PirABVP Toxin Binds to Epithelial Cells of the Digestive Tract and Produce Pathognomonic AHPND Lesions in Germ-Free Brine Shrimp
Abstract
1. Introduction
2. Results
2.1. PirAB Toxin Binds the Digestive Tract and Induces Sloughing of Epithelial Cells in Brine Shrimp (Artemia franciscana) Larvae
2.2. PirAB Toxin Induced Accumulation of Cellular Debris in the Digestive Tract Is of Epithelial Cell Origin
2.3. PirAB Toxin Binding Leads to Damage and Necrosis of the Digestive Tract in Brine Shrimp Larvae
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Preparation of Recombinant V. parahaemolyticus PirAVP and PirBVP
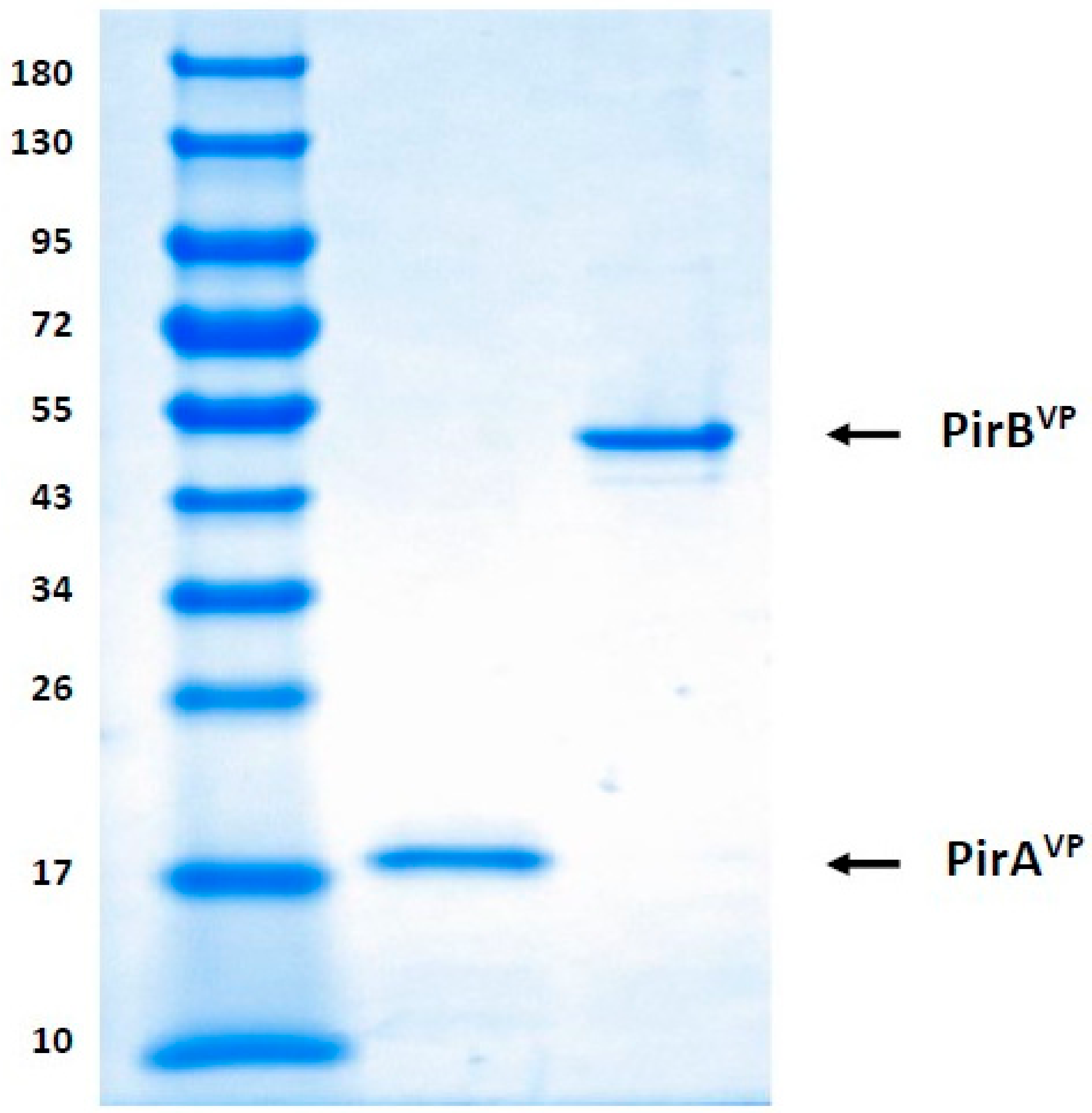
5.2. Detection of Recombinant PirAVP and PirBVP Toxins through SDS-PAGE
5.3. Axenic Brine Shrimp Hatching
5.4. Brine Shrimp Challenge Assay
5.5. Light Microscopical Analysis Using Immunohistochemistry
5.6. Transmission Electron Microscopical (TEM) Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dong, X.; Wang, H.; Xie, G.; Zou, P.; Guo, C.; Liang, Y.; Huang, J. An isolate of Vibrio campbellii carrying the pirVP gene causes acute hepatopancreatic necrosis disease. Emerg. Microbes Infect. 2017, 6, e2. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Bi, D.; Wang, H.; Zou, P.; Xie, G.; Wan, X.; Yang, Q.; Zhu, Y.; Chen, M.; Guo, C.; et al. pirABvp-Bearing Vibrio parahaemolyticus and Vibrio campbellii pathogens isolated from the Same AHPND-affected pond possess highly similar pathogenic plasmids. Front. Microbiol. 2017, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-T.; Chen, I.-T.; Yang, Y.-T.; Ko, T.-P.; Huang, Y.-T.; Huang, J.-Y.; Huang, M.-F.; Lin, S.-J.; Chen, C.-Y.; Lin, S.-S.; et al. The opportunistic marine pathogen Vibrio parahaemolyticus becomes virulent by acquiring a plasmid that expresses a deadly toxin. Proc. Natl. Acad. Sci. USA 2015, 112, 10798–10803. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Kinch, L.N.; Ray, A.; Dalia, A.B.; Cong, Q.; Nunan, L.M.; Camilli, A.; Grishin, N.V.; Salomon, D.; Orth, K. Acute hepatopancreatic necrosis disease-causing Vibrio parahaemolyticus strains maintain an antibacterial type VI secretion system with versatile effector repertoires. Appl. Environ. Microbiol. 2017, 83, e00737–17. [Google Scholar] [CrossRef] [PubMed]
- Han, J.E.; Tang, K.F.J.; Tran, L.H.; Lightner, D.V. Photorhabdus insect-related (Pir) toxin-like genes in a plasmid of Vibrio parahaemolyticus, the causative agent of acute hepatopancreatic necrosis disease (AHPND) of shrimp. Dis. Aquat. Organ. 2015, 113, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Nunan, L.; Lightner, D.; Pantoja, C.; Gomez-Jimenez, S. Detection of acute hepatopancreatic necrosis disease (AHPND) in Mexico. Dis. Aquat. Organ. 2014, 111, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Flegel, T.W. A future vision for disease control in shrimp aquaculture. J. World Aquac. Soc. 2019, 50, 249–266. [Google Scholar] [CrossRef]
