Meteorological and Nutrient Conditions Influence Microcystin Congeners in Freshwaters
Abstract
1. Introduction
2. Results
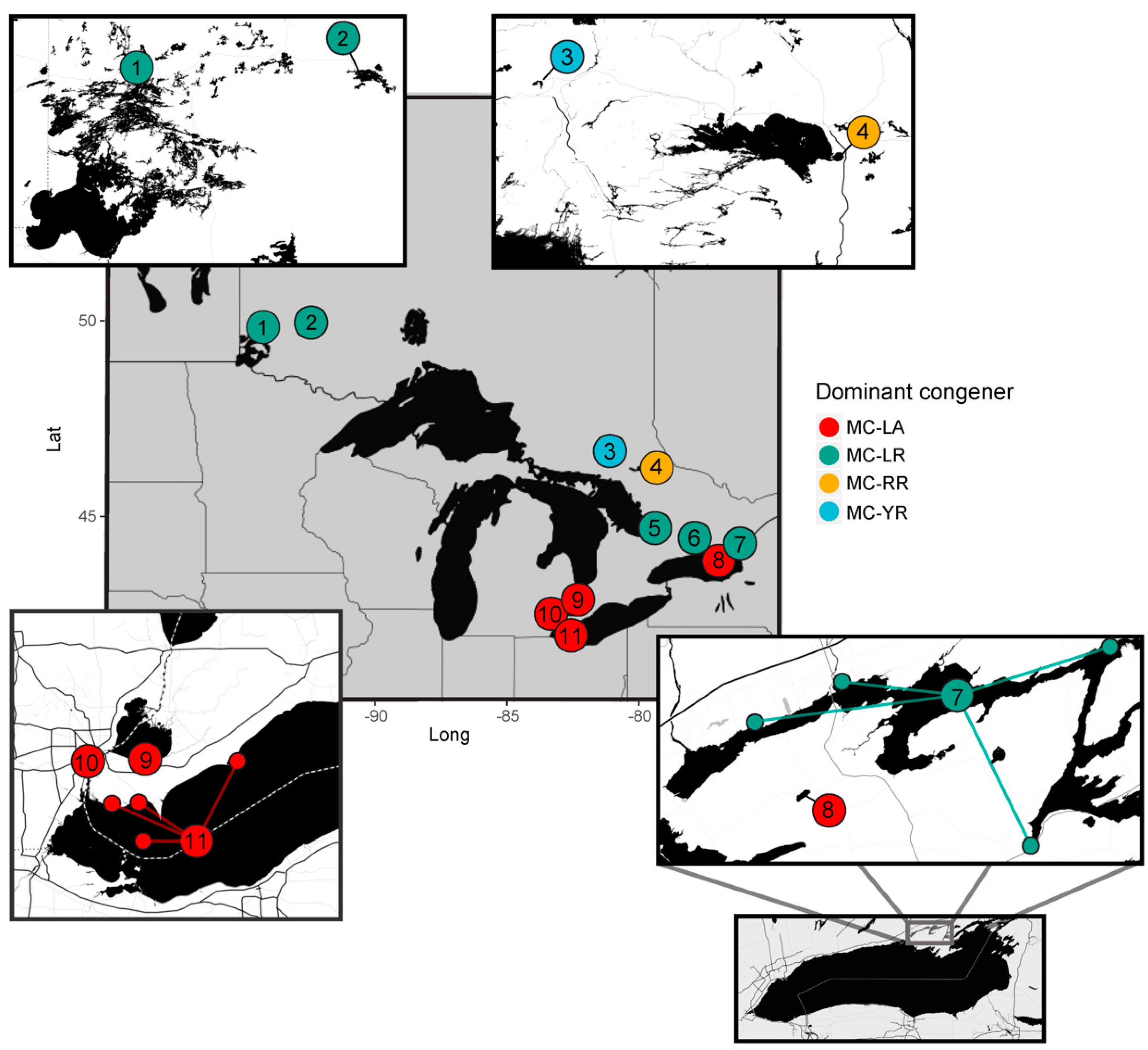
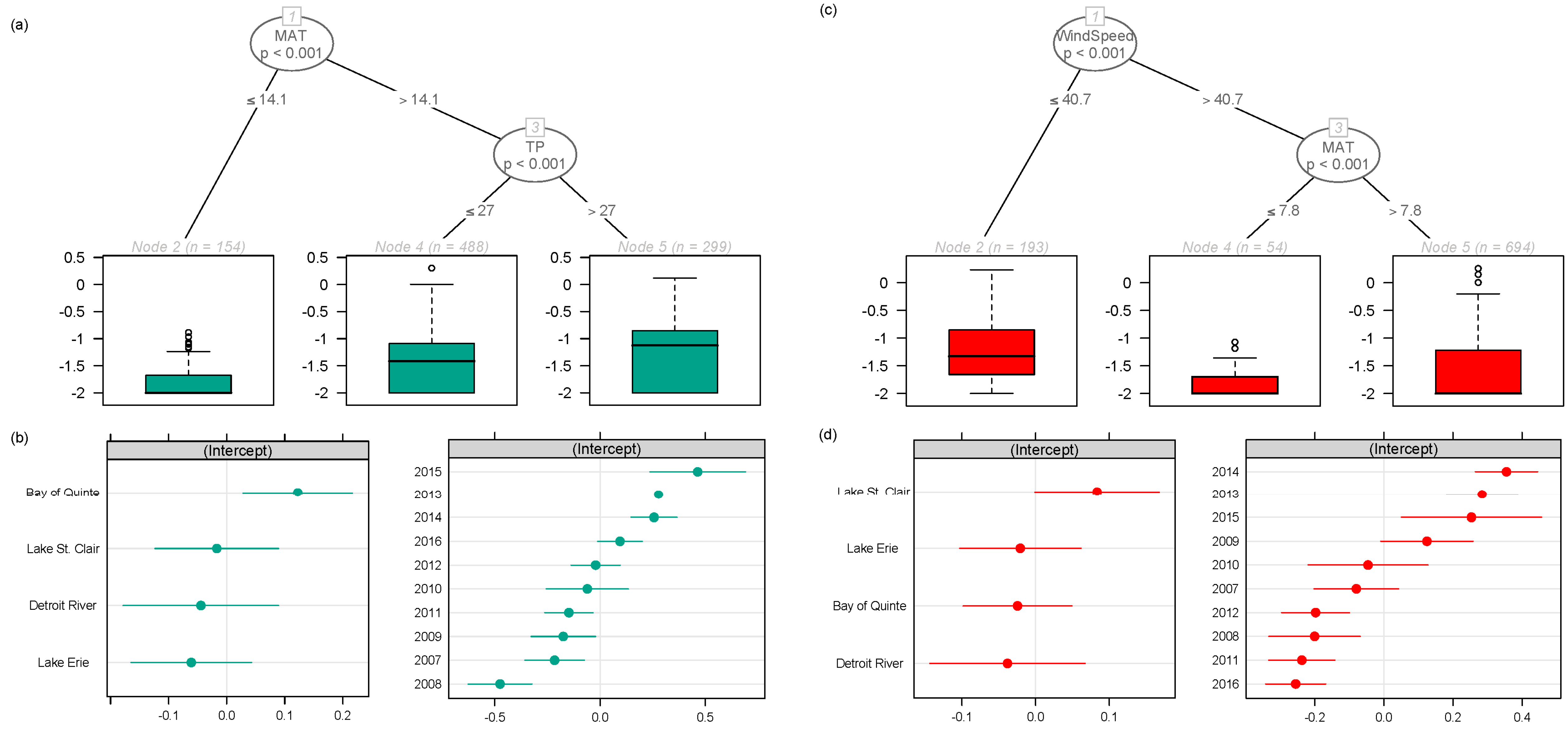
2.1. Regional Analysis of the Great Lakes Intake Sites
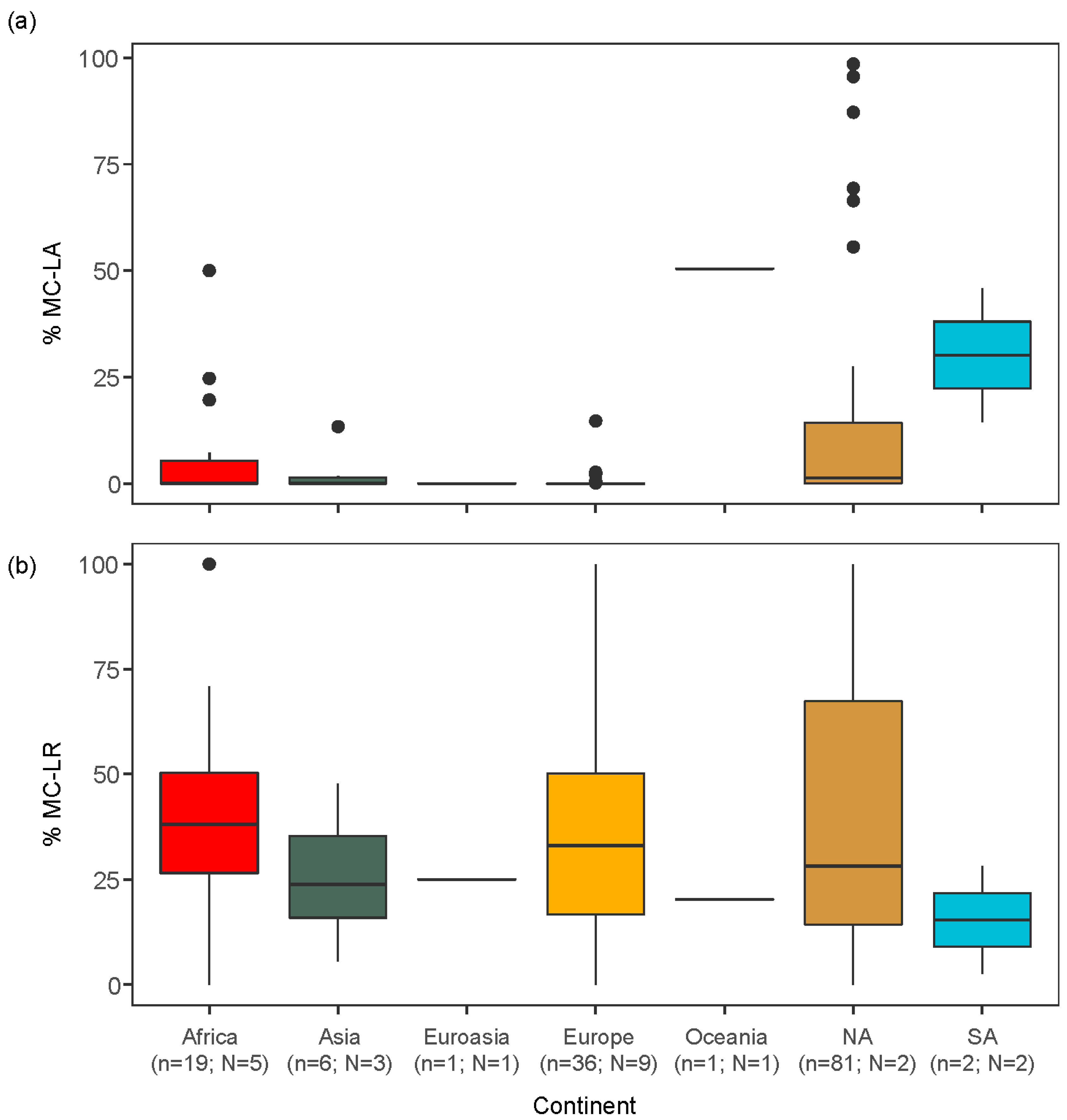
2.2. Global Analysis of the Peer-Reviewed Literature
3. Discussion
3.1. Regional Relationship between Congener Occurrence and Environmental Conditions
3.2. Global Relationships between Congener Occurrence and Environmental Conditions
4. Conclusions
- In the Laurentian Great Lakes, MC-LA was often more prevalent than MC-LR, and its concentration has increased over the last decade at several sites.
- The more toxic congeners (MC-LA and -LR) occurred under both low nutrient (MC-LA) and high nutrient (MC-LR) concentrations, while meteorological conditions (wind speed and precipitation) determined the relative concentration of each.
- Meso-oligotrophic waters with intermediate winds and frequent rain events showed greater percentage of MC-LA, while low winds or high winds combined with warm, nutrient-rich conditions showed greater percentage of MC-LR and -RR.
- Environmental conditions and related MC congener dominance were geographically distinct, with conditions that favoured MC-LA in the western part of our regional study (Lake St. Clair, Detroit River, and parts of Lake Erie), but windy, warm, eutrophic conditions that favoured MC-LR observed to the east in Lake Ontario’s Bay of Quinte.
- Globally, MC-LA and MC-LR also showed geographically distinct patterns, with a smaller percentage of MC-LA in Africa, Europe and Asia, compared to North and South America. These patterns of MC congener dominance were associated with differences in lake morphometry and nutrient concentrations: MC-LA tended to be more prevalent in smaller lakes while MC-LR peaked in eutrophic lakes.
5. Materials and Methods
5.1. Great Lakes Regional Analysis
5.1.1. Analytical Data
5.1.2. Statistical Analysis
5.2. Global Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Sinha, R.P.; Incharoensakdi, A. The cyanotoxin-microcystins: Current overview. Rev. Environ. Sci. Biotechnol. 2014, 13, 215–249. [Google Scholar] [CrossRef]
