Evolutionary Ecology of Fish Venom: Adaptations and Consequences of Evolving a Venom System
Abstract
:1. Introduction
A Brief Introduction to Venomous Fish: Morphology and Biochemistry
2. Evolving A Venom System
2.1. The Basics of Antagonistic Coevolution: The Classic “Arms Race”
2.1.1. Evolving Venom for Defense in Fish
2.1.2. Evolving Venom for Predation in Fish
2.1.3. Evolving Venom for Competition in Fish
3. Consequences of Evolving A Venom System
3.1. Aposematism
3.2. Mimicry
3.2.1. Batesian
3.2.2. Müllerian
4. Energetic Implications of Evolving A Venom System
5. Intersexual Variation in Venom
6. Ecological Niches, Life History, and Venom Evolution
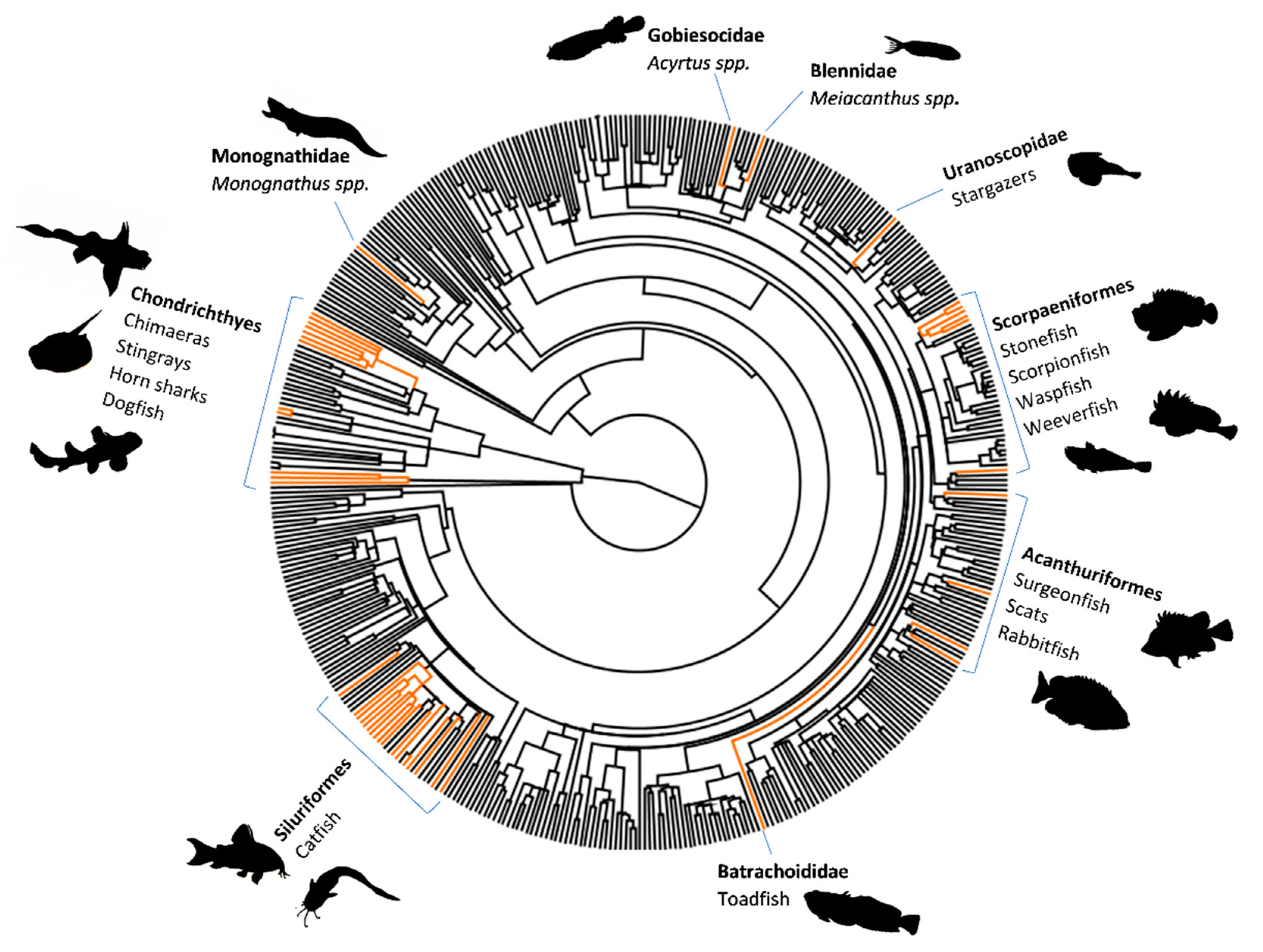
7. Diversification Rates and Venom Evolution
8. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fry, B.G.; Roelants, K.; Champagne, D.E.; Scheib, H.; Tyndall, J.D.; King, G.F.; Nevalainen, T.J.; Norman, J.A.; Lewis, R.J.; Norton, R.S. The toxicogenomic multiverse: Convergent recruitment of proteins into animal venoms. Ann. Rev. Genom. Human Genet. 2009, 10, 483–511. [Google Scholar] [CrossRef] [PubMed]
- Casewell, N.R.; Wüster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef]
- Harris, R.J.; Arbuckle, K. Tempo and mode of the evolution of venom and poison in tetrapods. Toxins 2016, 8, 193. [Google Scholar] [CrossRef] [PubMed]
- Dutertre, S.; Jin, A.-h.; Kaas, Q.; Jones, A.; Alewood, P.F.; Lewis, R.J. Deep venomics reveals the mechanism for expanded peptide diversity in cone snail venom. Mol. Cell. Proteom. 2013, 12, 312–329. [Google Scholar] [CrossRef]
- Dutertre, S.; Jin, A.-H.; Vetter, I.; Hamilton, B.; Sunagar, K.; Lavergne, V.; Dutertre, V.; Fry, B.G.; Antunes, A.; Venter, D.J. Evolution of separate predation-and defence-evoked venoms in carnivorous cone snails. Nat. Commun. 2014, 5, 3521. [Google Scholar] [CrossRef] [PubMed]
- Vetter, I.; J Lewis, R. Therapeutic potential of cone snail venom peptides (conopeptides). Curr. Top. Med. Chem. 2012, 12, 1546–1552. [Google Scholar] [CrossRef]
- Fry, B.G.; Vidal, N.; Norman, J.A.; Vonk, F.J.; Scheib, H.; Ramjan, S.F.R.; Kuruppu, S.; Fung, K.; Blair Hedges, S.; Richardson, M.K.; et al. Early evolution of the venom system in lizards and snakes. Nature 2005, 439, 584. [Google Scholar] [CrossRef]
- Clark, A.M. Natural Products as a Resource for New Drugs. Pharm. Res. 1996, 13, 1133–1141. [Google Scholar] [CrossRef]
- Fernandez, J.H.; Neshich, G.; Camargo, A.C.M. Using bradykinin-potentiating peptide structures to develop new antihypertensive drugs. Genet. Mol. Res. 2004, 3, 554–563. [Google Scholar]
- Triplitt, C.; Chiquette, E. Exenatide: From the Gila monster to the pharmacy. J. Am. Pharm. Assoc. 2006, 46, 44–55. [Google Scholar] [CrossRef]
- Ziegman, R.; Alewood, P. Bioactive components in fish venoms. Toxins 2015, 7, 1497–1531. [Google Scholar] [PubMed]
- Smith, W.L.; Stern, J.H.; Girard, M.G.; Davis, M.P. Evolution of Venomous Cartilaginous and Ray-Finned Fishes; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Smith, W.L.; Wheeler, W.C. Venom evolution widespread in fishes: A phylogenetic road map for the bioprospecting of piscine venoms. J. Hered. 2006, 97, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Gou, M.; Han, J.; Yuan, X.; Li, Y.; Li, T.; Jiang, Q.; Xiao, R.; Li, Q. Proteomic analysis of buccal gland secretion from fasting and feeding lampreys (Lampetra morii). Proteome Sci. 2018, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.J. Diversity, phylogenetic distribution, and origins of venomous catfishes. BMC Evol. Biol. 2009, 9, 282. [Google Scholar] [CrossRef] [PubMed]
- Revell, L.J. phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 2012, 3, 217–223. [Google Scholar] [CrossRef]
- Casewell, N.R.; Visser, J.C.; Baumann, K.; Dobson, J.; Han, H.; Kuruppu, S.; Morgan, M.; Romilio, A.; Weisbecker, V.; Mardon, K. The evolution of fangs, venom, and mimicry systems in blenny fishes. Curr. Biol. 2017, 27, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Baumann, K.; Casewell, N.R.; Ali, S.A.; Jackson, T.N.; Vetter, I.; Dobson, J.S.; Cutmore, S.C.; Nouwens, A.; Lavergne, V.; Fry, B.G. A ray of venom: Combined proteomic and transcriptomic investigation of fish venom composition using barb tissue from the blue-spotted stingray (Neotrygon kuhlii). J. Proteom. 2014, 109, 188–198. [Google Scholar] [CrossRef]