- Kumar, V.; Baruah, K.; Nguyen, D.V.; Smagghe, G.; Vossen, E.; Bossier, P. Phloroglucinol mediated Hsp70 production in crustaceans: Protection against Vibrio parahaemolyticus in Artemia franciscana and Macrobrachium rosenbergii. Front. Immunol. 2018, 9, 1091. [Google Scholar] [CrossRef]
- Kumar, V.; Viet, D.; Baruah, K.; Bossier, P. Probing the mechanism of VP AHPND extracellular proteins toxicity purified from Vibrio parahaemolyticus AHPND strain in germ-free Artemia test system. Aquaculture 2019, 504, 414–419. [Google Scholar] [CrossRef]
- Choi, M.; Stevens, A.M.; Smith, S.A.; Taylor, D.P.; Kuhn, D.D. Strain and dose infectivity of Vibrio parahaemolyticus: the causative agent of early mortality syndrome in shrimp. Aquac. Res. 2017, 48, 3719–3727. [Google Scholar] [CrossRef]
- Campa-Córdova, A.I.; León-Gallo, A.F.; Romero-Maldonado, A.; Ibarra-Serrano, A.C.; Rosales-Mendoza, S.; Hirono, I.; Angulo, C. Recombinant PirA-like toxin protects shrimp against challenge with Vibrio parahaemolyticus, the aetiological agent of acute hepatopancreatic necrosis disease. J. Fish Dis. 2017, 40, 1725–1729. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Lu, L.; Xu, D. Progress in research on acute hepatopancreatic necrosis disease (AHPND). Aquac. Int. 2016, 24, 577–593. [Google Scholar] [CrossRef]
- Lin, S.J.; Hsu, K.C.; Wang, H.C. Structural insights into the cytotoxic mechanism of Vibrio parahaemolyticus PirAvpand PirBvptoxins. Mar. Drugs 2017, 15, 373. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, J.; Terova, G.; Simó-Mirabet, P.; Rimoldi, S.; Folkedal, O.; Calduch-Giner, J.A.; Olsen, R.E.; Sitjà-Bobadilla, A. Skin mucus of gilthead sea bream (Sparus aurata L.) protein mapping and regulation in chronically stressed fish. Front. Physiol. 2017, 8, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Leung, T.L.F.; Bates, A.E. More rapid and severe disease outbreaks for aquaculture at the tropics: Implications for food security. J. Appl. Ecol. 2013, 50, 215–222. [Google Scholar] [CrossRef]
- Shinn, A.P.; Pratoomyot, J.; Griffiths, D.; Jiravanichpaisal, J.; Briggs, M. Asian shrimp production and the economic costs of disease. Asian Fish. Sci. J. 2018, 31S, 29–58. [Google Scholar]