- Sukenik, A.; Quesada, A.; Salmaso, N. Global expansion of toxic and non-toxic cyanobacteria: Effect on ecosystem functioning. Biodivers. Conserv. 2015, 24, 889–908. [Google Scholar] [CrossRef]
- Taranu, Z.E.; Gregory-Eaves, I.; Leavitt, P.R.; Bunting, L.; Buchaca, T.; Catalan, J.; Moorhouse, H. Acceleration of cyanobacteria dominance in north temperate-subarctic lakes during the Anthropocene. Ecol. Lett. 2015, 18, 375–384. [Google Scholar] [CrossRef]
- Huisman, J.M.; Codd, G.A.; Paerl, H.S.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Harke, M.J.; Davis, T.W.; Watson, S.B.; Gobler, C.J. Nutrient-controlled niche differentiation of western Lake Erie cyanobacterial populations revealed via metatranscriptomic surveys. Environ. Sci. Technol. 2016, 50, 604–615. [Google Scholar] [CrossRef]
- Pick, F.R. Blooming algae: A Canadian perspective on the rise of toxic cyanobacteria. Can. J. Fish. Aquat. Sci. 2016, 73, 1149–1158. [Google Scholar] [CrossRef]
- Taranu, Z.E.; Gregory-Eaves, I.; Steele, R.; Beaulieu, M.; Legendre, P. Predicting microcystin occurrences in US lakes and reservoirs: A new framework for modeling the drivers of an important health risk factor. Glob. Ecol. Biogeog. 2017, 26, 625–637. [Google Scholar] [CrossRef]
- Du, H.; Liu, H.; Yuan, L.; Wang, Y.; Ma, Y.; Wang, R.; Chen, X.; Losiewicz, M.D.; Guo, H.; Zhang, H. The diversity of cyanobacterial toxins on structural characterization, distribution and identification: A systematic review. Toxins 2019, 11, 530. [Google Scholar] [CrossRef]
- Spoof, L.; Catherine, A. Appendix 3: Tables of microcystins and nodularins. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; John Wiley & Sons, Ltd.: West Sussex, UK, 2017; pp. 526–537. [Google Scholar]
- Beversdorf, L.J.; Rude, K.; Weirich, C.A.; Bartlett, S.L.; Seaman, M.; Kozik, C.; Biese, P.; Gosz, T.; Suha, M.; Stempa, C.; et al. Analysis of cyanobacterial metabolites in surface and raw drinking waters reveals more than microcystin. Water Res. 2018, 140, 280–290. [Google Scholar] [CrossRef]
- Mantzouki, E.; Lürling, M.; Fastner, J.; de Senerpont Domis, L.; Wilk-Woźniak, E.; Koreivienė, J.; Walusiak, E. Temperature effects explain continental scale distribution of cyanobacterial toxins. Toxins 2018, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- Huisman, J.M.; Matthijs, H.C.P.; Visser, P.M. Harmful Cyanobacteria; Springer: Dordrecht, The Netherlands, 2005. [Google Scholar]
