Functional Analysis of FgNahG Clarifies the Contribution of Salicylic Acid to Wheat (Triticum aestivum) Resistance against Fusarium Head Blight
Abstract
:1. Introduction
2. Results
2.1. Deletion and Complementation of FgNahG in F. graminearum
2.2. Influence of FgNahG on Mycelial Growth
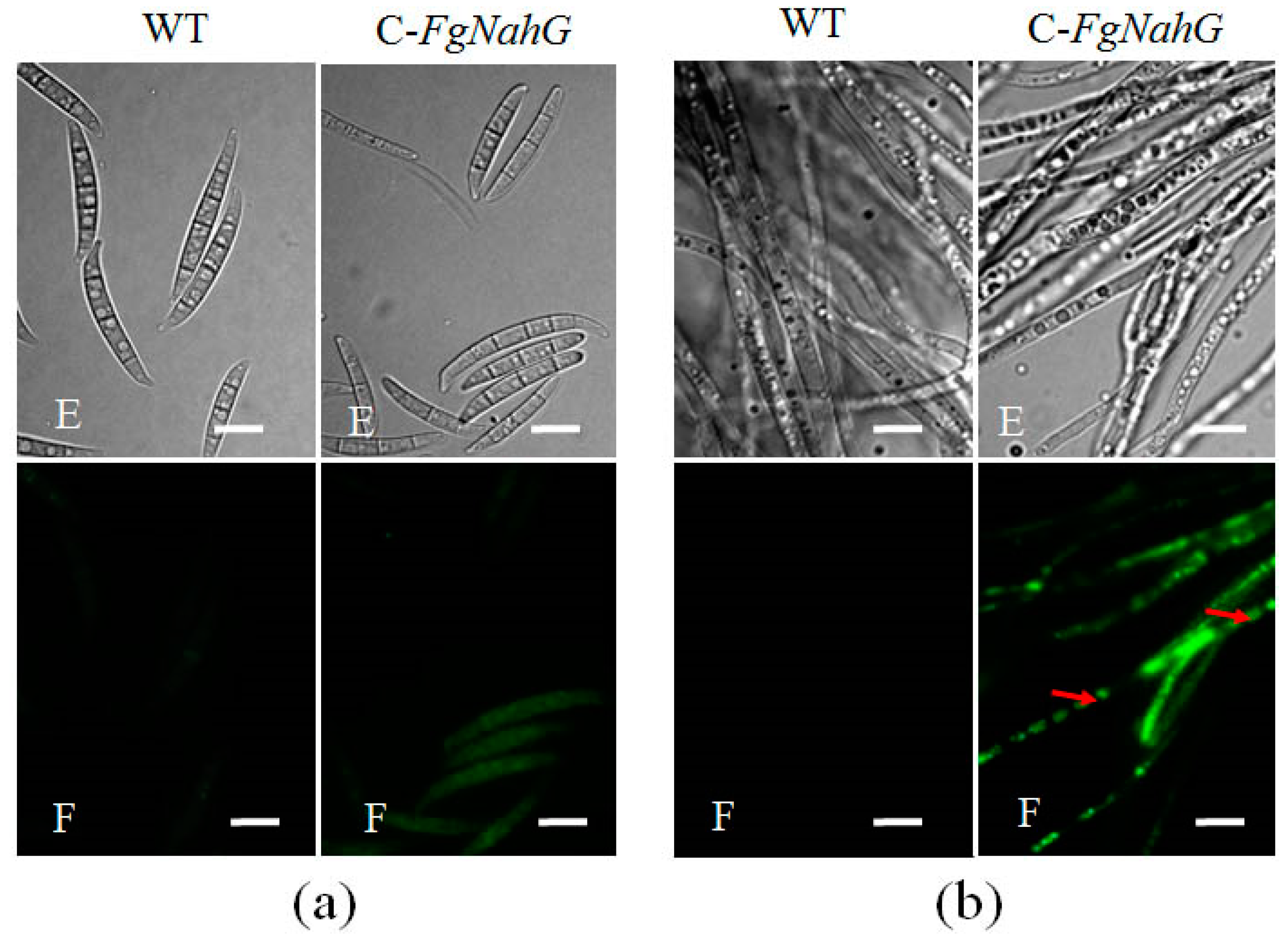
2.3. Subcellular Localization
2.4. Determination of the FgNahG Enzyme Activity In Vitro
2.5. Effect of FgNahG on Fungal Pathogenicity in Wheat
2.6. Expression of FgNahG in Transgenic A. thaliana
3. Discussion
4. Materials and Methods
4.1. Experimental Materials and Growth Conditions
4.2. Sequence Analysis and Primer Design
4.3. Transformation
4.4. In Vitro Expression of FgNahG in E. coli
4.5. Enzyme Activity Assay
4.6. Microscopy
4.7. Virulence Assay
4.8. Quantitative Real-Time PCR Analyses
4.9. Quantification of SA in Wheat Spikes and in A. thaliana Leaves
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ward, E.R.; Uknes, S.J.; Williams, S.C.; Dincher, S.S.; Wiederhold, D.L.; Alexander, D.C.; Ahl-Goy, P.; Metraux, J.P.; Ryals, J.A. Coordinate gene activity in response to agents that induce systemic acquired resistance. Plant Cell 1991, 3, 1085–1094. [Google Scholar] [CrossRef]
- Uknes, S.; Mauch-Mani, B.; Moyer, M.; Potter, S.; Williams, S.; Dincher, S.; Chandler, D.; Slusarenko, A.; Ward, E.; Ryals, J. Acquired resistance in Arabidopsis. Plant Cell 1992, 4, 645–656. [Google Scholar] [CrossRef]
- Dempsey, D.A.; Vlot, A.C.; Wildermuth, M.C.; Klessig, D.F. Salicylic Acid biosynthesis and metabolism. Arabidopsis Book 2011, 9, e0156. [Google Scholar] [CrossRef]
- Seyfferth, C.; Tsuda, K. Salicylic acid signal transduction: The initiation of biosynthesis, perception and transcriptional reprogramming. Front. Plant Sci. 2014, 5, 697. [Google Scholar] [CrossRef]
- Wildermuth, M.C.; Dewdney, J.; Wu, G.; Ausubel, F.M. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 2001, 414, 562–565. [Google Scholar] [CrossRef]
- Garcion, C.; Lohmann, A.; Lamodière, E.; Catinot, J.; Buchala, A.; Doermann, P.; Métraux, J.P. Characterization and biological function of the ISOCHORISMATE SYNTHASE2 gene of Arabidopsis. Plant Physiol. 2008, 147, 1279–1287. [Google Scholar] [CrossRef]
- León, J.; Shulaev, V.; Yalpani, N.; Lawton, M.A.; Raskin, I. Benzoic acid 2-hydroxylase, a soluble oxygenase from tobacco, catalyzes salicylic acid biosynthesis. Proc. Natl. Acad. Sci. USA 1995, 92, 10413–10417. [Google Scholar] [CrossRef]