- FAO. State of Fisheries and Aquaculture in the World; The Food and Agriculture Organization of the United Nations: Rome, Italy, 2018; ISBN 9789251305621. Available online: http://www.fao.org/3/i9540en/i9540en.pdf (accessed on 9 February 2019).
- Tran, L.; Nunan, L.; Redman, R.M.; Mohney, L.L.; Pantoja, C.R.; Fitzsimmons, K.; Lightner, D.V. Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting penaeid shrimp. Dis. Aquat. Organ. 2013, 105, 45–55. [Google Scholar] [CrossRef]
- Dong, X.; Wang, H.; Zou, P.; Chen, J.; Liu, Z.; Wang, X.; Huang, J. Complete genome sequence of Vibrio campbellii strain 20130629003S01 isolated from shrimp with acute hepatopancreatic necrosis disease. Gut Pathog. 2017, 9, 1–5. [Google Scholar] [CrossRef]
- Liu, L.; Xiao, J.; Xia, X.; Pan, Y.; Yan, S. Draft Genome Sequence of Vibrio owensii Strain SH-14, Which Causes. Genome Announc. 2015, 3, 3354. [Google Scholar] [CrossRef]
- Restrepo, L.; Bayot, B.; Arciniegas, S.; Bajaña, L.; Betancourt, I.; Panchana, F.; Reyes Muñoz, A. PirVP genes causing AHPND identified in a new Vibrio species (Vibrio punensis) within the commensal Orientalis clade. Sci. Rep. 2018, 8, 13080. [Google Scholar] [CrossRef]
- Wechprasit, P.; Panphloi, M.; Thitamadee, S.; Sritunyalucksana, K.; Prachumwat, A. Complete Genome Sequence of Shewanella sp. Strain TH2012, Isolated from Shrimp in a Cultivation Pond Exhibiting Early Mortality Syndrome. Microbiol. Resour. Announc. 2019, 8, e01703–18. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Kumar, V.; Bossier, P.; Norouzitallab, P.; Vanrompay, D. Phloroglucinol Treatment Induces Transgenerational Epigenetic Inherited Resistance Against Vibrio Infections and Thermal Stress in a Brine Shrimp (Artemia franciscana) Model. Front. Immunol. 2019, 10, 2745. [Google Scholar] [CrossRef]
- Baruah, K.; Huy, T.T.; Norouzitallab, P.; Niu, Y.; Gupta, S.K.; De Schryver, P.; Bossier, P. Probing the protective mechanism of poly-ß-hydroxybutyrate against vibriosis by using gnotobiotic Artemia franciscana and Vibrio campbellii as host-pathogen model. Sci. Rep. 2015, 5, 9427. [Google Scholar] [CrossRef] [PubMed]
- Baruah, K.; Duy Phong, H.P.P.; Norouzitallab, P.; Defoirdt, T.; Bossier, P. The gnotobiotic brine shrimp (Artemia franciscana) model system reveals that the phenolic compound pyrogallol protects against infection through its prooxidant activity. Free Radic. Biol. Med. 2015, 89, 593–601. [Google Scholar] [CrossRef]
- Norouzitallab, P.; Baruah, K.; Vanrompay, D.; Bossier, P. Teaching Shrimps Self-Defense to Fight Infections. Trends Biotechnol. 2019, 37, 16–19. [Google Scholar] [CrossRef]