- Han, H.; Baumann, K.; Casewell, N.R.; Ali, S.A.; Dobson, J.; Koludarov, I.; Debono, J.; Cutmore, S.C.; Rajapakse, N.W.; Jackson, T.N. The cardiovascular and neurotoxic effects of the venoms of six bony and cartilaginous fish species. Toxins 2017, 9, 67. [Google Scholar] [CrossRef]
- Borges, M.H.; Andrich, F.; Lemos, P.H.; Soares, T.G.; Menezes, T.N.; Campos, F.V.; Neves, L.X.; Castro-Borges, W.; Figueiredo, S.G. Combined proteomic and functional analysis reveals rich sources of protein diversity in skin mucus and venom from the Scorpaena plumieri fish. J. Proteom. 2018, 187, 200–211. [Google Scholar]
- Malacarne, P.F.; Menezes, T.N.; Martins, C.W.; Naumann, G.B.; Gomes, H.L.; Pires, R.G.; Figueiredo, S.G.; Campos, F.V. Advances in the characterization of the Scorpaena plumieri cytolytic toxin (Sp-CTx). Toxicon 2018, 150, 220–227. [Google Scholar] [CrossRef]
- Endler, J.A. Defense against predators. In Predator-Prey Relationships: Perspectives and Approaches from the Study in Lower Vertebrates; Feder, M.E., Lauder, G.V., Eds.; University of Chicago Press: Chicago, IL, USA, 1986. [Google Scholar]
- Endler, J.A. Interactions between predator and prey. Behav. Ecol. 1991, 169–196. [Google Scholar]
- Dawkins, R.; Krebs, J.R. Arms races between and within species. Proc. R. Soc. Lond. B 1979, 205, 489–511. [Google Scholar] [PubMed]
- Van Valen, L. A new evolutionary law. Evol. Theory 1973, 1, 1–30. [Google Scholar]
- Holding, M.L.; Biardi, J.E.; Gibbs, H.L. Coevolution of venom function and venom resistance in a rattlesnake predator and its squirrel prey. Proc. R. Soc. B 2016, 283, 20152841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margres, M.J.; Wray, K.P.; Hassinger, A.T.B.; Ward, M.J.; McGivern, J.J.; Moriarty Lemmon, E.; Lemmon, A.R.; Rokyta, D.R. Quantity, Not Quality: Rapid Adaptation in a Polygenic Trait Proceeded Exclusively through Expression Differentiation. Mol. Biol. Evol. 2017, 34, 3099–3110. [Google Scholar] [CrossRef] [PubMed]
- Brodie, E., Jr.; Formanowicz, D., Jr.; Brodie, E., III. Predator avoidance and antipredator mechanisms: Distinct pathways to survival. Ethol. Ecol. Evol. 1991, 3, 73–77. [Google Scholar] [CrossRef]
- Nelsen, D.R.; Nisani, Z.; Cooper, A.M.; Fox, G.A.; Gren, E.C.; Corbit, A.G.; Hayes, W.K. Poisons, toxungens, and venoms: Redefining and classifying toxic biological secretions and the organisms that employ them. Biol. Rev. 2014, 89, 450–465. [Google Scholar] [CrossRef]
- Wüster, W.; Allum, C.S.; Bjargardóttir, I.B.; Bailey, K.L.; Dawson, K.J.; Guenioui, J.; Lewis, J.; McGurk, J.; Moore, A.G.; Niskanen, M. Do aposematism and Batesian mimicry require bright colours? A test, using European viper markings. Proc. R. Soc. Lond. B Biol. Sci. 2004, 271, 2495–2499. [Google Scholar] [CrossRef] [Green Version]
- Mappes, J.; Marples, N.; Endler, J.A. The complex business of survival by aposematism. Trends Ecol. Evol. 2005, 20, 598–603. [Google Scholar]
- Speed, M.P.; Ruxton, G.D. Warning displays in spiny animals: One (more) evolutionary route to aposematism. Evolution 2005, 59, 2499–2508. [Google Scholar] [CrossRef]
- McCue, M.D. Cost of producing venom in three North American pitviper species. Copeia 2006, 2006, 818–825. [Google Scholar] [CrossRef]
- Morgenstern, D.; King, G.F. The venom optimization hypothesis revisited. Toxicon 2013, 63, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Arbuckle, K.; de la Vega, R.C.R.; Casewell, N.R. Coevolution takes the sting out of it: Evolutionary biology and mechanisms of toxin resistance in animals. Toxicon 2017, 140, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Barlow, A.; Pook, C.E.; Harrison, R.A.; Wüster, W. Coevolution of diet and prey-specific venom activity supports the role of selection in snake venom evolution. Proc. R. Soc. Lond. B Biol. Sci. 2009, 276, 2443–2449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyer, L.; Alagón, A.; Fry, B.; Jackson, T.; Sunagar, K.; Chippaux, J. Signs, symptoms and treatment of envenomation. Venom. Reptil. Their Toxins Evol. Pathophysiol. Biodiscovery 2015, 32–60. [Google Scholar]
