Tolerability Study of OnabotulinumtoxinA for Urinary Incontinence from Neurogenic Detrusor Overactivity. J. Urol. 2012, 187, 2131–2139. [Google Scholar] [CrossRef]
- Kennelly, M.; Dmochowski, R.; Ethans, K.; Karsenty, G.; Schulte-Baukloh, H.; Jenkins, B.; Thompson, C.; Li, D.; Haag-Molkenteller, C. Long-term Efficacy and Safety of OnabotulinumtoxinA in Patients With Urinary Incontinence Due to Neurogenic Detrusor Overactivity: An Interim Analysis. Urology 2013, 81, 491–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duchen, L.W. Motor nerve growth induced by botulinum toxin as a regenerative phenomenon. Proc. Roy. Soc. Med. 1972, 65, 196–197. [Google Scholar] [CrossRef] [PubMed]
- Rogozhin, A.A.; Pang, K.K.; Bukharaeva, E.; Young, C.; Slater, C.R. Recovery of mouse neuromuscular junctions from single and repeated injections of botulinum neurotoxin Am. J. Physiol. 2008, 586, 3163–3182. [Google Scholar] [CrossRef] [PubMed]
- Leitner, L.; Guggenbühl-Roy, S.; Knüpfer, S.C.; Walter, M.; Schneider, M.P.; Tornic, J.; Sammer, U.; Mehnert, U.; Kessler, T.M. More Than 15 Years of Experience with Intradetrusor Onabotulinumtoxin A Injections for Treating Refractory Neurogenic Detrusor Overactivity: Lessons to Be Learned. Eur. Urol. 2016, 70, 522–528. [Google Scholar] [CrossRef] [PubMed]
- De Groat, W.C.; Yoshimura, N. Anatomy and physiology of the lower urinary tract. Handb. Clin. Neurol. 2015, 130, 61–108. [Google Scholar] [PubMed]
- Daly, D.M.; Collins, V.M.; Chapple, C.R.; Grundy, D. The afferent system and its role in lower urinary tract dysfunction. Curr. Opin. Urol. 2011, 21, 268–274. [Google Scholar] [CrossRef]
- Andersson, K.E.; McCloskey, K.D. Lamina propria: The functional center of the bladder? Neurourol. Urodyn. 2014, 33, 9–16. [Google Scholar] [CrossRef]
- Chapple, C. Chapter 2: Pathophysiology of neurogenic detrusor overactivity and the symptom complex of “Overactive bladder”. Neurourol. Urodyn. 2014, 33, S6–S13. [Google Scholar] [CrossRef]
- Fry, C.H.; Vahabi, B. The Role of the Mucosa in Normal and Abnormal Bladder Function. Basic Clin. Pharmacol. Toxicol. 2016, 119, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Vannucchi, M.-G.; Traini, C. The telocytes/myofibroblasts 3-D network forms a stretch receptor in the human bladder mucosa. Is this structure involved in the detrusor overactive diseases? Ann. Anat. 2018, 218, 118–123. [Google Scholar] [CrossRef]
- Kanai, A.; Fry, C.; Ikeda, Y.; Kullmann, F.A.; Parsons, B.; Birder, L. Implications for bidirectional signaling between afferent nerves and urothelial cells-ICI-RS 2014. Neurourol. Urodyn. 2016, 35, 273–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckel, J.M.; Daugherty, S.L.; Tyagi, P.; Wolf-Johnston, A.S.; Birder, L.A.; Mitchell, C.H.; De Groat, W.C. Pannexin 1 channels mediate the release of ATP into the lumen of the rat urinary bladder. J. Physiol. 2015, 593, 1857–1871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLatchie, L.M.; Fry, C.H. ATP release from freshly isolated guinea-pig bladder urothelial cells: A quantification and study of the mechanisms involved. BJU Int. 2015, 115, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Hanna-Mitchell, A.T.; Wolf-Johnston, A.S.; Barrick, S.R.; Kanai, A.J.; Chancellor, M.B.; de Groat, W.C.; Birder, L.A. Effect of botulinum toxin A on urothelial-release of ATP and expression of SNARE targets within the urothelium. Neurourol. Urodyn. 2015, 34, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.P.; Vemulakonda, V.M.; Kiss, S.; Boone, T.B.; Somogyi, G.T. Enhanced ATP release from rat bladder urothelium during chronic bladder inflammation: Effect of botulinum toxin A. Neurochem. Int. 2005, 47, 291–297. [Google Scholar] [CrossRef]