- Miller, M.A.; Kudela, R.M.; Mekebri, A.; Crane, D.; Oates, S.C.; Tinker, M.T.; Hardin, D. Evidence for a novel marine harmful algal nloom: Cyanotoxin (microcystin) transfer from land to sea otters. PLoS ONE 2010, 5, e12576. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, B.; Gervais, M.C.; Chevalier, P.; Gauvin, D.; Anassour-Laouan-Sidi, E.; Gingras, S.; Fortin, N.; Brisson, G.; Greer, C.; Bird, D. Prospective study of acute health effects in relation to exposure to cyanobacteria. Sci. Total Environ. 2013, 466, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, W.W. Cyanobacterial secondary metabolites–the cyanotoxins. J. Appl. Bacteriol. 1992, 72, 445–459. [Google Scholar] [CrossRef]
- Ueno, T.; Nagata, S.; Tsutsumi, T.; Hasegawa, A.; Yoshida, F.; Suttajit, M.; Mebs, D.; Putsch, M.; Vasconcelos, V. Detection of microcystins, a blue-green algal hepatotoxin, in drinking water sampled in Haimen and Fusui, endemic areas of primary liver cancer in China, by highly senstive immunoassay. Carcinogenesis 1996, 17, 1317–1321. [Google Scholar] [CrossRef]
- Carmichael, W.W. The cyanotoxins. Adv. Bot. Res. 1997, 27, 211–256. [Google Scholar]
- Zhang, F.; Lee, J.; Shum, C.K. Cyanobacteria blooms and non-alcoholic liver disease: Evidence from a county level ecological study in the United States. Environ. Health 2015, 14, 41. [Google Scholar] [CrossRef]
- Sotton, B.; Guillard, J.; Anneville, O.; Maréchal, M.; Savichtcheva, O.; Domaizon, I. Trophic transfer of microcystins through the lake pelagic food web: Evidence for the role of zooplankton as a vector in fish contamination. Sci. Total Environ. 2014, 466, 152–163. [Google Scholar] [CrossRef]
- Ettoumi, A.; El Khalloufi, F.; El Ghazali, I.; Oudra, B.; Amrani, A.; Nasri, H.; Bouaïcha, N. Bioaccumulation of cyanobacterial toxins in aquatic organisms and its consequences for public health. In Zooplankton and Phytoplankton: Types, Characteristics and Ecology; Kattel, G., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2011. [Google Scholar]
