Ergochromes: Heretofore Neglected Side of Ergot Toxicity
Abstract
:1. Introduction
2. Results
2.1. Ergot Alkaloids and Ergochromes Content
2.2. Biological Activity In Vitro
3. Discussion
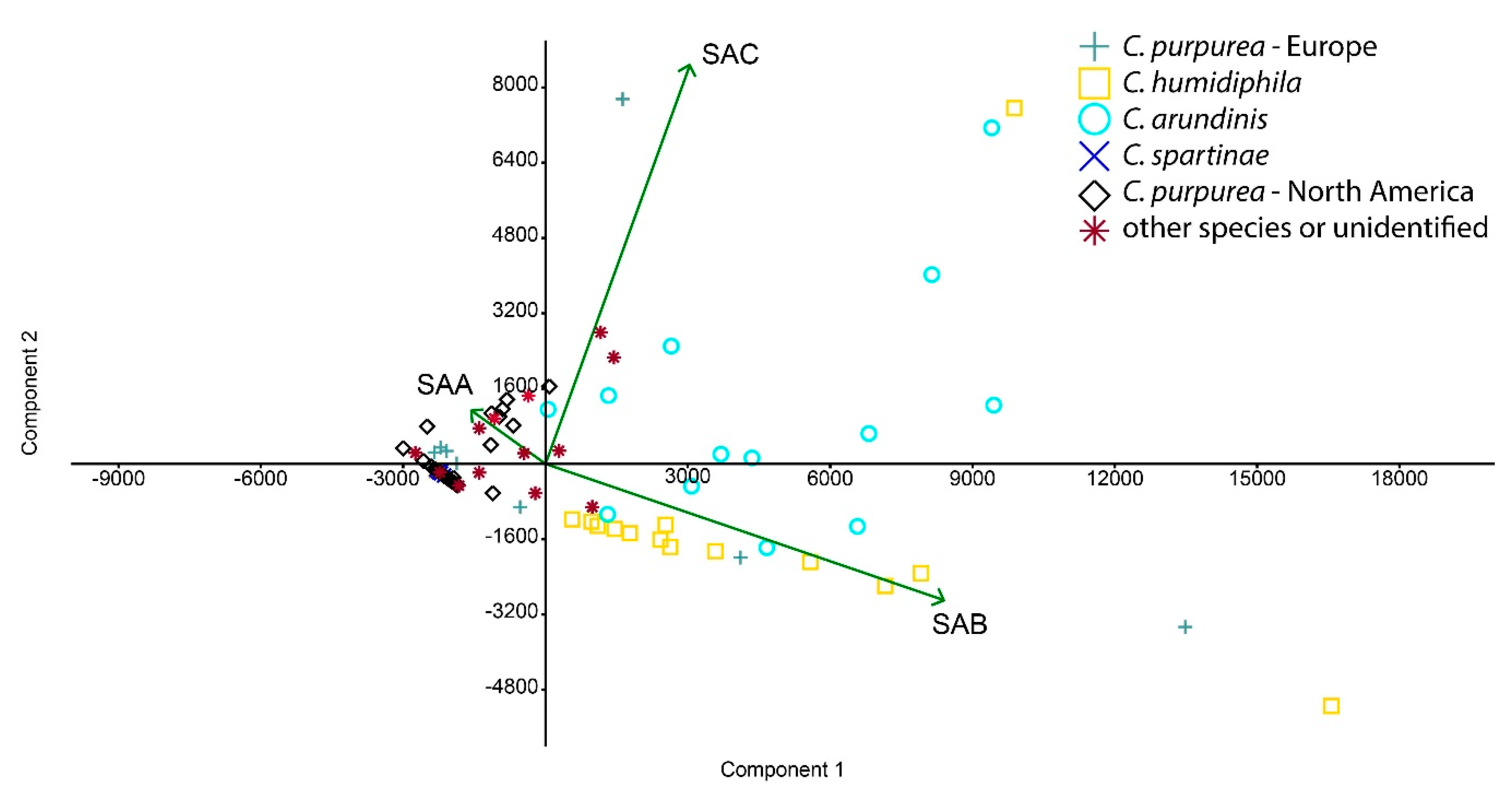
3.1. Ergochrome Quantity and Distribution Across the Species
3.2. Ecological Role of SAs
3.3. Ergochromes Toxicity and Significance
3.4. Mode of Action
4. Conclusions
5. Materials and Methods
5.1. Specimens Analyzed
5.2. Sample Preparation, Extraction, and HPLC Analyses
5.3. Ergot Alkaloids and Ergochromes Identification and Quantification
5.3.1. General Workflow
5.3.2. FTMS
5.3.3. NMR
5.3.4. Quantification of Ergot Alkaloids and Ergochromes
5.4. Biological Activity Testing
5.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Píchová, K.; Pažoutová, S.; Kostovčík, M.; Chudíčková, M.; Stodůlková, E.; Novák, P.; Flieger, M.; van der Linde, E.; Kolařík, M. Evolutionary history of ergot with a new infrageneric classification (Hypocreales: Clavicipitaceae: Claviceps). Mol. Phylogen. Evol. 2018, 123, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Pažoutová, S.; Pešicová, K.; Chudíčková, M.; Šrůtka, P.; Kolařík, M. Delimitation of cryptic species inside Claviceps purpurea. Fungal Biol. 2015, 119, 7–26. [Google Scholar] [CrossRef] [PubMed]
- Negård, M.; Uhlig, S.; Kauserud, H.; Andersen, T.; Høiland, K.; Vrålstad, T. Links between genetic groups, indole alkaloid profiles and ecology within the grass-parasitic Claviceps purpurea species complex. Toxins (Basel) 2015, 7, 1431–1456. [Google Scholar] [CrossRef] [PubMed]
- Shoukouhi, P.; Hicks, C.; Menzies, J.G.; Popovic, Z.; Chen, W.; Seifert, K.A.; Assabgui, R.; Liu, M. Phylogeny of Canadian ergot fungi and a detection assay by real-time polymerase chain reaction. Mycologia 2019, 111, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Manzanares, N.; Gámiz-Gracia, L.; García-Campaña, A.M.; Diana Di Mavungu, J.; De Saeger, S. Ergot alkaloids: Chemistry, biosynthesis, bioactivity, and methods of analysis. In Fungal Metabolites; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer: Berlin, Germany, 2017; pp. 887–929. [Google Scholar]
