Efficacy of Divinylbenzenic Resin in Removing Indoxyl Sulfate and P-cresol Sulfate in Hemodialysis Patients: Results from an In Vitro Study and an In Vivo Pilot Trial (xuanro4-Nature 3.2)
Abstract
:1. Introduction
2. Results
2.1. Removal of IS and PCS by DVB-PVP and Cellulose Resins: In Vitro Study
2.2. Clinical Trial
2.3. Effects of Synbiotic on IS and PCS Plasma Levels
2.4. Effects of the Combined Synbiotic with Innovative Dialysis Treatment on IS and PCS Plasma Levels
2.5. Effects of the Innovative Dialysis Treatment on IS and PCS Plasma Levels
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cellulose and DVB-PVP Resins
4.3. In Vitro Adsorption Assay
4.4. Clinical Trial: Patients and Study Design
4.5. Dialysis Modalities
4.6. Blood Analyses
4.7. LC-MS/MS for Quantification of PCS and IS
4.8. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Itoh, Y.; Ezawa, A.; Kikuchi, K.; Tsuruta, Y.; Niwa, T. Protein-bound uremic toxins in hemodialysis patients measured by liquid chromatography/tandem mass spectrometry and their effects on endothelial ROS production. Anal. Bioanal. Chem. 2012, 403, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Ezawa, A.; Kikuchi, K.; Tsuruta, Y.; Niwa, T. Correlation between Serum Levels of Protein-Bound Uremic Toxins in Hemodialysis Patients Measured by LC/MS/MS. Mass Spectrom. (Tokyo, Japan) 2013, 2, S0017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pretorius, C.J.; McWhinney, B.C.; Sipinkoski, B.; Johnson, L.A.; Rossi, M.; Campbell, K.L.; Ungerer, J.P.J. Reference ranges and biological variation of free and total serum indoxyl- and p-cresyl sulphate measured with a rapid UPLC fluorescence detection method. Clin. Chim. Acta 2013, 419, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Liabeuf, S.; Drüeke, T.B.; Massy, Z.A. Protein-bound uremic toxins: New insight from clinical studies. Toxins 2011, 3, 911–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.J.; Wu, C.J.; Pan, C.F.; Chen, Y.C.; Sun, F.J.; Chen, H.H. Serum protein-bound uraemic toxins and clinical outcomes in haemodialysis patients. Nephrol. Dial. Transplant. 2010, 25, 3693–3700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.-J.; Pan, C.-F.; Liu, H.-L.; Chuang, C.-K.; Jayakumar, T.; Wang, T.-J.; Chen, H.-H.; Wu, C.-J. The role of protein-bound uremic toxins on peripheral artery disease and vascular access failure in patients on hemodialysis. Atherosclerosis 2012, 225, 173–179. [Google Scholar] [CrossRef]
- Lin, C.J.; Chuang, C.K.; Jayakumar, T.; Liu, H.L.; Pan, C.F.; Wang, T.J.; Chen, H.H.; Wu, C.J. Serum p-cresyl sulfate predicts cardiovascular disease and mortality in elderly hemodialysis patients. Arch. Med. Sci. 2013, 9, 662–668. [Google Scholar] [CrossRef]
- Cosola, C.; Rocchetti, M.T.; Cupisti, A.; Gesualdo, L. Microbiota metabolites: Pivotal players of cardiovascular damage in chronic kidney disease. Pharmacol. Res. 2018, 130, 132–142. [Google Scholar] [CrossRef]
- Lin, C.J.; Wu, V.; Wu, P.C.; Wu, C.J. Meta-analysis of the associations of p-cresyl sulfate (PCS) and indoxyl sulfate (IS) with cardiovascular events and all-cause mortality in patients with chronic renal failure. PLoS ONE 2015, 10, e0132589. [Google Scholar] [CrossRef]
- Wu, I.W.; Hsu, K.H.; Hsu, H.J.; Lee, C.C.; Sun, C.Y.; Tsai, C.J.; Wu, M.S. Serum free p-cresyl sulfate levels predict cardiovascular and all-cause mortality in elderly hemodialysis patients-A prospective cohort study. Nephrol. Dial. Transplant. 2012, 27, 1169–1175. [Google Scholar] [CrossRef] [Green Version]
- Lesaffer, G.; De Smet, R.; Lameire, N.; Dhondt, A.; Duym, P.V.R. Intradialytic removal of protein-bound uraemic toxins: Role of solute characteristics and of dialyser membrane. Nephrol. Dial. Transplant. 2000, 15, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Panichi, V.; Rocchetti, M.T.; Scatena, A.; Rosati, A.; Migliori, M.; Pizzarelli, F.; Gesualdo, L.; Giusti, R.; Ferro, G.; Malagnino, E.; et al. Long term variation of serum levels of uremic toxins in patients treated by post-dilution high volume on-line hemodiafiltration in comparison to standard low-flux bicarbonate dialysis: Results from the REDERT study. J. Nephrol. 2017, 30, 583–591. [Google Scholar] [CrossRef]