- Inceoglu, B.; Lango, J.; Jing, J.; Chen, L.; Doymaz, F.; Pessah, I.N.; Hammock, B.D. One scorpion, two venoms: Prevenom of Parabuthus transvaalicus acts as an alternative type of venom with distinct mechanism of action. Proc. Natl. Acad. Sci. USA 2003, 100, 922–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whittington, C.M.; Papenfuss, A.T.; Bansal, P.; Torres, A.M.; Wong, E.S.; Deakin, J.E.; Graves, T.; Alsop, A.; Schatzkamer, K.; Kremitzki, C. Defensins and the convergent evolution of platypus and reptile venom genes. Genome Res. 2008, 18, 986–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sismour, E.N.; Nellis, S.C.; Newton, S.H.; Mays, D.A.; Fine, M.L. An experimental study of consumption of channel catfish Ictalurus punctatus by largemouth bass Micropterus salmoides when alternative prey are available. Copeia 2013, 2013, 277–283. [Google Scholar] [CrossRef]
- Wright, J.J. Adaptive significance of venom glands in the tadpole madtom Noturus gyrinus (Siluriformes: Ictaluridae). J. Exp. Biol. 2012, 215, 1816–1823. [Google Scholar] [CrossRef] [Green Version]
- Margres, M.J.; Wray, K.P.; Seavy, M.; McGivern, J.J.; Sanader, D.; Rokyta, D.R. Phenotypic integration in the feeding system of the eastern diamondback rattlesnake (Crotalus adamanteus). Mol. Ecol. 2015, 24, 3405–3420. [Google Scholar] [CrossRef]
- Strickland, J.L.; Smith, C.F.; Mason, A.J.; Schield, D.R.; Borja, M.; Castañeda-Gaytán, G.; Spencer, C.L.; Smith, L.L.; Trápaga, A.; Bouzid, N.M. Evidence for divergent patterns of local selection driving venom variation in Mojave Rattlesnakes (Crotalus scutulatus). Sci. Rep. 2018, 8, 17622. [Google Scholar] [CrossRef]
- Cameron, A.M.; Endean, R. Epidermal secretions and the evolution of venom glands in fishes. Toxicon 1973, 11, 401–410. [Google Scholar] [CrossRef]
- Colorni, A.; Ullal, A.; Heinisch, G.; Noga, E. Activity of the antimicrobial polypeptide piscidin 2 against fish ectoparasites. J. Fish Dis. 2008, 31, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Rajanbabu, V.; Chen, J.-Y. Applications of antimicrobial peptides from fish and perspectives for the future. Peptides 2011, 32, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Ángeles Esteban, M. An overview of the immunological defenses in fish skin. ISRN Immunol. 2012. [Google Scholar] [CrossRef]
- Ellisdon, A.M.; Reboul, C.F.; Panjikar, S.; Huynh, K.; Oellig, C.A.; Winter, K.L.; Dunstone, M.A.; Hodgson, W.C.; Seymour, J.; Dearden, P.K. Stonefish toxin defines an ancient branch of the perforin-like superfamily. Proc. Natl. Acad. Sci. USA 2015, 112, 15360–15365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gratzer, B.; Millesi, E.; Walzl, M.; Herler, J. Skin toxins in coral-associated G obiodon species (T eleostei: G obiidae) affect predator preference and prey survival. Mar. Ecol. 2015, 36, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Munday, P.L.; Caley, M.J.; Jones, G.P.; Llewellyn, L.E. The toxicity of skin secretions from coral-dwelling gobies and their potential role as a predator deterrent. Environ. Biol. Fishes 2003, 67, 359–367. [Google Scholar] [CrossRef]
- Junqueira, M.E.P.; Grund, L.Z.; Orii, N.M.; Saraiva, T.C.; de Magalhães Lopes, C.A.; Lima, C.; Lopes-Ferreira, M. Analysis of the inflammatory reaction induced by the catfish (Cathorops spixii) venoms. Toxicon 2007, 49, 909–919. [Google Scholar] [CrossRef]
- Monteiro-dos-Santos, J.; Conceição, K.; Seibert, C.S.; Marques, E.E.; Silva, P.I., Jr.; Soares, A.B.; Lima, C.; Lopes-Ferreira, M. Studies on pharmacological properties of mucus and sting venom of Potamotrygon cf. henlei. Int. Immunopharmacol. 2011, 11, 1368–1377. [Google Scholar] [CrossRef] [Green Version]