- Young, C.A.; Schardl, C.L.; Panaccione, D.G.; Florea, S.; Takach, J.E.; Charlton, N.D.; Moore, N.; Webb, J.S.; Jaromczyk, J. Genetics, genomics and evolution of ergot alkaloid diversity. Toxins (Basel) 2015, 7, 1273–1302. [Google Scholar] [CrossRef]
- EFSA. Scientific opinion on ergot alkaloids in food and feed. EFSA J. 2012, 10, 158. [Google Scholar] [CrossRef]
- Belser-Ehrlich, S.; Harper, A.; Hussey, J.; Hallock, R. Human and cattle ergotism since 1900: Symptoms, outbreaks, and regulations. Toxicol. Ind. Health 2013, 29, 307–316. [Google Scholar] [CrossRef]
- Klotz, J. Activities and effects of ergot alkaloids on livestock physiology and production. Toxins (Basel) 2015, 7, 2801–2821. [Google Scholar] [CrossRef]
- Bauer, J.I.; Gross, M.; Cramer, B.; Wegner, S.; Hausmann, H.; Hamscher, G.; Usleber, E. Detection of the tremorgenic mycotoxin paxilline and its desoxy analog in ergot of rye and barley: A new class of mycotoxins added to an old problem. Anal. Bioanal. Chem. 2017, 409, 5101–5112. [Google Scholar] [CrossRef]
- Miedaner, T.; Dänicke, S.; Schmiedchen, B.; Wilde, P.; Wortmann, H.; Dhillon, B.; Geiger, H.; Mirdita, V. Genetic variation for ergot (Claviceps purpurea) resistance and alkaloid concentrations in cytoplasmic-male sterile winter rye under pollen isolation. Euphytica 2010, 173, 299–306. [Google Scholar] [CrossRef]
- Mulder, P.; Van Raamsdonk, L.; Van Egmond, H.; Van Der Horst, T.; De Jong, J. Dutch survey ergot alkaloids and sclerotia in animal feeds. In Report/RIKILT 2012; Institute of Food Safety: Wageningen, The Netherlands, 2012; Available online: http://edepot.wur.nl/234699 (accessed on 24 July 2019).