- Smith, C.P.; Gangitano, D.A.; Munoz, A.; Salas, N.A.; Boone, T.B.; Aoki, K.R.; Francis, J.; Somogyi, G.T. Botulinum toxin type A normalizes alterations in urothelial ATP and NO release induced by chronic spinal cord injury. Neurochem. Int. 2008, 52, 1068–1075. [Google Scholar] [CrossRef] [Green Version]
- Birder, L.A.; Apodaca, G.; De Groat, W.C.; Kanai, A.J. Adrenergic- and capsaicin-evoked nitric oxide release from urothelium and afferent nerves in urinary bladder. Am. J. Physiol.. 1998, 275, F226–F229. [Google Scholar] [CrossRef]
- Birder, L.A.; Nealen, M.L.; Kiss, S.; de Groat, W.C.; Caterina, M.J.; Wang, E.; Apodaca, G.; Kanai, A.J. Beta-adrenoceptor agonists stimulate endothelial nitric oxide synthase in rat urinary bladder and urothelial cells. J. Neurosci. 2002, 22, 8063–8070. [Google Scholar] [CrossRef]
- Jeremy, J.Y.; Tsang, V.; Mikhailidis, D.P.; Rogers, H.; Morgan, R.J.; Dandona, P. Eicosanoid Synthesis by Human Urinary Bladder Mucosa: Pathological Implications. BJU Int. 1987, 59, 36–39. [Google Scholar] [CrossRef]
- Wang, X.; Momota, Y.; Yanase, H.; Narumiya, S.; Maruyama, T.; Kawatani, M. Urothelium EP1 receptor facilitates the micturition reflex in mice. Biomed. Res. 2008, 29, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Nile, C.J.; De Vente, J.; Gillespie, J.I. Stretch independent regulation of prostaglandin E2production within the isolated guinea-pig lamina propria. BJU Int. 2010, 105, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Hanna-Mitchell, A.T.; Beckel, J.M.; Barbadora, S.; Kanai, A.J.; De Groat, W.C.; Birder, L.A. Non-neuronal acetylcholine and urinary bladder urothelium. Life Sci. 2007, 80, 2298–2302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLatchie, L.M.; Young, J.S.; Fry, C.H. Regulation of ACh release from guinea pig bladder urothelial cells: Potential role in bladder filling sensations. Br. J. Pharmacol. 2014, 171, 3394–3403. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Zabbarova, I.V.; Birder, L.A.; De Groat, W.C.; McCarthy, C.J.; Hanna-Mitchell, A.T.; Kanai, A.J. Botulinum neurotoxin serotype A suppresses neurotransmitter release from afferent as well as efferent nerves in the urinary bladder. Eur. Urol. 2012, 62, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, O.J.; Fowler, C.J.; Landon, D.N. The role of the human bladder lamina propria myofibroblast. BJU Int. 2003, 91, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, M.-G.; Traini, C.; Guasti, D.; Giulio, D.P.; Faussone-Pellegrini, M.-S. Telocytes subtypes in human urinary bladder. J. Cell. Mol. Med. 2014, 18, 2000–2008. [Google Scholar] [CrossRef] [PubMed]
- Traini, C.; Fausssone-Pellegrini, M.S.; Guasti, D.; Del Popolo, G.; Frizzi, J.; Serni, S.; Vannucchi, M.G. Adaptive changes of telocytes in the urinary bladder of patients affected by neurogenic detrusor overactivity. J. Cell. Mol. Med. 2018, 22, 195–206. [Google Scholar] [CrossRef]
- Saban, R.; Undem, B.J.; Keith, I.M.; Saban, M.R.; Tengowski, M.W.; Graziano, F.M.; Bjorling, D.E. Differential Release of Prostaglandins and Leukotrienes by Sensitized Guinea Pig Urinary Bladder Layers Upon Antigen Challenge. J. Urol. 1994, 152, 544–549. [Google Scholar] [CrossRef]
- Cooley, L.F.; Kielb, S. A Review of Botulinum Toxin A for the Treatment of Neurogenic Bladder. PM R 2019, 11, 192–200. [Google Scholar] [CrossRef]
- Ku, J.H. The management of neurogenic bladder and quality of life in spinal cord injury. BJU Int. 2006, 98, 739–745. [Google Scholar] [CrossRef]