- Ramos, A.D.; Conceição, K.; Silva, P.I., Jr.; Richardson, M.; Lima, C.; Lopes-Ferreira, M. Specialization of the sting venom and skin mucus of Cathorops spixii reveals functional diversification of the toxins. Toxicon 2012, 59, 651–665. [Google Scholar] [CrossRef]
- Baracchi, D.; Francese, S.; Turillazzi, S. Beyond the antipredatory defence: Honey bee venom function as a component of social immunity. Toxicon 2011, 58, 550–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grow, N.B.; Nekaris, K. Does toxic defence in Nycticebus spp. relate to ectoparasites? The lethal effects of slow loris venom on arthropods. Toxicon 2015, 95, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Ferreira, M.; Ramos, A.D.; Martins, I.A.; Lima, C.; Conceição, K.; Haddad, V., Jr. Clinical manifestations and experimental studies on the spine extract of the toadfish Porichthys porosissimus. Toxicon 2014, 86, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Jared, C.; Mailho-Fontana, P.L.; Antoniazzi, M.M.; Mendes, V.A.; Barbaro, K.C.; Rodrigues, M.T.; Brodie, E.D. Venomous Frogs Use Heads as Weapons. Curr. Biol. 2015, 25, 2166–2170. [Google Scholar] [PubMed] [Green Version]
- Mangoni, M.L.; Rinaldi, A.C.; Di Giulio, A.; Mignogna, G.; Bozzi, A.; Barra, D.; Simmaco, M. Structure–function relationships of temporins, small antimicrobialpeptides from amphibian skin. Eur. J. Biochem. 2000, 267, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, A.C. Antimicrobial peptides from amphibian skin: An expanding scenario: Commentary. Curr. Opin. Chem. Biol. 2002, 6, 799–804. [Google Scholar] [CrossRef]
- Paxton, J.R.; Eschmeyer, W.N.; Kirshner, D. Encyclopedia of Fishes; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Mumby, P.J.; Harborne, A.R.; Brumbaugh, D.R. Grouper as a natural biocontrol of invasive lionfish. PLoS ONE 2011, 6, e21510. [Google Scholar] [CrossRef] [PubMed]
- Ruxton, G.D.; Sherratt, T.N.; Speed, M.P.; Speed, M.P.; Speed, M. Avoiding Attack: The Evolutionary Ecology of Crypsis, Warning Signals and Mimicry; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Chuang, P.-S.; Shiao, J.-C. Toxin gene determination and evolution in scorpaenoid fish. Toxicon 2014, 88, 21–33. [Google Scholar] [PubMed]
- Hundt, P.J.; Nakamura, Y.; Yamaoka, K. Diet of combtooth blennies (Blenniidae) in Kochi and Okinawa, Japan. Ichthyol. Res. 2014, 61, 76–82. [Google Scholar] [CrossRef]
- Baxter, E.W. Observations on the Buccal Glands of Lampreys (Petromyzonidae). Proc. Zool. Soc. Lond. 1956, 127, 95–118. [Google Scholar]
- Dayton, P.; Hessler, R. Role of Biological Disturbance in Maintaining Diversity in the Deep Sea; Deep Sea Research and Oceanographic Abstracts; Elsevier: Amsterdam, The Netherlands, 1972; pp. 199–208. [Google Scholar]
- Ribeiro, J. Role of saliva in blood-feeding by arthropods. Ann. Rev. Entomol. 1987, 32, 463–478. [Google Scholar] [CrossRef]
- Apitz-Castro, R.; Beguin, S.; Tablante, A.; Bartoli, F.; Holt, J.C.; Hemker, H.C. Purification and partial characterization of draculin, the anticoagulant factor present in the saliva of vampire bats (Desmodus rotundus). Thromb. Haemost. 1995, 73, 094–100. [Google Scholar] [CrossRef]
- Francischetti, I.M. Platelet aggregation inhibitors from hematophagous animals. Toxicon 2010, 56, 1130–1144. [Google Scholar] [CrossRef] [Green Version]
- Gage, S.H.; Gage-Day, M. The anti-coagulating action of the secretion of the buccal glands of the lampreys (Petromyzon, Lampetra and Entosphenus). Science 1927, 66, 282–284. [Google Scholar] [CrossRef]
- Ito, N.; Mita, M.; Takahashi, Y.; Matsushima, A.; Watanabe, Y.G.; Hirano, S.; Odani, S. Novel cysteine-rich secretory protein in the buccal gland secretion of the parasitic lamprey, Lethenteron japonicum. Biochem. Biophys. Res. Commun. 2007, 358, 35–40. [Google Scholar]
- Meckley, T.D.; Wagner, C.M.; Gurarie, E. Coastal movements of migrating sea lamprey (Petromyzon marinus) in response to a partial pheromone added to river water: Implications for management of invasive populations. Can. J. Fish. Aquat. Sci. 2014, 71, 533–544. [Google Scholar]