- Franzmann, C.; Wächter, J.; Dittmer, N.; Humpf, H.-U. Ricinoleic acid as a marker for ergot impurities in rye and rye products. J. Agric. Food Chem. 2010, 58, 4223–4229. [Google Scholar] [CrossRef] [PubMed]
- Appelt, M.; Ellner, F.M. Investigations into the occurrence of alkaloids in ergot and single sclerotia from the 2007 and 2008 harvests. Mycotoxin Res. 2009, 25, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, J.; Dexter, J.; Roscoe, M.; Nowicki, T. Retention of ergot alkaloids in wheat during processing1, 2. Cereal Chem. 1995, 72, 291–298. [Google Scholar]
- Pažoutová, S.; Olšovská, J.; Linka, M.; Kolínská, R.; Flieger, M. Chemoraces and habitat specialization of Claviceps purpurea populations. Appl. Environ. Microbiol. 2000, 66, 5419–5425. [Google Scholar] [CrossRef] [PubMed]
- Aboling, S.; Drotleff, A.; Cappai, M.; Kamphues, J. Contamination with ergot bodies (Claviceps purpurea sens ulato) of two horse pastures in Northern Germany. Mycotoxin Res. 2016, 32, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, S.; Vikøren, T.; Ivanova, L.; Handeland, K. Ergot alkaloids in Norwegian wild grasses: A mass spectrometric approach. Rapid Commun. Mass Spectrom. 2007, 21, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Stoll, A.; Renz, J.; Brack, A. Über gelbe Farbstoffe im Mutterkorn. 11. Mitteilung über antibakterielle Stoffe. Helv. Chim. Acta 1952, 35, 2022–2034. [Google Scholar] [CrossRef]
- Franck, B. Structure and biosynthesis of the ergot pigments. Angew. Chem. Int. Ed. Engl. 1969, 8, 251–260. [Google Scholar] [CrossRef]
- Buchta, M.; Cvak, L. Ergot alkaloids and other metabolites of the genus Claviceps. In Ergot: The Genus Claviceps; Křen, V., Cvak, L., Eds.; Harwood Academic Publishers: Amsterdam, The Netherlands, 1999; pp. 173–200. [Google Scholar]
- Masters, K.-S.; Bräse, S. Xanthones from fungi, lichens, and bacteria: The natural products and their synthesis. Chem. Rev. 2012, 112, 3717–3776. [Google Scholar] [CrossRef]
- Wezeman, T.; Bräse, S.; Masters, K.-S. Xanthone dimers: A compound family which is both common and privileged. Nat. Prod. Rep. 2015, 32, 6–28. [Google Scholar] [CrossRef]
- Ciegler, A.; Hayes, A.W.; Vesonder, R.F. Production and biological activity of secalonic acid D. Appl. Environ. Microbiol. 1980, 39, 285–287. [Google Scholar] [PubMed]
- Reddy, C.; Reddy, R.; Hayes, A.; Ciegler, A. Teratogenicity of secalonic acid D in mice. J. Toxicol. Environ. Health A 1981, 7, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-Y.; Tao, L.-Y.; Liang, Y.-J.; Yan, Y.-Y.; Dai, C.-L.; Xia, X.-K.; She, Z.-G.; Lin, Y.-C.; Fu, L.-W. Secalonic acid D induced leukemia cell apoptosis and cell cycle arrest of G1 with involvement of GSK-3β/β-catenin/c-Myc pathway. Cell Cycle 2009, 8, 2444–2450. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, M.; Maebayshi, Y.; Miyaki, K. The isolation of secalonic acid A from Aspergillus ochraceus cultured on rice. Chem. Pharm. Bull. (Tokyo) 1971, 19, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Steffens, J.C.; Robeson, D.J. Secalonic acid A, a vivotoxin in pink root-infected onion. Phytochemistry 1987, 26, 1599–1602. [Google Scholar] [CrossRef]
- Andersen, R.; Buechi, G.; Kobbe, B.; Demain, A.L. Secalonic acids D and F are toxic metabolites of Aspergillus aculeatus. J. Org. Chem. 1977, 42, 352–353. [Google Scholar] [CrossRef]
- Elsaid, A.; Sallam, A.; Ashour, A.; Ebrahim, W.; Lahloub, M.F.; Saad, H.-E. Biologically active metabolites from Penicillium sp., An endophyte isolated from Glaucium arabicum. J. Am. Sci. 2016, 12, 33–41. [Google Scholar] [CrossRef]