- Hardy, F.J.; Johnson, A.; Hamel, K.; Preece, E.P. Cyanotoxin bioaccumulation in freshwater fish, Washington state, USA. Environ. Monit. Assess. 2015, 187, 667. [Google Scholar] [CrossRef]
- Chorus, I. Cyanotoxin occurrence in freshwaters: A summary of survey results from different countries. In Cyanotoxins: Occurrence, Causes, Consequences; Springer: Berlin, Germany, 2001. [Google Scholar]
- Graham, J.L.; Loftin, K.A.; Meyer, M.T.; Ziegler, A.C. Cyanotoxin mixtures and taste and odour compounds in cyanobacterial blooms from the Midwestern United States. Environ. Sci. Technol. 2010, 44, 7361–7368. [Google Scholar] [CrossRef]
- Jones, G.J.; Falconer, I.F.; Wilkins, R.M. Persistence of cyclic peptide toxins in dried cyanobacterial crusts from Lake Mokoan, Australia. Environ. Toxicol. Water Qual. 1995, 10, 19–24. [Google Scholar] [CrossRef]
- Falconer, I.; Bartram, J.; Chorus, I.; Kuiper-Goodman, T.; Utkilen, H.; Burch, M.; Codd, G. Safe levels and practices. In Toxic Cyanobacteria in Water, A Guide to Their Public Health Consequences, Monitoring and Management, Chorus, I., Bartam, J., Eds.; E & FN Spon: London, UK, 1999; pp. 155–178. [Google Scholar]
- Srivastava, A.; Choi, G.G.; Ahn, C.Y.; Oh, H.M.; Ravi, A.K.; Asthana, R.K. Dynamics of microcystin production and quantification of potentially toxigenic Microcystis sp. using real-time PCR. Water Res. 2012, 46, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Monchamp, M.-E.; Pick, F.R.; Beisner, B.E.; Maranger, R. Nitrogen forms influence microcystin concentration and composition via changes in cyanobacterial community structure. PLoS ONE 2014, 9, e85573. [Google Scholar] [CrossRef] [PubMed]
- Zastepa, A.; Taranu, Z.E.; Kimpe, L.E.; Blais, J.M.; Gregory-Eaves, I.; Pick, F.R. Reconstructing a long-term record of microcystins from the analysis of lake sediments. Sci. Total Environ. 2017, 579, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Health Canada. Guidelines for Canadian Drinking Water Quality: Guideline Technical Document-Cyanobacterial Toxins. 2018. Available online: https://www.canada.ca/en/health-canada/services/publications/healthy-living/guidelines-canadian-drinking-water-quality-guideline-technical-document-cyanobacterial-toxins-document.html (accessed on 25 October 2019).