- Waldman, J.; Grunwald, C.; Wirgin, I. Sea lamprey Petromyzon marinus: An exception to the rule of homing in anadromous fishes. Biol. Lett. 2008, 4, 659–662. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Araújo, M.J.; Bao, M.; Mucientes, G.; Cobo, F. The haematophagous feeding stage of anadromous populations of sea lamprey Petromyzon marinus: Low host selectivity and wide range of habitats. Hydrobiologia 2014, 734, 187–199. [Google Scholar] [CrossRef]
- Bergstedt, R.A.; Seelye, J.G. Evidence for Lack of Homing by Sea Lampreys. Trans. Am. Fish. Soc. 1995, 124, 235–239. [Google Scholar] [CrossRef]
- Buchheim, J.R.; Hixon, M.A. Competition for shelter holes in the coral-reef fish Acanthemblemaria spinosa Metzelaar. J. Exp. Mar. Biol. Ecol. 1992, 164, 45–54. [Google Scholar] [CrossRef]
- Koppel, V.H. Habitat selection and space partitioning among two Mediterranean blenniid species. Mar. Ecol. 1988, 9, 329–346. [Google Scholar] [CrossRef]
- Stephens, J.S., Jr.; Johnson, R.K.; Key, G.S.; McCosker, J.E. The comparative ecology of three sympatric species of California blennies of the genus Hypsoblennius Gill (Teleostomi, Blenniidae). Ecol. Monogr. 1970, 40, 213–233. [Google Scholar] [CrossRef]
- Enzor, L.; Wilborn, R.; Bennett, W. Toxicity and metabolic costs of the Atlantic stingray (Dasyatis sabina) venom delivery system in relation to its role in life history. J. Exp. Mar. Biol. Ecol. 2011, 409, 235–239. [Google Scholar] [CrossRef]
- Hughes, R.; Pedersen, K.; Huskey, S. The kinematics of envenomation by the yellow stingray, Urobatis jamaicensis. Zoomorphology 2018, 1–10. [Google Scholar] [CrossRef]
- Cott, H.B. Adaptive Coloration in Animals; Methuen: London, UK, 1940. [Google Scholar]
- Lewis, D.B. Studies of the biology of the lesser weever fish Trachinus vipera Cuvier: I. Adaptations to a benthic habit. J. Fish Biol. 1976, 8, 127–138. [Google Scholar] [CrossRef]
- Inbar, M.; Lev-Yadun, S. Conspicuous and aposematic spines in the animal kingdom. Naturwissenschaften 2005, 92, 170–172. [Google Scholar] [CrossRef]
- Kauppinen, J.; Mappes, J. Why are wasps so intimidating: Field experiments on hunting dragonflies (Odonata: Aeshna grandis). Anim. Behav. 2003, 66, 505–511. [Google Scholar] [CrossRef]
- Schuler, W.; Hesse, E. On the function of warning coloration: A black and yellow pattern inhibits prey-attack by naive domestic chicks. Behav. Ecol. Sociobiol. 1985, 16, 249–255. [Google Scholar] [CrossRef]
- Noonan, B.P.; Comeault, A.A. The role of predator selection on polymorphic aposematic poison frogs. Biol. Lett. 2009, 5, 51–54. [Google Scholar] [Green Version]
- Hoese, F.; Law, E.; Rao, D.; Herberstein, M. Distinctive yellow bands on a sit-and-wait predator: Prey attractant or camouflage? Behaviour 2006, 143, 763–781. [Google Scholar]
- Iniesta, L.F.; Ratton, P.; Guerra, T.J. Avian predators avoid attacking artificial aposematic millipedes in Brazilian Atlantic Forest. J. Trop. Ecol. 2017, 33, 89–93. [Google Scholar] [CrossRef]
- Hughes, A.E.; Troscianko, J.; Stevens, M. Motion dazzle and the effects of target patterning on capture success. BMC Evol. Biol. 2014, 14, 201. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.; Searle, W.T.L.; Seymour, J.E.; Marshall, K.L.; Ruxton, G.D. Motion dazzle and camouflage as distinct anti-predator defenses. BMC Biol. 2011, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Dolenska, M.; Nedved, O.; Vesely, P.; Tesarova, M.; Fuchs, R. What constitutes optical warning signals of ladybirds (Coleoptera: Coccinellidae) towards bird predators: Colour, pattern or general look? Biol. J. Linn. Soc. 2009, 98, 234–242. [Google Scholar]
- Mäthger, L.M.; Bell, G.R.; Kuzirian, A.M.; Allen, J.J.; Hanlon, R.T. How does the blue-ringed octopus (Hapalochlaena lunulata) flash its blue rings? J. Exp. Biol. 2012, 215, 3752–3757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cronin, T.W.; Marshall, N.J.; Caldwell, R.L. Spectral tuning and the visual ecology of mantis shrimps. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000, 355, 1263–1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gačić, Z.; Damjanović, I.; Mićković, B.; Hegediš, A.; Nikčević, M. Spectral sensitivity of the dogfish shark (Scyliorhinus canicula). Fish Physiol. Biochem. 2007, 33, 21. [Google Scholar] [CrossRef]