- Naude, T.W.; Botha, C.; Vorster, J.H.; Roux, C.; van der Linde, E.; van der Walt, S.I.; Rottinghaus, G.; van Jaarsveld, L.; Lawrence, A.N. Claviceps cyperi, a new cause of severe ergotism in dairy cattle consuming maize silage and teff hay contaminated with ergotised Cyperus esculentus (nut sedge) on the Highveld of South Africa. Onderstepoort J. Vet. Res. 2005, 72, 23–37. [Google Scholar] [CrossRef]
- Cerri, M.; Reale, L.; Moretti, C.; Buonaurio, R.; Coppi, A.; Ferri, V.; Foggi, B.; Gigante, D.; Lastrucci, L.; Quaglia, M. Claviceps arundinis identification and its role in the die-back syndrome of Phragmites australis populations in central Italy. Plant Biosyst. 2018, 152, 818–824. [Google Scholar] [CrossRef]
- Boestfleisch, C.; Drotleff, A.M.; Ternes, W.; Nehring, S.; Pažoutová, S.; Papenbrock, J. The invasive ergot Claviceps purpurea var. spartinae recently established in the European Wadden Sea on common cord grass is genetically homogeneous and the sclerotia contain high amounts of ergot alkaloids. Eur. J. Plant Pathol. 2015, 141, 445–461. [Google Scholar] [CrossRef]
- van der Linde, E.J.; Pešicová, K.; Pažoutová, S.; Stodůlková, E.; Flieger, M.; Kolařík, M. Ergot species of the Claviceps purpurea group from South Africa. Fungal Biol. 2016, 120, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Brady, L. Phylogenetic distribution of parasitism by Claviceps species. Lloydia 1962, 25, 1–36. [Google Scholar]
- Franck, B.; Gottschalk, E.M.; Ohnsorge, U.; Hüper, F. Mutterkorn-Farbstoffe, XII. Trennung, Struktur und absolute Konfiguration der diastereomeren Secalonsäuren A, B and C. Chem. Ber. 1966, 99, 3842–3862. [Google Scholar] [CrossRef]
- McClymont Peace, D.; Harwig, J. Screening for ergot particles in grain products by light microscopy. Food Res. Int. 1982, 15, 147–149. [Google Scholar] [CrossRef]
- Maríne Font, A.; Moreno Martin, F.; Costes, C. Study of the pigments of ergot. New method for studying ergot in flours. Ann. Falsif. Expert. Chim. 1971, 64, 80. [Google Scholar]
- Neubauer, L.; Dopstadt, J.; Humpf, H.-U.; Tudzynski, P. Identification and characterization of the ergochrome gene cluster in the plant pathogenic fungus Claviceps purpurea. Fungal Biol. Biotechnol. 2016, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Komarova, E.; Tolkachev, O. The chemistry of peptide ergot alkaloids. Part 1. Classification and chemistry of ergot peptides. Pharm. Chem. J. 2001, 35, 504–513. [Google Scholar] [CrossRef]
- Wäli, P.P.; Wäli, P.R.; Saikkonen, K.; Tuomi, J. Is the pathogenic ergot fungus a conditional defensive mutualist for its host grass? PLoS ONE 2013, 8, e69249. [Google Scholar] [CrossRef]
- Zhang, W.; Krohn, K.; Egold, H.; Draeger, S.; Schulz, B. Diversity of antimicrobial pyrenophorol derivatives from an endophytic fungus, Phoma sp. Eur. J. Org. Chem. 2008, 4320–4328. [Google Scholar] [CrossRef]
- Pettit, G.R.; Meng, Y.; Herald, D.L.; Graham, K.A.; Pettit, R.K.; Doubek, D.L. Isolation and structure of ruprechstyril from Ruprechtia tangarana. J. Nat. Prod. 2003, 66, 1065–1069. [Google Scholar] [CrossRef]
- Menzies, J.; Turkington, T. An overview of the ergot (Claviceps purpurea) issue in western Canada: Challenges and solutions. Can. J. Plant Pathol. 2015, 37, 40–51. [Google Scholar] [CrossRef]
- Greatorex, J.; Mantle, P. Experimental ergotism in sheep. Res. Vet. Sci. 1973, 15, 337–346. [Google Scholar] [CrossRef]