- Chorus, I.; Bartram, J. Toxic Cyanobacteria Water: A Guide Public Health Conseq. Monitoring. Manag.; E.&FN Spon: London, UK, 1999. [Google Scholar]
- Newcombe, G.; Cook, D.; Brooke, S.; Ho, L.; Slyman, N. Treatment options for microcystin toxins: Similarities and differences between variants. Environ. Technol. 2003, 24, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Zastepa, A.; Pick, F.R.; Blais, J. Fate and persistence of particulate and dissolved microcystin-LA from Microcystis blooms. Hum. Ecol. Risk Assess. 2014, 20, 1670–1686. [Google Scholar] [CrossRef]
- Namikoshi, M.; Rinehart, K.L.; Sakai, R.; Stotts, R.R.; Dahlem, A.M.; Beasley, V.R.; Carmichael, W.W.; Evans, W.R. Identification of 12 hepatotoxins from a Homer Lake bloom of the cyanobacteria Microcystis aeruginosa, Microcystis viridis, and Microcystis wesenbergii: Nine new microcystins. J. Org. Chem. 1992, 57, 866–872. [Google Scholar] [CrossRef]
- Namikoshi, M.; Sun, F.; Choi, B.W.; Rinehart, K.L.; Carmichael, W.W.; Evans, W.R.; Beasley, V.R. Seven more microcystins from Homer Lake cells: Application of the general method for structure assignment of peptides containing α,β-dehydroamino acid unit(s). J. Org. Chem. 1995, 60, 3671–3679. [Google Scholar] [CrossRef]
- Puddick, J.; Prinsep, M.R.; Wood, S.A.; Kaufononga, S.A.; Cary, S.C.; Hamilton, D.P. High levels of structural diversity observed in microcystins from Microcystis CAWBG 11 and characterization of six new microcystin congeners. Mar. Drug. 2014, 12, 5372–5395. [Google Scholar] [CrossRef]
- Ibelings, B.W.; Backerb, L.C.W.; Kardinaa, E.A.; Chorus, I. Current approaches to cyanotoxin risk assessment and risk management around the globe. Harmful Algae 2014, 40, 63–74. [Google Scholar] [CrossRef]
- Fischer, A.; Hoeger, S.J.; Stemmer, K.; Feurstein, D.J.; Knobeloch, D.; Nussler, A.; Dietrich, D.R. The role of organic anion transporting polypeptides (OATPs/SLCOs) in the toxicity of different microcystin congeners in vitro: A comparison of primary human hepatocytes and OATP-transfected HEK293 cells. Toxicol. Appl. Pharm. 2010, 245, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Jetoo, S.; Grover, V.I.; Krantzberg, G. The Toledo drinking water advisory: Suggested application of the water safety planning approach. Sustainability 2015, 7, 9787–9808. [Google Scholar] [CrossRef]
- Jones, G.J.; Orr, P. Release and degradation of microcystin following algicide treatment of a Microcystis aeruginosa bloom in a recreational lake, as determined by HPLC and protein phosphatase inhibition assay. Water Res. 1994, 28, 871–876. [Google Scholar] [CrossRef]
- Walsby, A.E.; Hayes, P.K.; Boje, R.; Stal, L.J. The selective advantage of buoyancy provided by gas vesibles for planktonic cyanobacteria in the Baltic Sea. New Phytol. 1997, 136, 407–417. [Google Scholar] [CrossRef]
- Wagner, C.; Adrian, R. Cyanobacteria dominance: Quantifying the effects of climate change. Limnol. Oceanogr. 2009, 54, 2460–2468. [Google Scholar] [CrossRef]
- Posch, T.; Köster, O.; Salcher, M.M.; Pernthaler, J. Harmful filamentous cyanobacteria favoured by reduced water turnover with lake warming. Nat. Clim. Chang. 2012, 2, 809–813. [Google Scholar] [CrossRef]
- Downing, J.A.; Watson, S.B.; McCauley, E. Predicting cyanobacteria dominance in lakes. Can. J. Fish. Aquat. Sci. 2001, 58, 1905–1908. [Google Scholar] [CrossRef]
- Dolman, A.M.; Rücker, J.; Pick, F.R.; Fastner, J.; Rohrlack, T.; Mischke, U.; Wiedner, C. Cyanobacteria and cyanotoxins: The influence of nitrogen versus phosphorus. PLoS ONE 2012, 7, e38757. [Google Scholar] [CrossRef]
- Rosenblum, L.; Zaffiro, A.; Adams, W.A.; Wendelken, S.C. Effect of chlorination by-producs on the quantitation of microcystins in finished drinking water. Toxicon 2017, 138, 138–144. [Google Scholar] [CrossRef]
- Knoll, L.B.; Sarnelle, O.; Hamilton, S.K.; Kissman, C.E.H.; Wilson, A.E.; Rose, J.B.; Morgan, M.R. Invasive zebra mussels (Dreissena polymorpha) increase cyanobacterial toxin concentrations in low-nutrient lakes. Can. J. Fish. Aquat. Sci. 2008, 65, 448–455. [Google Scholar] [CrossRef]