- Hart, N.S. Microspectrophotometry of visual pigments and oil droplets in a marine bird, the wedge-tailed shearwater Puffinus pacificus: Topographic variations in photoreceptor spectral characteristics. J. Exp. Biol. 2004, 207, 1229–1240. [Google Scholar]
- Levenson, D.H.; Ponganis, P.J.; Crognale, M.A.; Deegan, J.F.; Dizon, A.; Jacobs, G.H. Visual pigments of marine carnivores: Pinnipeds, polar bear, and sea otter. J. Comp. Physiolo. A 2006, 192, 833–843. [Google Scholar] [CrossRef]
- Nisani, Z.; Dunbar, S.G.; Hayes, W.K. Cost of venom regeneration in Parabuthus transvaalicus (Arachnida: Buthidae). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 147, 509–513. [Google Scholar] [CrossRef]
- Arbuckle, K.; Speed, M.P. Antipredator defenses predict diversification rates. Proc. Natl. Acad. Sci. USA 2015, 112, 13597–13602. [Google Scholar] [CrossRef] [PubMed]
- Speed, M.P.; Franks, D.W. Antagonistic evolution in an aposematic predator–prey signaling system. Evolution 2014, 68, 2996–3007. [Google Scholar] [CrossRef] [PubMed]
- Skelhorn, J.; Halpin, C.G.; Rowe, C. Learning about aposematic prey. Behav. Ecol. 2016, 27, 955–964. [Google Scholar] [CrossRef] [Green Version]
- Pasteur, G. A classificatory review of mimicry systems. Ann. Rev. Ecol. Syst. 1982, 13, 169–199. [Google Scholar] [CrossRef]
- Sheppard, P. The Evolution of Mimicry; a Problem in Ecology and Genetics; Cold Spring Harbor Symposia on Quantitative Biology; Cold Spring Harbor Laboratory Press: Woodbury, NY, USA, 1959; pp. 131–140. [Google Scholar]
- Alexandrou, M.A.; Oliveira, C.; Maillard, M.; McGill, R.A.; Newton, J.; Creer, S.; Taylor, M.I. Competition and phylogeny determine community structure in Müllerian co-mimics. Nature 2011, 469, 84. [Google Scholar] [CrossRef]
- Greene, H.W.; McDiarmid, R.W. Coral snake mimicry: Does it occur? Science 1981, 213, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Caley, J.M.; Schluter, D. Predators favour mimicry in a tropical reef fish. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 667–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheney, K.L. Multiple selective pressures apply to a coral reef fish mimic: A case of Batesian–aggressive mimicry. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2010, 277, 1849–1855. [Google Scholar] [CrossRef]
- Fujisawa, M.; Sakai, Y.; Kuwamura, T. Aggressive mimicry of the cleaner wrasse by Aspidontus taeniatus functions mainly for small blennies. Ethology 2018, 124, 432–439. [Google Scholar] [CrossRef]
- Cheney, K.L.; Côté, I.M. Frequency-dependent success of aggressive mimics in a cleaning symbiosis. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2005, 272, 2635–2639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowland, H.M.; Ihalainen, E.; Lindström, L.; Mappes, J.; Speed, M.P. Co-mimics have a mutualistic relationship despite unequal defences. Nature 2007, 448, 64. [Google Scholar] [CrossRef] [PubMed]
- Rowland, H.M.; Mappes, J.; Ruxton, G.D.; Speed, M.P. Mimicry between unequally defended prey can be parasitic: Evidence for quasi-Batesian mimicry. Ecol. Lett. 2010, 13, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.I. Evolution: Fangtastic Venoms Underpin Parasitic Mimicry. Curr. Biol. 2017, 27, R295–R298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, J.J. Conservative coevolution of Müllerian mimicry in a group of rift lake catfish. Evolution 2011, 65, 395–407. [Google Scholar] [PubMed]
- Marek, P.E.; Bond, J.E. A Müllerian mimicry ring in Appalachian millipedes. Proc. Natl. Acad. Sci. USA 2009, 106, 9755–9760. [Google Scholar] [CrossRef] [PubMed]
- Symula, R.; Schulte, R.; Summers, K. Molecular phylogenetic evidence for a mimetic radiation in Peruvian poison frogs supports a Müllerian mimicry hypothesis. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2001, 268, 2415–2421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balogh, A.C.; Gamberale-Stille, G.; Leimar, O. Learning and the mimicry spectrum: From quasi-Bates to super-Müller. Anim. Behav. 2008, 76, 1591–1599. [Google Scholar] [CrossRef]