- Tonnessen, B.W.; Manosalva, P.; Lang, J.M.; Baraoidan, M.; Bordeos, A.; Mauleon, R.; Oard, J.; Hulbert, S.; Leung, H.; Leach, J.E. Rice phenylalanine ammonia-lyase gene OsPAL4 is associated with broad spectrum disease resistance. Plant Mol. Biol. 2015, 87, 273–286. [Google Scholar] [CrossRef]
- Catinot, J.; Buchala, A.; Abou-Mansour, E.; Métraux, J.P. Salicylic acid production in response to biotic and abiotic stress depends on isochorismate in Nicotiana benthamiana. FEBS. Lett. 2008, 582, 473–478. [Google Scholar] [CrossRef]
- Hao, Q.; Wang, W.; Han, X.; Wu, J.; Lyu, B.; Chen, F.; Caplan, A.; Li, C.; Wu, J.; Wang, W.; et al. Isochorismate-based salicylic acid biosynthesis confers basal resistance to Fusarium graminearum in barley. Mol. Plant Pathol. 2018, 19, 1995–2010. [Google Scholar] [CrossRef]
- Wright, J.D. Fungal degradation of benzoic acid and related compounds. World J. Microbiol. Biotechnol. 1993, 9, 9–16. [Google Scholar] [CrossRef]
- Dodge, A.G.; Wackett, L.P. Metabolism of bismuth subsalicylate and intracellular accumulation of bismuth by Fusarium sp. strain BI. Appl. Environ. Microbiol. 2005, 71, 876–882. [Google Scholar] [CrossRef]
- Rabe, F.; Ajami-Rashidi, Z.; Doehlemann, G.; Kahmann, R.; Djamei, A. Degradation of the plant defence hormone salicylic acid by the biotrophic fungus Ustilago maydis. Mol. Microbiol. 2013, 89, 179–188. [Google Scholar] [CrossRef]
- Ambrose, K.V.; Tian, Z.; Wang, Y.; Smith, J.; Zylstra, G.; Huang, B.; Belanger, F.C. Functional characterization of salicylate hydroxylase from the fungal endophyte Epichloë festucae. Sci. Rep. 2015, 9, 10939. [Google Scholar] [CrossRef]
- Li, J.; Pang, Z.; Trivedi, P.; Zhou, X.; Ying, X.; Jia, H.; Wang, N. ‘Candidatus liberibacter asiaticus’ encodes a functional salicylic acid (SA) hydroxylase that degrades SA to suppress plant defenses. Mol. Plant Microbe Interact. 2017, 30, 620–630. [Google Scholar] [CrossRef]
- Qi, P.F.; Johnston, A.; Balcerzak, M.; Rocheleau, H.; Harris, L.J.; Long, X.Y.; Wei, Y.M.; Zheng, Y.L.; Ouellet, T. Effect of salicylic acid on Fusarium graminearum, the major causal agent of fusarium head blight in wheat. Fungal Biol. 2012, 116, 413–426. [Google Scholar] [CrossRef]
- Parry, D.W.; Jenkinson, P.; Mcleod, L. Fusarium ear blight (scab) in small grain cereals—A review. Plant Pathol. 1995, 44, 207–238. [Google Scholar] [CrossRef]
- Windels, C.E. Economic and social impacts of fusarium head blight: Changing farms and rural communities in the northern Great Plains. Phytopathology 2000, 90, 17–21. [Google Scholar] [CrossRef]