- Simpson, L.A.; Eng, J.J.; Hsieh, J.T.; Wolfe, D.L.; Spinal Cord Injury Rehabilitation Evidence Scire Research Team. The health and life priorities of individuals with spinal cord injury: A systematic review. J. Neurotrauma 2012, 29, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, J.T. Detrusor sphincter dyssynergia: A review of physiology, diagnosis, and treatment strategies. Transl. Androl. Urol. 2016, 5, 127–135. [Google Scholar] [PubMed]
- Stevens, L.A.; Chapple, C.R.; Chess-Williams, R. Human Idiopathic and Neurogenic Overactive Bladders and the Role of M2 Muscarinic Receptors in Contraction. Eur. Urol. 2007, 52, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Madersbacher, H.; Mürtz, G.; Stöhrer, M. Neurogenic detrusor overactivity in adults: A review on efficacy, tolerability and safety of oral antimuscarinics. Spinal Cord 2013, 51, 432–441. [Google Scholar] [CrossRef]
- Shenot, P.J.; Mark, J.R. Intradetrusorial onabotulinum toxin A injection: How to do it. Can. J. Urol. 2013, 20, 6650–6655. [Google Scholar]
- Moore, D.C.; Cohn, J.A.; Dmochowski, R.R. Use of botulinum toxin A in the treatment of lower urinary tract disorders: A review of the litteraure. Toxins 2016, 8, 88. [Google Scholar] [CrossRef]
- Rovner, E.; Kohan, A.; Chartier-Kastler, E.; Jünemann, K.-P.; Del Popolo, G.; Herschorn, S.; Joshi, M.; Magyar, A.; Nitti, V. Long-Term Efficacy and Safety of OnabotulinumtoxinA in Patients with Neurogenic Detrusor Overactivity Who Completed 4 Years of Treatment. J. Urol. 2016, 196, 801–808. [Google Scholar] [CrossRef]
- Lombardi, G.; Musco, S.; Bacci, G.; Celso, M.; Bellio, V.; Del Popolo, G. Long-term response of different Botulinum toxins in refractory neurogenic detrusor overactivity due to spinal cord injury. Int. Braz. J. Urol. 2017, 43, 721–729. [Google Scholar] [CrossRef] [Green Version]
- Knispel, H.H.; Schulte-Baukloh, H.; Schulte-Baukloh, H. A minimally invasive technique for outpatient local anaesthetic administration of intradetrusor botulinum toxin in intractable detrusor overactivity. BJU Int. 2005, 95, 454. [Google Scholar]
- Coelho, A.; Dinis, P.; Pinto, R.; Gorgal, T.; Silva, C.; Silva, A.; Silva, J.; Cruz, C.D.; Cruz, F.; Avelino, A. Distribution of the High-Affinity Binding Site and Intracellular Target of Botulinum Toxin Type A in the Human Bladder. Eur. Urol. 2010, 57, 884–890. [Google Scholar] [CrossRef]
- Malde, S.; Fry, C.; Schurch, B.; Marcelissen, T.; Averbeck, M.; Digesu, A.; Sahai, A. What is the exact working mechanism of botulinum toxin A and sacral nerve stimulation in the treatment of overactive bladder/detrusor overactivity? ICI-RS 2017. Neurourol. Urodyn. 2018, 37, S108–S116. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.P.; Franks, M.E.; McNeil, B.K.; Ghosh, R.; De Groat, W.C.; Chancellor, M.B.; Somogyi, G.T. Effect of Botulinum Toxin A on the Autonomic Nervous System of the Rat Lower Urinary Tract. J. Urol. 2003, 169, 1896–1900. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, C. Purinergic signaling in the lower urinary tract. Acta Phisiol. 2013, 207, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Lucioni, A.; Bales, G.T.; Lotan, T.L.; McGehee, D.S.; Cook, S.P.; Rapp, D.E. Botulinum toxin type A inhibits sensory neuropeptide release in rat bladder models of acute injury and chronic inflammation. BJU Int. 2008, 101, 366–370. [Google Scholar] [CrossRef]
- Morrison, J. The activation of bladder wall afferent nerves. Exp. Physiol. 1999, 84, 131–136. [Google Scholar] [CrossRef]
- Chancellor, M.B.; Fowler, C.J.; Apostolidis, A.; De Groat, W.C.; Smith, C.P.; Somogyi, G.T.; Aoki, K.R. Drug Insight: Biological effects of botulinum toxin A in the lower urinary tract. Nat. Clin. Pract. Urol. 2008, 5, 319–328. [Google Scholar] [CrossRef]
- Vemulakonda, V.M.; Somogyi, G.T.; Kiss, S.; Salas, N.A.; Boone, T.B.; Smith, C.P. Inhibitory effect of intravesically applied botulinum toxin A in chronic bladder inflammation. J. Urol. 2005, 173, 621–624. [Google Scholar] [CrossRef]
