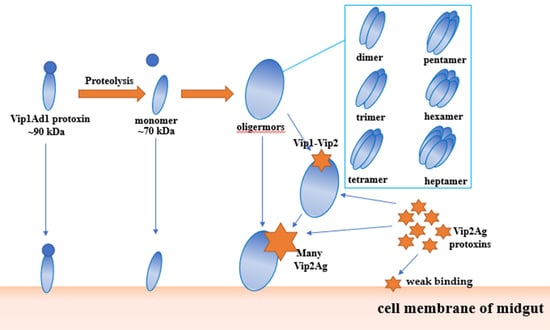
Bacillus thuringiensis Vip1 Functions as a Receptor of Vip2 Toxin for Binary Insecticidal Activity against Holotrichia parallela
Abstract
Share and Cite
Geng, J.; Jiang, J.; Shu, C.; Wang, Z.; Song, F.; Geng, L.; Duan, J.; Zhang, J. Bacillus thuringiensis Vip1 Functions as a Receptor of Vip2 Toxin for Binary Insecticidal Activity against Holotrichia parallela. Toxins 2019, 11, 440. https://doi.org/10.3390/toxins11080440
Geng J, Jiang J, Shu C, Wang Z, Song F, Geng L, Duan J, Zhang J. Bacillus thuringiensis Vip1 Functions as a Receptor of Vip2 Toxin for Binary Insecticidal Activity against Holotrichia parallela. Toxins. 2019; 11(8):440. https://doi.org/10.3390/toxins11080440
Chicago/Turabian StyleGeng, Jianxun, Jian Jiang, Changlong Shu, Zeyu Wang, Fuping Song, Lili Geng, Jiangyan Duan, and Jie Zhang. 2019. "Bacillus thuringiensis Vip1 Functions as a Receptor of Vip2 Toxin for Binary Insecticidal Activity against Holotrichia parallela" Toxins 11, no. 8: 440. https://doi.org/10.3390/toxins11080440
APA StyleGeng, J., Jiang, J., Shu, C., Wang, Z., Song, F., Geng, L., Duan, J., & Zhang, J. (2019). Bacillus thuringiensis Vip1 Functions as a Receptor of Vip2 Toxin for Binary Insecticidal Activity against Holotrichia parallela. Toxins, 11(8), 440. https://doi.org/10.3390/toxins11080440