- Chandler, E.A.; Simpson, D.R.; Thomsett, M.A.; Nicholson, P. Development of PCR assays to TRI7 and TRI13 trichothecene biosynthetic genes, and characterization of chemotypes of Fuarium graminearum, Fuarium culomrum and Fuarium cerealis. Physiol. Mol. Plant. Pathol. 2003, 62, 355–367. [Google Scholar] [CrossRef]
- Goswami, R.S.; Kistler, H.C. Heading for disaster: Fusarium graminearum on cereal crops. Mol. Plant Pathol. 2004, 5, 515–525. [Google Scholar] [CrossRef]
- Qi, P.F.; Balcerzak, M.; Rocheleau, H.; Leung, W.; Wei, Y.M.; Zheng, Y.L.; Ouellet, T. Jasmonic acid and abscisic acid play important roles in host-pathogen interaction between Fusarium graminearum and wheat during the early stages of fusarium head blight. Physiol. Mol. Plant. Pathol. 2016, 93, 39–48. [Google Scholar] [CrossRef]
- Wang, J.R.; Wang, L.; Gulden, S.; Rocheleau, H.; Balcerzak, M.; Hattori, J.; Cao, W.; Han, F.; Zheng, Y.L.; Fedak, G.; et al. RNA profiling of fusarium head blight-resistant wheat addition lines containing the Thinopyrum elongtum chromosome 7E. Can. J. Plant Pathol. 2010, 32, 188–214. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Chen, Q.; Liu, C.H.; Liu, Y.B.; Yi, P.; Niu, K.X.; Wang, Y.Q.; Wang, A.Q.; Yu, H.Y.; Pu, Z.E.; et al. Chitin synthase gene FgCHS8 affects virulence and fungal cell wall sensitivity to environmental stress in Fusarium graminearum. Fungal Biol. 2016, 120, 764–774. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Wei, Z.Z.; Liu, C.H.; Chen, Q.; Xu, B.J.; Guo, Z.R.; Cao, Y.L.; Wang, Y.; Han, Y.N.; Chen, C.; et al. Linoleic acid isomerase gene FgLAI12 affects sensitivity to salicylic acid, mycelial growth and virulence of Fusarium graminearum. Sci. Rep. 2017, 7, 46129. [Google Scholar] [CrossRef]
- Qi, P.F.; Zhang, Y.Z.; Liu, C.H.; Zhu, J.; Chen, Q.; Guo, Z.R.; Wang, Y.; Xu, B.J.; Zheng, T.; Jiang, Y.F.; Wang, J.P.; et al. Fusarium graminearum ATP-Binding Cassette Transporter Gene FgABCC9 Is Required for Its Transportation of Salicylic Acid, Fungicide Resistance, Mycelial Growth and Pathogenicity towards Wheat. Int. J. Mol. Sci. 2018, 19, 2351. [Google Scholar] [CrossRef]
- Makandar, R.; Nalam, V.J.; Lee, H.; Trick, H.N.; Dong, Y.; Shah, J. Salicylic acid regulates basal resistance to Fusarium head blight in wheat. Mol. Plant Microbe Interact. 2012, 25, 431–439. [Google Scholar] [CrossRef]