- Currier, R.B.; Calvete, J.J.; Sanz, L.; Harrison, R.A.; Rowley, P.D.; Wagstaff, S.C. Unusual stability of messenger RNA in snake venom reveals gene expression dynamics of venom replenishment. PLoS ONE 2012, 7, e41888. [Google Scholar] [CrossRef]
- Nisani, Z.; Boskovic, D.S.; Dunbar, S.G.; Kelln, W.; Hayes, W.K. Investigating the chemical profile of regenerated scorpion (Parabuthus transvaalicus) venom in relation to metabolic cost and toxicity. Toxicon 2012, 60, 315–323. [Google Scholar] [CrossRef]
- Saggiomo, S.L.; Zelenka, C.; Seymour, J. Relationship between food and venom production in the estuarine stonefish Synanceia horrida. Toxicon 2017, 125, 19–23. [Google Scholar] [CrossRef]
- Fu, S.-J.; Zeng, L.-Q.; Li, X.-M.; Pang, X.; Cao, Z.-D.; Peng, J.-L.; Wang, Y.-X. The behavioural, digestive and metabolic characteristics of fishes with different foraging strategies. J. Exp. Biol. 2009, 212, 2296–2302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nisani, Z.; Hayes, W.K. Defensive stinging by Parabuthus transvaalicus scorpions: Risk assessment and venom metering. Anim. Behav. 2011, 81, 627–633. [Google Scholar] [CrossRef]
- Young, B.A.; Zahn, K. Venom flow in rattlesnakes: Mechanics and metering. J. Exp. Biol. 2001, 204, 4345–4351. [Google Scholar] [PubMed]
- Amorim, F.G.; Costa, T.R.; Baiwir, D.; De Pauw, E.; Quinton, L.; Sampaio, S.V. Proteopeptidomic, Functional and Immunoreactivity Characterization of Bothrops moojeni Snake Venom: Influence of Snake Gender on Venom Composition. Toxins 2018, 10, 177. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Ferreira, M.; Sosa-Rosales, I.; Bruni, F.M.; Ramos, A.D.; Portaro, F.C.V.; Conceição, K.; Lima, C. Analysis of the intersexual variation in Thalassophryne maculosa fish venoms. Toxicon 2016, 115, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.J.; Ellsworth, S.A.; Hogan, M.P.; Nystrom, G.S.; Martinez, P.; Budhdeo, A.; Zelaya, R.; Perez, A.; Powell, B.; He, H. Female-biased population divergence in the venom of the Hentz striped scorpion (Centruroides hentzi). Toxicon 2018, 152, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Zobel-Thropp, P.A.; Bulger, E.A.; Cordes, M.H.; Binford, G.J.; Gillespie, R.G.; Brewer, M.S. Sexually dimorphic venom proteins in long-jawed orb-weaving spiders (Tetragnatha) comprise novel gene families. PeerJ 2018, 6, e4691. [Google Scholar] [CrossRef]
- Gross, M.R.; Sargent, R.C. The evolution of male and female parental care in fishes. Am. Zool. 1985, 25, 807–822. [Google Scholar] [CrossRef]
- Hoffman, S.G.; Robertson, D.R. Foraging and reproduction of two Caribbean reef toadfishes (Batrachoididae). Bull. Mar. Sci. 1983, 33, 919–927. [Google Scholar]
- Herzig, V.; Ward, R.J.; dos Santos, W.F. Intersexual variations in the venom of the Brazilian ‘armed’spider Phoneutria nigriventer (Keyserling, 1891). Toxicon 2002, 40, 1399–1406. [Google Scholar] [CrossRef]
- Ehrlich, P.R.; Raven, P.H. Butterflies and plants: A study in coevolution. Evolution 1964, 18, 586–608. [Google Scholar] [CrossRef]
- Arbuckle, K.; Brockhurst, M.; Speed, M.P. Does chemical defence increase niche space? A phylogenetic comparative analysis of the Musteloidea. Evol. Ecol. 2013, 27, 863–881. [Google Scholar] [CrossRef]
- Hodge, J.R.; Alim, C.; Bertrand, N.G.; Lee, W.; Price, S.A.; Tran, B.; Wainwright, P.C. Ecology shapes the evolutionary trade-off between predator avoidance and defence in coral reef butterflyfishes. Ecol. Lett. 2018, 21, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Layman, C.A.; Allgeier, J.E. Characterizing trophic ecology of generalist consumers: A case study of the invasive lionfish in The Bahamas. Mar. Ecol. Prog. Ser. 2012, 448, 131–141. [Google Scholar] [CrossRef]
- Betancur-R, R.; Hines, A.; Acero P, A.; Ortí, G.; Wilbur, A.E.; Freshwater, D.W. Reconstructing the lionfish invasion: Insights into Greater Caribbean biogeography. J. Biogeogr. 2011, 38, 1281–1293. [Google Scholar] [CrossRef]