- Griffith, R.; Grauwiler, J.; Hodel, C.; Leist, K.; Matter, B. Toxicologic considerations. In Ergot Alkaloids and Related Compounds; Berde, B., Schild, H.O., Eds.; Springer: Berlin, Germany, 1978; pp. 805–851. [Google Scholar]
- Tor-Agbidye, J.; Blythe, L.; Craig, A. Correlation of endophyte toxins (ergovaline and lolitrem B) with clinical disease: Fescue foot and perennial ryegrass staggers. Vet. Hum. Toxicol. 2001, 43, 140–146. [Google Scholar] [PubMed]
- Merrill, M.; Bohnert, D.; Harmon, D.; Craig, A.; Schrick, F. The ability of a yeast-derived cell wall preparation to minimize the toxic effects of high-ergot alkaloid tall fescue straw in beef cattle. J. Anim. Sci. 2007, 85, 2596–2605. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Yano, S.; Watanabe, H.; Yamazaki, M.; Miyaki, K. Phlogistic activity of secalonic acid A. Chem. Pharm. Bull. (Tokyo) 1974, 22, 1600–1606. [Google Scholar] [CrossRef]
- Steyn, P.S. The isolation, structure and absolute configuration of secalonic acid D, the toxic metabolite of Penicillium oxalicum. Tetrahedron 1970, 26, 51–57. [Google Scholar] [CrossRef]
- Mayura, K.; Wallace Hayes, A.; Berndt, W.O. Teratogenicity of secalonic acid d in rats. Toxicology 1982, 25, 311–322. [Google Scholar] [CrossRef]
- Hanumegowda, U.M.; Dhulipala, V.C.; Reddy, C.S. Mechanism of secalonic acid D-induced inhibition of transcription factor binding to cyclic AMP response element in the developing murine palate. Toxicol. Sci. 2002, 70, 55–62. [Google Scholar] [CrossRef]
- Zhai, A.; Zhang, Y.; Zhu, X.; Liang, J.; Wang, X.; Lin, Y.; Chen, R. Secalonic acid A reduced colchicine cytotoxicity through suppression of JNK, p38 MAPKs and calcium influx. Neurochem. Int. 2011, 58, 85–91. [Google Scholar] [CrossRef]
- Zhai, A.; Zhu, X.; Wang, X.; Chen, R.; Wang, H. Secalonic acid A protects dopaminergic neurons from 1-methyl-4-phenylpyridinium (MPP+)-induced cell death via the mitochondrial apoptotic pathway. Eur. J. Pharmacol. 2013, 713, 58–67. [Google Scholar] [CrossRef]
- Kurobane, I.; Iwahashi, S.; Fukuda, A. Cytostatic activity of naturally isolated isomers of secalonic acids and their chemically rearranged dimers. Drugs Exp. Clin. Res. 1987, 13, 339–344. [Google Scholar] [PubMed]
- Millot, M.; Tomasi, S.; Studzinska, E.; Rouaud, I.; Boustie, J. Cytotoxic constituents of the lichen Diploicia canescens. J. Nat. Prod. 2009, 72, 2177–2180. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-P.; Tao, L.-Y.; Wang, F.; Zhang, J.-Y.; Liang, Y.-J.; Fu, L.-W. Secalonic acid D reduced the percentage of side populations by down-regulating the expression of ABCG2. Biochem. Pharmacol. 2013, 85, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Franck, B.; Flasch, H. Die Ergochrome (Physiologie, Isolierung, Struktur und Biosynthese). In Fortschritte der Chemie Organischer Naturstoffe; Grisebach, H., Kirby, G.W., Herz, W., Eds.; Springer: Berlin, Germany, 1973; pp. 151–206. [Google Scholar]
- Reddy, C.; Hayes, A.; Williams, W.; Ciegler, A. Toxicity of secalonic acid D. J. Toxicol. Environ. Health A 1979, 5, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, Y.-P.; Li, X.-X.; Lu, Z.-H.; Zheng, Q.-H.; Liu, Q.-Y. Isolation of 4, 4′-bond secalonic acid D from the marine-derived fungus Penicillium oxalicum with inhibitory property against hepatocellular carcinoma. J. Antibiot. 2019, 72, 34. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Sun, H.; Liu, D.; Zhang, J.; Zhang, J.; Yan, M.; Pan, X. Secalonic acid-F inhibited cell growth more effectively than 5-fluorouracil on hepatocellular carcinoma in vitro and in vivo. Neoplasma 2017, 64, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Nakamaru, T.; Maebayashi, Y.; Nozawa, Y.; Yamazaki, M. Inhibition by secalonic acid D of oxidative phosphorylation and Ca2+-induced swelling in mitochondria isolated from rat livers. Appl. Environ. Microbiol. 1983, 46, 793–796. [Google Scholar] [Green Version]