- Morenilla-Palao, C.; Planells-Cases, R.; Garcia-Sanz, N.; Ferrer-Montiel, A. Regulated Exocytosis Contributes to Protein Kinase C Potentiation of Vanilloid Receptor Activity. J. Boil. Chem. 2004, 279, 25665–25672. [Google Scholar] [CrossRef] [Green Version]
- Apostolidis, A.; Popat, R.; Yiangou, Y.; Cockayne, D.; Ford, A.; Davis, J.; Dasgupta, P.; Fowler, C.; Anand, P. Decreased Sensory Receptors P2x 3 And Trpv1 In Suburothelial Nerve Fibers Following Intradetrusor Injections Of Botulinum Toxin For Human Detrusor Overactivity. J. Urol. 2005, 174, 977–983. [Google Scholar] [CrossRef]
- Dolly, J.O.; Lawrence, G.W. Chapter 3: Molecular basis for the therapeutic effectiveness of botulinum neurotoxin type A. Neurourol. Urodyn. 2014, 33, S14–S20. [Google Scholar] [CrossRef]
- Apostolidis, A.; Dasgupta, P.; Fowler, C.J. Proposed Mechanism for the Efficacy of Injected Botulinum Toxin in the Treatment of Human Detrusor Overactivity. Eur. Urol. 2006, 49, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Masunaga, K.; Satoji, Y.; Maeda, Y.; Nagata, T.; Inadome, A. Basic and clinical aspects of non-neuronal acetylcholine: Expression of non-neuronal acetylcholine in urothelium and its clinical significance. J. Pharmacol. Sci. 2008, 106, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Collins, V.M.; Daly, D.M.; Liaskos, M.; McKay, N.G.; Sellers, D.; Chapple, C.; Grundy, D. OnabotulinumtoxinA significantly attenuates bladder afferent nerve firing and inhibits ATP release from the urothelium. BJU Int. 2013, 112, 1018–1026. [Google Scholar] [CrossRef] [Green Version]
- Khera, M.; Somogyi, G.T.; Kiss, S.; Boone, T.B.; Smith, C.P. Botulinum toxin A inhibits ATP release from bladder urothelium after chronic spinal cord injury. Neurochem. Int. 2004, 45, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Cretoiu, S.M.; Popescu, L.M. Telocytes revisited. Biomol. Concepts 2014, 5, 353–369. [Google Scholar] [CrossRef]
- Gillespie, J.I.; Markerink-van Ittersum, M.; de Vente, J. cGMP-generating cellsin the bladder wall: Identification of distinct networks of interstitial cells. BJU Int. 2004, 94, 1114–1124. [Google Scholar] [CrossRef]
- Ost, D.; Roskams, T.; Van Der Aa, F.; De Ridder, D. Topography of the Vanilloid Receptor in the Human Bladder: More Than Just the Nerve Fibers. J. Urol. 2002, 168, 293–297. [Google Scholar] [CrossRef]
- Mukerji, G.; Yiangou, Y.; Grogono, J.; Underwood, J.; Agarwal, S.K.; Khullar, V.; Anand, P. Localization of M2 and M3 muscarinic receptors in human bladderdisorders and their clinical correlations. J. Urol. 2006, 176, 367–373. [Google Scholar] [CrossRef]
- Svennersten, K.; Hallén-Grufman, K.; De Verdier, P.J.; Wiklund, N.P.; Poljakovic, M. Localization of P2X receptor subtypes 2, 3 and 7 in human urinary bladder. BMC Urol. 2015, 15, 81. [Google Scholar] [CrossRef]
- Wu, C.; Sui, G.P.; Fry, C.H. Purinergic regulation of guinea pig suburothelial myofibroblasts. J. Physiol. 2004, 559, 231–243. [Google Scholar] [CrossRef] [Green Version]
- Sui, G.-P.; Wu, C.; Roosen, A.; Ikeda, Y.; Kanai, A.J.; Fry, C.H. Modulation of bladder myofibroblast activity: Implications for bladder function. Am. J. Physiol. Physiol. 2008, 295, F688–F697. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.M. Enteric Inhibitory Neurotransmission, Starting Down Under. Adv. Exp. Med. Biol. 2016, 891, 21–29. [Google Scholar] [PubMed]
- Saberi, F.A.; Dressler, D. Botulinum Toxin: Mechanisms of Action. Eur. Neurol. 2005, 53, 3–9. [Google Scholar]