- Cure, K.; Benkwitt, C.E.; Kindinger, T.L.; Pickering, E.A.; Pusack, T.J.; McIlwain, J.L.; Hixon, M.A. Comparative behavior of red lionfish Pterois volitans on native Pacific versus invaded Atlantic coral reefs. Mar. Ecol. Prog. Ser. 2012, 467, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Phillips, B.L.; Brown, G.P.; Webb, J.K.; Shine, R. Invasion and the evolution of speed in toads. Nature 2006, 439, 803. [Google Scholar] [PubMed]
- Daltry, J.C.; Wüster, W.; Thorpe, R.S. Diet and snake venom evolution. Nature 1996, 379, 537. [Google Scholar] [CrossRef] [PubMed]
- Prator, C.A.; Murayama, K.M.; Schulz, J.R. Venom variation during prey capture by the cone snail, Conus textile. PLoS ONE 2014, 9, e98991. [Google Scholar]
- Losey, G.S. Predation protection in the poison-fang blenny, Meiacanthus atrodorsalis, and its mimics, Ecsenius bicolor and Runula laudandus (Blenniidae). Pac. Sci. 1972, 26, 129–139. [Google Scholar]
- Li, M.; Fry, B.; Kini, R.M. Eggs-only diet: Its implications for the toxin profile changes and ecology of the marbled sea snake (Aipysurus eydouxii). J. Mol. Evol. 2005, 60, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Fry, B.G.; Kini, R.M. Putting the brakes on snake venom evolution: The unique molecular evolutionary patterns of Aipysurus eydouxii (Marbled sea snake) phospholipase A2 toxins. Mol. Biol. Evol. 2005, 22, 934–941. [Google Scholar] [PubMed]
- Santos, J.C.; Cannatella, D.C. Phenotypic integration emerges from aposematism and scale in poison frogs. Proc. Natl. Acad. Sci. USA 2011, 108, 6175–6180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosher, B.T.; Newton, S.H.; Fine, M.L. The spines of the channel catfish, Ictalurus punctatus, as an anti-predator adaptation: An experimental study. Ethology 2006, 112, 188–195. [Google Scholar] [CrossRef]
- Hossie, T.; Hassall, C.; Knee, W.; Sherratt, T. Species with a chemical defence, but not chemical offence, live longer. J. Evol. Biol. 2013, 26, 1598–1602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco, M.A.; Sherman, P.W. Maximum longevities of chemically protected and non-protected fishes, reptiles, and amphibians support evolutionary hypotheses of aging. Mech. Ageing Dev. 2005, 126, 794–803. [Google Scholar] [CrossRef]
- Blount, J.D.; Speed, M.P.; Ruxton, G.D.; Stephens, P.A. Warning displays may function as honest signals of toxicity. Proc. R. Soc. Lond. B Biol. Sci. 2009, 276, 871–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arbuckle, K. Phylogenetic Comparative Methods can Provide Important Insights into the Evolution of Toxic Weaponry. Toxins 2018, 10, 518. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-Y.V.; Frederich, B.; Lavoué, S.; Chang, J.; Erdmann, M.V.; Mahardika, G.N.; Barber, P.H. Buccal venom gland associates with increased of diversification rate in the fang blenny fish Meiacanthus (Blenniidae; Teleostei). Mol. Phylogenet. Evol. 2018, 125, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, B.D.; Moreau, C.S. Defensive traits exhibit an evolutionary trade-off and drive diversification in ants. Evolution 2017, 71, 315–328. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harris, R.J.; Jenner, R.A. Evolutionary Ecology of Fish Venom: Adaptations and Consequences of Evolving a Venom System. Toxins 2019, 11, 60. https://doi.org/10.3390/toxins11020060
Harris RJ, Jenner RA. Evolutionary Ecology of Fish Venom: Adaptations and Consequences of Evolving a Venom System. Toxins. 2019; 11(2):60. https://doi.org/10.3390/toxins11020060
Chicago/Turabian StyleHarris, Richard J., and Ronald A. Jenner. 2019. "Evolutionary Ecology of Fish Venom: Adaptations and Consequences of Evolving a Venom System" Toxins 11, no. 2: 60. https://doi.org/10.3390/toxins11020060
APA StyleHarris, R. J., & Jenner, R. A. (2019). Evolutionary Ecology of Fish Venom: Adaptations and Consequences of Evolving a Venom System. Toxins, 11(2), 60. https://doi.org/10.3390/toxins11020060