- Pazoutova, S.; Olsovska, J.; Sulc, M.; Chudickova, M.; Flieger, M. Claviceps nigricans and Claviceps grohii: Their alkaloids and phylogenetic placement. J. Nat. Prod. 2008, 71, 1085–1088. [Google Scholar] [CrossRef]
- Osmundson, T.W.; Eyre, C.A.; Hayden, K.M.; Dhillon, J.; Garbelotto, M.M. Back to basics: An evaluation of NaOH and alternative rapid DNA extraction protocols for DNA barcoding, genotyping, and disease diagnostics from fungal and oomycete samples. Mol. Ecol. Resour. 2013, 13, 66–74. [Google Scholar] [CrossRef]
- Flieger, M.; Banďouchová, H.; Černý, J.; Chudíčková, M.; Kolařík, M.; Kováčová, V.; Martínková, N.; Novák, P.; Šebesta, O.; Stodůlková, E. Vitamin B2 as a virulence factor in Pseudogymnoascus destructans skin infection. Sci. Rep. 2016, 6, 33200. [Google Scholar] [CrossRef]
- Stodůlková, E.; Císařová, I.; Kolařík, M.; Chudíčková, M.; Novák, P.; Man, P.; Kuzma, M.; Pavlů, B.; Černý, J.; Flieger, M. Biologically active metabolites produced by the basidiomycete Quambalaria cyanescens. PLoS ONE 2015, 10, e0118913. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.; Ryan, P. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]





| Taxon | # Samples | Total Secalonic Acids (mg/g) | Endocrocin, Ergochrysin (µg/g) | EAs (mg/g) |
|---|---|---|---|---|
| C. purpurea—Europe | 13 | 1.66–5.05 (4.87)–17.96 | 0.00–17.51 (29.64)–97.58 | 0.00–2.69 (1.65)–5.17 |
| C. purpurea—NA | 46 | 0.02–1.91 (1.43)–6.33 | 0.00–3.13 (13.75)–84.82 | 0.02–2.96 (1.99)–9.71 |
| C. purpurea—all | 61 | 0.02–2.59 (2.81)–17.96 | 0.00–6.09 (18.96)–97.58 | 0.00–3.03 (2.03)–9.71 |
| C. humidiphila | 15 | 2.56–7.54 (6.04)–20.90 | 0.00–14.96 (16.07)–50.31 | 1.70–4.77 (2.38)–10.16 |
| C. arundinis | 13 | 3.70–9.67 (5.08)–20.23 | 0.00–50.28 (34.18)–126.93 | 2.18–5.32 (2.77)–12.87 |
| C. spartinae | 7 | 1.67–2.29 (0.49)–3.15 | 0.00–56.84 (68.65)–205.13 | 1.55–4.20 (1.42)–6.07 |
| other spp. | 15 | 0.03–2.66 (1.93)–6.50 | 0.00 | 0.00–1.77 (3.20)–11.99 |
| all samples | 111 | 0.02–4.08 (4.39)–20.90 | 0.00–9.01 (24.55)–205.13 | 0.00–3.57 (2.46)–12.87 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flieger, M.; Stodůlková, E.; Wyka, S.A.; Černý, J.; Grobárová, V.; Píchová, K.; Novák, P.; Man, P.; Kuzma, M.; Cvak, L.; et al. Ergochromes: Heretofore Neglected Side of Ergot Toxicity. Toxins 2019, 11, 439. https://doi.org/10.3390/toxins11080439
Flieger M, Stodůlková E, Wyka SA, Černý J, Grobárová V, Píchová K, Novák P, Man P, Kuzma M, Cvak L, et al. Ergochromes: Heretofore Neglected Side of Ergot Toxicity. Toxins. 2019; 11(8):439. https://doi.org/10.3390/toxins11080439
Chicago/Turabian StyleFlieger, Miroslav, Eva Stodůlková, Stephen A. Wyka, Jan Černý, Valéria Grobárová, Kamila Píchová, Petr Novák, Petr Man, Marek Kuzma, Ladislav Cvak, and et al. 2019. "Ergochromes: Heretofore Neglected Side of Ergot Toxicity" Toxins 11, no. 8: 439. https://doi.org/10.3390/toxins11080439
APA StyleFlieger, M., Stodůlková, E., Wyka, S. A., Černý, J., Grobárová, V., Píchová, K., Novák, P., Man, P., Kuzma, M., Cvak, L., Broders, K. D., & Kolařík, M. (2019). Ergochromes: Heretofore Neglected Side of Ergot Toxicity. Toxins, 11(8), 439. https://doi.org/10.3390/toxins11080439