- Mangera, A.; Apostolidis, A.; Andersson, K.E.; Dasgupta, P.; Giannantoni, A.; Roehrborn, C.; Novara, G.; Chapple, C. An Updated Systematic Review and Statistical Comparison of Standardised Mean Outcomes for the Use of Botulinum Toxin in the Management of Lower Urinary Tract Disorders. Eur. Urol. 2014, 65, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Compérat, E.; Reitz, A.; Delcourt, A.; Capron, F.; Denys, P.; Chartier-Kastler, E. Histologic Features in the Urinary Bladder Wall Affected from Neurogenic Overactivity—A Comparison of Inflammation, Oedema and Fibrosis With and Without Injection of Botulinum Toxin Type A. Eur. Urol. 2006, 50, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Haferkamp, A.; Schurch, B.; Reitz, A.; Krengel, U.; Grosse, J.; Kramer, G.; Schumacher, S.; Bastian, P.; Büttner, R.; Müller, S.; et al. Lack of Ultrastructural Detrusor Changes Following Endoscopic Injection of Botulinum Toxin Type A in Overactive Neurogenic Bladder. Eur. Urol. 2004, 46, 784–791. [Google Scholar] [CrossRef]
- Apostolidis, A.; Jacques, T.S.; Freeman, A.; Kalsi, V.; Popat, R.; Gonzales, G.; Datta, S.N.; Ghazi-Noori, S.; Elneil, S.; Dasgupta, P.; et al. Histological Changes in the Urothelium and Suburothelium of Human Overactive Bladder following Intradetrusor Injections of Botulinum Neurotoxin Type A for the Treatment of Neurogenic or Idiopathic Detrusor Overactivity. Eur. Urol. 2008, 53, 1245–1253. [Google Scholar] [CrossRef]
- Traini, C.; Del Popolo, G.; Faussone-Pellegrini, M.; Guasti, D.; Catarinicchia, S.; Vannucchi, M.G. Nerve sprouting and neurogenic inflammation characterize the neurogenic detrusor overactive bladder of patients no longer responsive to drug therapies. J. Cell. Mol. Med. 2019, 23, 4076–4087. [Google Scholar] [CrossRef]
- Drake, M.; Gardner, B.; Brading, A.; Drake, M. Innervation of the detrusor muscle bundle in neurogenic detrusor overactivity. BJU Int. 2003, 91, 702–710. [Google Scholar] [CrossRef]
- Birder, L.A.; Kullmann, F.A. Role of neurogenic inflammation in local communication in the visceral mucosa. Semin. Immunopathol. 2018, 40, 1–19. [Google Scholar] [CrossRef]
- Steers, W.D.; Tuttle, J. Neurogenic inflammation and nerve growth factor: Possible roles in interstitial cystitis. In Interstitial Cystitis; Sant, G.R., Ed.; Lippincott-Raven: Philadelphia, PA, USA, 1997; pp. 67–76. [Google Scholar]
- Sorkin, L.S.; Eddinger, K.A.; Woller, S.A.; Yaksh, T.L. Origins of antidromic activity in sensory afferent fibers and neurogenic inflammation. Semin. Immunopathol. 2018, 40, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.E.; Di Nardo, A. Skin neurogenic inflammation. Semin. Immunopathol. 2018, 40, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Gouin, O.; Lebonvallet, N.; L’Herondelle, K.; Buhé, V.; Carré, J.-L.; Lefeuvre, L.; Misery, L.; Le Gall-Ianotto, C.; Plée-Gautier, E.; Le Gall-Ianotto, C.; et al. Self-maintenance of neurogenic inflammation contributes to a vicious cycle in skin. Exp. Dermatol. 2015, 24, 723–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Traini, C.; Vannucchi, M.G. The Botulinum Treatment of Neurogenic Detrusor Overactivity: The Double-Face of the Neurotoxin. Toxins 2019, 11, 614. https://doi.org/10.3390/toxins11110614
Traini C, Vannucchi MG. The Botulinum Treatment of Neurogenic Detrusor Overactivity: The Double-Face of the Neurotoxin. Toxins. 2019; 11(11):614. https://doi.org/10.3390/toxins11110614
Chicago/Turabian StyleTraini, Chiara, and Maria Giuliana Vannucchi. 2019. "The Botulinum Treatment of Neurogenic Detrusor Overactivity: The Double-Face of the Neurotoxin" Toxins 11, no. 11: 614. https://doi.org/10.3390/toxins11110614
APA StyleTraini, C., & Vannucchi, M. G. (2019). The Botulinum Treatment of Neurogenic Detrusor Overactivity: The Double-Face of the Neurotoxin. Toxins, 11(11), 614. https://doi.org/10.3390/toxins11110614