- Ameye, M.; Audenaert, K.; De Zutter, N.; Steppe, K.; Van Meulebroek, L.; Vanhaecke, L.; De Vleesschauwer, D.; Haesaert, G.; Smagghe, G. Priming of wheat with the green leaf volatile Z-3-hexenyl acetate enhances defense against Fusarium graminearum but boosts deoxynivalenol production. Plant Physiol. 2015, 167, 1671–1684. [Google Scholar] [CrossRef]
- Li, G.; Yen, Y. Jasmonate and ethylene signaling pathway may mediate Fusarium head blight resistance in wheat. Crop Sci. 2008, 48, 1888–1896. [Google Scholar] [CrossRef]
- Rocheleau, H.; Al-harthi, R.; Ouellet, T. Degradation of salicylic acid by Fusarium graminearum. Fungal Biol. 2018. [CrossRef]
- You, I.S.; Ghosal, D.; Gunsalus, I.C. Nucleotide sequence analysis of the Pseudomonas putida PpG7 salicylate hydroxylase gene (nahG) and its 3’-flanking region. Biochemistry 1991, 30, 1635–1641. [Google Scholar] [CrossRef]
- Vlot, A.C.; Dempsey, D.A.; Klessig, D.F. Salicylic Acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef]
- Rivas-San Vicente, M.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef]
- Martínez, C.; Pons, E.; Prats, G.; León, J. Salicylic acid regulates flowering time and links defence responses and reproductive development. Plant J. 2004, 37, 209–217. [Google Scholar] [CrossRef]
- Morris, K.; MacKerness, S.A.; Page, T.; John, C.F.; Murphy, A.M.; Carr, J.P.; Buchanan-Wollaston, V. Salicylic acid has a role in regulating gene expression during leaf senescence. Plant J. 2000, 23, 677–685. [Google Scholar] [CrossRef] [Green Version]
- Wada, K.C.; Yamada, M.; Shiraya, T.; Takeno, K. Salicylic acid and the flowering gene FLOWERING LOCUS T homolog are involved in poor-nutrition stress-induced flowering of Pharbitis nil. J. Plant Physiol. 2010, 167, 447–452. [Google Scholar] [CrossRef]
- Ding, L.; Xu, H.; Yi, H.; Yang, L.; Kong, Z.; Zhang, L.; Xue, S.; Jia, H.; Ma, Z. Resistance to hemi-biotrophic F. graminearum infection is associated with coordinated and ordered expression of diverse defense signaling pathways. PLoS ONE 2011, 6, e19008. [Google Scholar] [CrossRef]
- Makandar, R.; Nalam, V.; Chaturvedi, R.; Jeannotte, R.; Sparks, A.A.; Shah, J. Involvement of salicylate and jasmonate signaling pathways in Arabidopsis interaction with Fusarium graminearum. Mol. Plant Microbe Interact. 2010, 23, 861–870. [Google Scholar] [CrossRef]
- Trail, F. For blighted waves of grain: Fusarium graminearum in the postgenomics era. Plant Physiol. 2009, 149, 103–110. [Google Scholar] [CrossRef]
- Koornneef, A.; Pieterse, C.M.J. Cross talk in defense signaling. Plant Physiol. 2008, 146, 839–844. [Google Scholar] [CrossRef]