- Ffrench-Constant, R.H.; Waterfield, N.; Burland, V.; Perna, N.T.; Daborn, P.J.; Bowen, D.; Blattner, F.R. A genomic sample sequence of the entomopathogenic bacterium Photorhabdus luminescens W14: Potential implications for virulence. Appl. Environ. Microbiol. 2000, 66, 3310–3329. [Google Scholar] [CrossRef]
- Duchaud, E.; Rusniok, C.; Frangeul, L.; Buchrieser, C.; Givaudan, A.; Taourit, S.; Bocs, S.; Boursaux-Eude, C.; Chandler, M.; Charles, J.F.; et al. The genome sequence of the entomopathogenic bacterium Photorhabdus luminescens. Nat. Biotechnol. 2003, 21, 1307–1313. [Google Scholar] [CrossRef]
- Waterfield, N.; Kamita, S.G.; Hammock, B.D.; Ffrench-Constant, R. The Photorhabdus Pir toxins are similar to a developmentally regulated insect protein but show no juvenile hormone esterase activity. FEMS Microbiol. Lett. 2005, 245, 47–52. [Google Scholar] [CrossRef]
- Blackburn, M.B.; Farrar, R.R.; Novak, N.G.; Lawrence, S.D. Remarkable susceptibility of the diamondback moth (Plutella xylostella) to ingestion of Pir toxins from Photorhabdus luminescens. Entomol. Exp. Appl. 2006, 121, 31–37. [Google Scholar] [CrossRef]
- Ahantarig, A.; Chantawat, N.; Waterfield, N.R.; Ffrench-Constant, R.; Kittayapong, P. PirAB toxin from Photorhabdus asymbiotica as a larvicide against dengue vectors. Appl. Environ. Microbiol. 2009, 75, 4627–4629. [Google Scholar] [CrossRef]
- Kondo, H.; Tinwongger, S.; Proespraiwong, P.; Mavichak, R.; Unajak, S.; Nozaki, R.; Hirono, I. Draft Genome Sequences of Six Strains of Vibrio parahaemolyticus Isolated from Early Mortality Syndrome/Acute Hepatopancreatic Necrosis Disease Shrimp in Thailand. Genome Announc. 2014, 2, e00221–14. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.J.; Chen, Y.F.; Hsu, K.C.; Chen, Y.L.; Ko, T.P.; Lo, C.F.; Wang, H.C.; Wang, H.C. Structural insights to the heterotetrameric interaction between the vibrio parahaemolyticus pirAvp and pirBvp toxins and activation of the cry-like pore-forming domain. Toxins 2019, 11, E233. [Google Scholar] [CrossRef] [PubMed]
- Sirikharin, R.; Taengchaiyaphum, S.; Sanguanrut, P.; Chi, T.D.; Mavichak, R.; Proespraiwong, P.; Nuangsaeng, B.; Thitamadee, S.; Flegel, T.W.; Sritunyalucksana, K. Characterization and PCR detection of binary, pir-like toxins from vibrio parahaemolyticus isolates that cause acute hepatopancreatic necrosis disease (AHPND) in shrimp. PLoS ONE 2015, 10, e0126987. [Google Scholar] [CrossRef] [PubMed]
- Magin, T.M.; Vijayaraj, P.; Leube, R.E. Structural and regulatory functions of keratins. Exp. Cell Res. 2007, 313, 2021–2032. [Google Scholar] [CrossRef]