- Jöhnk, K.D.; Huisman, J.; Sharples, J.; Sommeijer, B.; Visser, P.M.; Strooms, J.M. Summer heatwaves promote blooms of harmful cyanobacteria. Glob. Chang. Biol. 2008, 14, 495–512. [Google Scholar] [CrossRef]
- Dokulil, M.T.; Teubner, K. Cyanobacterial dominance in lakes. Hydrobiol. 2000, 438, 1–12. [Google Scholar] [CrossRef]
- Michalak, A.M.; Anderson, E.J.; Beletsky, D.; Boland, S.; Bosch, N.S. Record-setting algal bloom in Lake Erie caused by agricultural and meteorological trends consistent with expected future conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 6448–6452. [Google Scholar] [CrossRef] [PubMed]
- Kelly, N.E.; Javed, A.J.; Shimoda, Y.; Zastepa, A.; Watson, S.; Mugalingam, S.; Arhonditsis, G.B. A Bayesian risk assessment framework for microcystin violations of drinking water and recreational standards in the Bay of Quinte, Lake Ontario, Canada. Water Res. 2019, 162, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Bartolai, A.M.; He, L.; Hurst, A.E.; Mortsch, L.; Paehlke, R.; Scavia, D. Climate change as a driver of change in the Great Lakes St. Lawrence River basin. J. Great Lakes Res. 2015, 41, 45–58. [Google Scholar] [CrossRef]
- Webster, I.T.; Hutchinson, P.A. Effects of wind on the distribution of phytoplankton cells in lakes-revisited. Limnol. Oceanogr. 1994, 39, 365–373. [Google Scholar] [CrossRef]
- Šejnohová, L.; Maršálek, B.; Microcystin. Ecology of Cyanobacteria II: Their Diversity in Space and Time; Whitton, B.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 195–228. [Google Scholar]
- Tromas, N.; Taranu, Z.E.; Martin, B.D.; Willis, A.; Fortin, N.; Greer, C.W.; Shapiro, B.J. Niche separation increases with genetic distance among bloom-forming cyanobacteria. Front. Microbiol. 2018. [Google Scholar] [CrossRef]
- Amé, M.; Wunderlin, D. Effects of iron, ammonium and temperature on microcystin content by a natural concentrated Microcystis aeruginosa population. Water Air Soil Pollut. 2005, 168, 235–248. [Google Scholar] [CrossRef]
- Tonk, L.; Visser, P.M.; Christiansen, G.; Dittmann, E.; Snelder, E.O.F.M.; Wiedner, C.; Mur, L.R.; Huisman, J. The microcystin composition of the cyanobacterium Planktothrix agardhii changes toward a more toxic variant with increasing light intensity. Appl. Environ. Microbiol. 2005, 71, 5177–5181. [Google Scholar] [CrossRef]
- Agha, R.; Cirés, S.; Wömer, L.; Quesada, A. Limited stability of microcystins in oligopeptide compositions of Microcystis aeruginos (Cyanobacteria): Implications in the definition of chemotypes. Toxins 2013, 5, 1089–1104. [Google Scholar] [CrossRef]
- Van de Waal, D.B.; Ferreruela, G.; Tonk, L.; Van Donk, E.; Huisman, J.; Visser, P.M.; Matthijs, H.C. Pulsed nitrogen supply induces dynamic changes in the amino acid composition and microcystin production of the harmful cyanobacterium Planktothrix agardhii. FEMS Microbiol. Ecol. 2010, 74, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Puddick, J.; Prinsep, M.R.; Wood, S.A.; Cary, S.C.; Hamilton, D.P. Modulation of microcystin congener abundance following nitrogen depletion of a Microcystis batch culture. Aquat. Ecol. 2016, 50, 235–246. [Google Scholar] [CrossRef]
- Rinta-Kanto, J.M.; Wilhelm, S.W. Diversity of microcystin-producing cyanobacteria in spatially isolated regions of Lake Erie. Appl. Environ. Microbiol. 2006, 72, 5083–5085. [Google Scholar] [CrossRef] [PubMed]
- Kutovaya, O.A.; McKay, R.M.L.; Beall, B.F.N.; Wihlem, S.W.; Kane, D.D.; Chaffin, J.D.; Bridgeman, T.B.; Bullerjahn, G.S. Evidence against fluvial seeding of recurrent toxic blooms of Microcystis spp. in Lake Erie’s Western basin. Harmful Algae 2012, 15, 71–77. [Google Scholar] [CrossRef]
- Rohrlack, T.; Bente, E.; Skulberg, R.; Halstvedt, C.B.; Utkilen, H.C.; Ptacnik, R.; Skulberg, O.M. Oligopeptide chemotypes of the toxic freshwater cyanobacterium Planktothrix can form sub-populations with dissimilar ecological traits. Limnol. Oceanogr. 2008, 53, 1279–1293. [Google Scholar] [CrossRef]