- Capellini, R.A.; Peterson, J.L. Macroconidium formation in submerged cultures by a non-sporulating strain of Gibberella zeae. Mycologia 1965, 57, 962–966. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [Green Version]
- Remans, T.; Smeets, K.; Opdenakker, K.; Mathijsen, D.; Vangronsveld, J.; Cuypers, A. Normalisation of real-time RT-PCR gene expression measurements in Arabidopsis thaliana exposed to increased metal concentrations. Planta 2008, 227, 1343–1349. [Google Scholar] [CrossRef]
- Lodhi, M.A.; Ye, G.N.; Weeden, N.F.; Reisch, B.I. A simple and efficient method for DNA extraction from grapevine cultivars and Vitis species. Plant Mol. Biol. Rep. 1994, 12, 6–13. [Google Scholar] [CrossRef]
- Frandsen, R.J.; Andersson, J.A.; Kristensen, M.B.; Giese, H. Efficient four fragment cloning for the construction of vectors for targeted gene replacement in filamentous fungi. BMC Mol. Biol. 2008, 9, 70. [Google Scholar] [CrossRef]
- Frandsen, R.J.; Frandsen, M.; Giese, H. Targeted gene replacement in fungal pathogens via Agrobacterium tumefaciens-mediated transformation. Methods Mol. Biol. 2012, 835, 17–45. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Bertani, G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 1951, 62, 293–300. [Google Scholar]
- Qi, P.F.; Wei, Y.M.; Chen, Q.; Ouellet, T.; Ai, J.; Chen, G.Y.; Li, W.; Zheng, Y.L. Identification of novel α-gliadin genes. Genome 2011, 54, 244–252. [Google Scholar] [CrossRef]
- Bosch, R.; Moore, E.R.; García-Valdés, E.; Pieper, D.H. NahW a novel, inducible salicylate hydroxylase involved in mineralization of naphthalene by Pseudomonas stutzeri AN10. J. Bacteriol. 1999, 181, 2315–2322. [Google Scholar]
- Miller, D.; Blackwell, B.A. Biosynthesis of 3-acetyldeoxynivalenol and other metabolites by Fusarium culmorum HLK 1503 in a stirred jar fermentor. Can. J. Bot. 1986, 64, 1–5. [Google Scholar] [CrossRef]
- Siciliano, I.; Amaral Carneiro, G.; Spadaro, D.; Garibaldi, A.; Gullino, M.L. Jasmonic acid, abscisic acid, and salicylic acid are involved in the phytoalexin responses of rice to Fusarium fujikuroi, a high gibberellin producer pathogen. J. Agric. Food Chem. 2015, 63, 8134–8142. [Google Scholar] [CrossRef]







| Primer | Sequence (5′–3′) | Reference |
|---|---|---|
| FgNahG-up-F | GGAAGCTTCACCCACTTTCTCACCA | this study |
| FgNahG-up-R | GGACTAGTCGAACTTTCTTCCGTATTG | |
| FgNahG-down-F | GCGGGCCCATCAGGATAGTAAACAGAA | this study |
| FgNahG-down-R | GCGAGCTCCGACTGGTTCCACTAC | |
| ∆J-up-F | TTTGAGCGGCATTCTTG | this study |
| ∆J-up-R | TGTTTCGGCGTGGGTA | |