- Gunasekara, R.A.Y.S.A.; Defoirdt, T.; Rekecki, A.; Decostere, A.; Cornelissen, M.; Sorgeloos, P.; Bossier, P.; Van den Broeck, W. Light and transmission electron microscopy of Vibrio campbellii infection in gnotobiotic Artemia franciscana and protection offered by a yeast mutant with elevated cell wall glucan. Vet. Microbiol. 2012, 158, 337–343. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Baruah, K.; Cam, D.T.V.; Dierckens, K.; Wille, M.; Defoirdt, T.; Sorgeloos, P.; Bossier, P. In vivo effects of single or combined N-acyl homoserine lactone quorum sensing signals on the performance of Macrobrachium rosenbergii larvae. Aquaculture 2009, 288, 233–238. [Google Scholar] [CrossRef]
- Baruah, K.; Norouzitallab, P.; Roberts, R.J.; Sorgeloos, P.; Bossier, P. A novel heat-shock protein inducer triggers heat shock protein 70 production and protects Artemia franciscana nauplii against abiotic stressors. Aquaculture 2012, 334, 152–158. [Google Scholar] [CrossRef]
- Gunasekara, R.A.Y.S.A.; Rekecki, A.; Baruah, K.; Bossier, P.; Van den Broeck, W. Evaluation of probiotic effect of Aeromonas hydrophila on the development of the digestive tract of germ-free Artemia franciscana nauplii. J. Exp. Mar. Biol. Ecol. 2010, 393, 78–82. [Google Scholar] [CrossRef]
- Gunasekara, R.A.Y.S.A.; Rekecki, A.; Cornillie, P.; Cornelissen, M.; Sorgeloos, P.; Simoens, P.; Bossier, P.; Van den Broeck, W. Morphological characteristics of the digestive tract of gnotobiotic Artemia franciscana nauplii. Aquaculture 2011, 321, 1–7. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, V.; Bels, L.D.; Couck, L.; Baruah, K.; Bossier, P.; Broeck, W.V.d. PirABVP Toxin Binds to Epithelial Cells of the Digestive Tract and Produce Pathognomonic AHPND Lesions in Germ-Free Brine Shrimp. Toxins 2019, 11, 717. https://doi.org/10.3390/toxins11120717
Kumar V, Bels LD, Couck L, Baruah K, Bossier P, Broeck WVd. PirABVP Toxin Binds to Epithelial Cells of the Digestive Tract and Produce Pathognomonic AHPND Lesions in Germ-Free Brine Shrimp. Toxins. 2019; 11(12):717. https://doi.org/10.3390/toxins11120717
Chicago/Turabian StyleKumar, Vikash, Lobke De Bels, Liesbeth Couck, Kartik Baruah, Peter Bossier, and Wim Van den Broeck. 2019. "PirABVP Toxin Binds to Epithelial Cells of the Digestive Tract and Produce Pathognomonic AHPND Lesions in Germ-Free Brine Shrimp" Toxins 11, no. 12: 717. https://doi.org/10.3390/toxins11120717
APA StyleKumar, V., Bels, L. D., Couck, L., Baruah, K., Bossier, P., & Broeck, W. V. d. (2019). PirABVP Toxin Binds to Epithelial Cells of the Digestive Tract and Produce Pathognomonic AHPND Lesions in Germ-Free Brine Shrimp. Toxins, 11(12), 717. https://doi.org/10.3390/toxins11120717