- Bogialli, S.; di Gregorio, F.N.; Lucentini, L.; Ferretti, E.; Ottaviani, M.; Ungaro, N.; Cannarozzi de Grazia, M. Management of a toxic cyanobacterium bloom (Planktothrix rubescens) affecting an Italian drinking water basin: A case study. Environ. Sci. Technol. 2013, 47, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Ferranti, P.; Fabbrocino, S.; Chiaravalle, E.; Bruno, M.; Adriana Basile, A.; Serpe, L.; Gallo, P. Profiling microcystin contamination in a water reservoir by MALDI-TOF and liquid chromatography coupled to Q/TOF tandem mass spectrometry. Food Res. Int. 2013, 54, 1321–1330. [Google Scholar] [CrossRef]
- Van de Waal, D.B.; Verspagen, J.M.; Lürling, M.; Van Donk, E.; Visser, P.M.; Huisman, J. The ecological stoichiometry of toxins produced by harmful cyanobacteria: An experimental test of the carbon-nutrient balance hypothesis. Ecol. Lett. 2009, 12, 1326–1335. [Google Scholar] [CrossRef]
- Zastepa, A.; Pick, F.; Blais, J.M. Distribution and flux of microcystin congeners in lake sediments. Lake Res. Manag. 2017, 33, 444–451. [Google Scholar] [CrossRef]
- Conroy, J.D.; Edwards, W.J.; Pontius, R.A.; Kane, D.D.; Zhang, H.; Shea, J.F.; Richey, J.N.; Culver, D.A. Soluble nitrogen and phosphorus excretion of exotic freshwater mussels (Dreissena spp.): Potential impacts for nutrient remineralization in western Lake Erie. Freshw. Biol. 2005, 50, 1146–1162. [Google Scholar] [CrossRef]
- Bykova, O.; Laursen, A.; Bostan, V.; Bautista, J.; McCarthy, L. Do zebra mussels (Dreissena polymorpha) alter lake water chemistry in a way that favors Microcystis growth? Sci. Total Environ. 2006, 371, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Sarnelle, O.; White, J.D.; Horst, G.P.; Hamilton, S.K. Phosphorus addition reverses the positive effect of zebra mussels (Dreissena polymorpha) on the toxic cyanobacterium, Microcystis aeruginosa. Water Res. 2011, 46, 3471–3478. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.D.; Wilhelm, F.; Graham, J.L.; Loftin, K.A. Experimental manipulation of TN:TP ratios suppress cyanobacterial biovolume and microcystin concentration in large-scale in situ mesocosms. Lake Res. Menag. 2014, 30, 72–83. [Google Scholar] [CrossRef]
- Kalff, J. Limnology: Inland Water Ecosystems; Upper: Saddle River, NJ, USA, 2002. [Google Scholar]
- Håkanson, L.A. Manual of Lake Morphometry; Springer: Berlin, Germany, 1981. [Google Scholar]
- Ortiz, X.; Korenkova, E.; Jobst, K.J.; MacPherson, K.A.; Reiner, E.J. A high throughput targeted and non-targeted method for the analysis of microcystins and anatoxin-A using on-line solid phase extraction coupled to liquid chromatography-quadrupole time-of-flight high resolution mass spectrometry. Anal. Bioanal. Chem. 2017, 409, 4959–4969. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. vegan: Community Ecology Package. R package, version 2.5-4. 2019. Available online: https://CRAN.R-project.org/package=vegan (accessed on 25 October 2019).
- Legendre, P.; Legendre, L.F. Numerical Ecology; Elsevier: Amsterdam, The Netherlands, 2002; pp. 209–213. [Google Scholar]
- Therneau, T.M.; Atkinson, B. mvpart: Multivariate partitioning. R package, version 1.6-2. 2014. Available online: https://CRAN.R-project.org/package=mvpart (accessed on 25 October 2019).
- Ouellette, M.-H.; Legendre, P. MVPARTwrap: Additional features for package mvpart. R package, version 0.1-9.2. 2013. Available online: https://CRAN.R-project.org/package=MVPARTwrap (accessed on 25 October 2019).
- De’Ath, G. Multivariate regression trees: A new technique for modeling species–environment relationships. Ecology 2009, 83, 1105–1117. [Google Scholar]
- Fokkema, M.; Smits, N.; Zeileis, A.; Hothorn, T.; Kelderman, H. Detecting Treatment-Subgroup Interactions in Clustered Data with Generalized Linear Mixed-Effects Model Trees. Working Papers in Economics and Statistics, Research Platform Empirical and Experimental Economics, Universitaet Innsbruck. Available online: http://EconPapers.RePEc.org/RePEc:inn:wpaper:2015-10 (accessed on 25 October 2019).