| ∆J-down-F | CAGTTGCCTAAATGAACC | this study |
| ∆J-down-R | CTATCGTCAACGGGTCT | |
| R-FgNahG -F | CGAGCTCGAGGATCTGCCACAACAC | this study |
| R-FgNahG -R | AACTGCAGGATTGCTTCCAACAAACT | |
| 30a-FgNahG-F | GGGGTACCATCATTGGAGCTGGCCTCTCA | this study |
| 30a-FgNahG-R | ACGAGCTCTCAATTGCTTCCAACAAAC | |
| Fg-GAPDH-F | TGACTTGACTGTTCGCCTCGAGAA | [16] |
| Fg-GAPDH-R | ATGGAGGAGTTGGTGTTGCCGTTA | |
| Fg-β-tubulin-F | GTTGATCTCCAAGATCCGTG | [16] |
| Fg-β-tubulin-R | CATGCAAATGTCGTAGAGGG | |
| Fg-Factor1-F | CCTCCAGGATGTCTACAAGA | [16] |
| Fg-Factor1-R | CTCAACGGACTTGACTTCAG | |
| Rj-FgNahG-F | AGAAGGGAAGGCAGGAGCG | this study |
| Rj-FgNahG-R | TCATTCAAATCAACCGAGTAAAGC | |
| ACT2-F | CTTGCACCAAGCAGCATGAA | [42] |
| ACT2-R | CCGATCCAGACACTGTACTTCCTT | |
| Aox-F | GACTTGTCATGGTAGATGCCTG | [16] |
| Aox-R | CAGGACGAGCATAACCATTCTC | |
| w-GAPDH-F | AACTGTTCATGCCATCACTGCCAC | [16] |
| w-GAPDH-R | AGGACATACCAGTGAGCTTGCCAT | |
| hn-RNP-Q-F | TCACCTTCGCCAAGCTCAGAACTA | [16] |
| hn-RNP-Q-R | AGTTGAACTTGCCCGAAACATGCC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, P.-F.; Zhang, Y.-Z.; Liu, C.-H.; Chen, Q.; Guo, Z.-R.; Wang, Y.; Xu, B.-J.; Jiang, Y.-F.; Zheng, T.; Gong, X.; et al. Functional Analysis of FgNahG Clarifies the Contribution of Salicylic Acid to Wheat (Triticum aestivum) Resistance against Fusarium Head Blight. Toxins 2019, 11, 59. https://doi.org/10.3390/toxins11020059
Qi P-F, Zhang Y-Z, Liu C-H, Chen Q, Guo Z-R, Wang Y, Xu B-J, Jiang Y-F, Zheng T, Gong X, et al. Functional Analysis of FgNahG Clarifies the Contribution of Salicylic Acid to Wheat (Triticum aestivum) Resistance against Fusarium Head Blight. Toxins. 2019; 11(2):59. https://doi.org/10.3390/toxins11020059
Chicago/Turabian StyleQi, Peng-Fei, Ya-Zhou Zhang, Cai-Hong Liu, Qing Chen, Zhen-Ru Guo, Yan Wang, Bin-Jie Xu, Yun-Feng Jiang, Ting Zheng, Xi Gong, and et al. 2019. "Functional Analysis of FgNahG Clarifies the Contribution of Salicylic Acid to Wheat (Triticum aestivum) Resistance against Fusarium Head Blight" Toxins 11, no. 2: 59. https://doi.org/10.3390/toxins11020059
APA StyleQi, P.-F., Zhang, Y.-Z., Liu, C.-H., Chen, Q., Guo, Z.-R., Wang, Y., Xu, B.-J., Jiang, Y.-F., Zheng, T., Gong, X., Luo, C.-H., Wu, W., Kong, L., Deng, M., Ma, J., Lan, X.-J., Jiang, Q.-T., Wei, Y.-M., Wang, J.-R., & Zheng, Y.-L. (2019). Functional Analysis of FgNahG Clarifies the Contribution of Salicylic Acid to Wheat (Triticum aestivum) Resistance against Fusarium Head Blight. Toxins, 11(2), 59. https://doi.org/10.3390/toxins11020059