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2018; p. 453. [Google Scholar]



| Environmental Factor | Percentile | Stn near L Ontario (N = 456) | Stn near L St. Clair and Detroit R (N = 236 & 118) | Stn near L Erie (N = 476) |
|---|---|---|---|---|
| MAT | min | −7.5 | −9.3 | −13.7 |
| 5th | −2.6 | −4.4 | −9.2 | |
| 35th | 8.8 | 6.7 | 2.3 | |
| 50th | 14.3 | 13.0 | 10.3 | |
| 75th | 23.5 | 20.9 | 21.4 | |
| 95th | 27.4 | 24.8 | 25.5 | |
| max | 28.8 | 26.5 | 27.5 | |
| Precipitation | min | 0.8 | 0.8 | 0.6 |
| 5th | 1.3 | 1.9 | 1.4 | |
| 35th | 48.1 | 54.1 | 5.5 | |
| 50th | 70.5 | 73.8 | 42.6 | |
| 75th | 98.0 | 110.2 | 73.2 | |
| 95th | 125.3 | 166.3 | 122.7 | |
| max | 195.6 | 207.0 | 150.0 | |
| Wind direction | min | 2.0 | 1.0 | 1.0 |
| 5th | 4.0 | 7.2 | 2.8 | |
| 35th | 24.0 | 21.0 | 19.0 | |
| 50th | 25.0 | 23.0 | 20.5 | |
| 75th | 28.0 | 26.0 | 22.8 | |
| 95th | 31.0 | 29.0 | 34.3 | |
| max | 35.0 | 34.0 | 36.0 | |
| Wind speed | min | 32.3 | 36.1 | 36.2 |
| 5th | 33.7 | 37.3 | 39.3 | |
| 35th | 52.5 | 52.0 | 45.1 | |
| 50th | 57.0 | 60.0 | 51.0 | |
| 75th | 69.0 | 74.0 | 65.0 | |
| 95th | 91.0 | 95.3 | 78.5 | |
| max | 100.0 | 111.0 | 100.0 |
| Water Source | Percentile | L Ontario (N = 771 n sites = 4) | L St. Clair (N = 128 n sites = 2) | Detroit R (N = 30 n site = 1) | L Erie (N = 195 n sites = 4) |
|---|---|---|---|---|---|
| TN | min | 25.0 | 79.0 | 112.0 | 104.0 |
| 5th | 180.2 | 209.0 | 139.6 | 170.6 | |
| 35th | 460.0 | 390.0 | 320.2 | 330.0 | |
| 50th | 510.0 | 490.0 | 345.0 | 416.5 | |
| 75th | 610.0 | 888.0 | 413.0 | 576.0 | |
| 95th | 869.8 | 1982.0 | 558.8 | 730.9 | |
| max | 2896.0 | 4020.0 | 1340.0 | 1244.0 | |
| TP | min | 6.0 | 6.0 | 6.0 | 5.0 |
| 5th | 12.0 | 10.0 | 7.0 | 7.0 | |
| 35th | 22.0 | 15.0 | 11.0 | 12.0 | |
| 50th | 27.0 | 18.0 | 12.0 | 14.0 | |
| 75th | 39.0 | 25.0 | 15.5 | 19.0 | |
| 95th | 63.0 | 36.0 | 28.5 | 42.2 | |
| max | 675.0 | 66.0 | 179.0 | 139.0 |
| Great Lakes Basin Dataset | Global Dataset | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| All Intake Sites | N & S America | Africa, Asia, Europe & Oceana | |||||||
| Depth at Intake (m) | Distance from Shore (m) | TP (µg/L) | Surface Area (ha) | Maximum Depth (m) | TP (µg/L) | Surface Area (ha) | Maximum Depth (m) | TP (µg/L) | |
| Range | 0–12 | 0–1300 | 5–675 | 4.38–72,500 | 1–44 | 2–2047 | 0.7–305,000 | 1–175 | 2–14,420 |
| Median | 5 | 340 | 20 | 127 | 5.4 | 41.5 | 521 | 7.4 | 69 |
| Mean | 5.1 | 320 | 27 | 1633 | 7.7 | 150 | 11065 | 17 | 700.7 |
| N | 17 | 16 | 17 | 65 | 69 | 60 | 108 | 110 | 95 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taranu, Z.E.; Pick, F.R.; Creed, I.F.; Zastepa, A.; Watson, S.B. Meteorological and Nutrient Conditions Influence Microcystin Congeners in Freshwaters. Toxins 2019, 11, 620. https://doi.org/10.3390/toxins11110620
Taranu ZE, Pick FR, Creed IF, Zastepa A, Watson SB. Meteorological and Nutrient Conditions Influence Microcystin Congeners in Freshwaters. Toxins. 2019; 11(11):620. https://doi.org/10.3390/toxins11110620
Chicago/Turabian StyleTaranu, Zofia E., Frances R. Pick, Irena F. Creed, Arthur Zastepa, and Sue B. Watson. 2019. "Meteorological and Nutrient Conditions Influence Microcystin Congeners in Freshwaters" Toxins 11, no. 11: 620. https://doi.org/10.3390/toxins11110620
APA StyleTaranu, Z. E., Pick, F. R., Creed, I. F., Zastepa, A., & Watson, S. B. (2019). Meteorological and Nutrient Conditions Influence Microcystin Congeners in Freshwaters. Toxins, 11(11), 620. https://doi.org/10.3390/toxins11110620