- Sirich, T.L.; Luo, F.J.G.; Plummer, N.S.; Hostetter, T.H.; Meyer, T.W. Selectively increasing the clearance of protein-bound uremic solutes. Nephrol. Dial. Transplant. 2012, 27, 1574–1579. [Google Scholar] [CrossRef]
- Basile, C.; Libutti, P.; Di Turo, A.L.; Casino, F.G.; Vernaglione, L.; Tundo, S.; Maselli, P.; De Nicol, E.V.; Ceci, E.; Teutonico, A.; et al. Removal of uraemic retention solutes in standard bicarbonate haemodialysis and long-hour slow-flow bicarbonate haemodialysis. Nephrol. Dial. Transplant. 2011, 26, 1296–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornelis, T.; Eloot, S.; Vanholder, R.; Glorieux, G.; Van Der Sande, F.M.; Scheijen, J.L.; Leunissen, K.M.; Kooman, J.P.; Schalkwijk, C.G. Protein-bound uraemic toxins, dicarbonyl stress and advanced glycation end products in conventional and extended haemodialysis and haemodiafiltration. Nephrol. Dial. Transplant. 2015, 30, 1395–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krieter, D.H.; Hackl, A.; Rodriguez, A.; Chenine, L.; Moragues, H.L.; Lemke, H.D.; Wanner, C.; Canaud, B. Protein-bound uraemic toxin removal in haemodialysis and post-dilution haemodiafiltration. Nephrol. Dial. Transplant. 2010, 25, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Marquez, I.O.; Tambra, S.; Luo, F.Y.; Li, Y.; Plummer, N.S.; Hostetter, T.H.; Meyer, T.W. Contribution of residual function to removal of protein-bound solutes in hemodialysis. Clin. J. Am. Soc. Nephrol. 2011, 6, 290–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, S.; Kazama, J.J.; Omori, K.; Matsuo, K.; Takahashi, Y.; Kawamura, K.; Matsuto, T.; Watanabe, H. Continuous Reduction of Protein- Bound Uraemic Toxins with Improved Oxidative Stress by Using the Oral Charcoal Adsorbent AST-120 in Haemodialysis Patients. Sci. Rep. 2015, 5, 14381. [Google Scholar] [CrossRef]
- Grandi, F.; Bolasco, P.; Palladino, G.; Sereni, L.; Caiazzo, M.; Atti, M.G.P. Adsorption in Extracorporeal Blood Purification: How to Enhance Solutes Removal Beyond Diffusion and Convection. In Hemodialysis; Suzuki, H., Ed.; InTech: Rijeka, Croatia, 2013. [Google Scholar]
- Pomarè, D.; Ankawi, G.; Lorenzin, A.; Neri, M.; Caprara, C.; Ronco, C. Biocompatibility and Cytotoxic Evaluation of New Sorbent Cartridges for Blood Hemoperfusion. Blood Purif. 2018, 46, 187–195. [Google Scholar]
- Yamamoto, S.; Sato, M.; Sato, Y.; Wakamatsu, T.; Takahashi, Y.; Iguchi, A.; Omori, K.; Suzuki, Y.; Ei, I. Adsorption of Protein-Bound Uremic Toxins Through Direct Hemoperfusion With Hexadecyl-Immobilized Cellulose Beads in Patients Undergoing Hemodialysis. Artif. Organs 2017, 42, 88–93. [Google Scholar] [CrossRef]
- Sartori, M.; Sharma, A.; Neri, M.; Garzotto, F.; Nalesso, F.; Giavarina, D.; Zancato, M.R.C. New option for the treatment of hyperbilirubinemia: In vitro direct hemoperfusion with the Lixelle S-35. Int. J. Artif. Organs 2014, 37, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Gemelli, C.; Cuoghi, A.; Magnani, S.; Atti, M.; Ricci, D.; Siniscalchi, A.; Mancini, E.F.S. Removal of Bilirubin with a New Adsorbent System: In Vitro Kinetics. Blood Purif. 2019, 47, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Winchester, J.F.; Kellum, J.A.; Ronco, C. Sorbents in acute renal failure and the systemic inflammatory response syndrome. Blood Purif. 2003, 21, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Friesecke, S.; Stecher, S.; Gross, S.; Felix, S.B.N.A. Extracorporeal cytokine elimination as rescue therapy in refractory septic shock: A prospective single-center study. J. Artif. Organs 2017, 20, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Cosola, C.; Rocchetti, M.T.; Sabatino, A.; Fiaccadori, E.; Di Iorio, B.R.; Gesualdo, L. Microbiota issue in CKD: How promising are gut-targeted approaches? J. Nephrol. 2019, 32, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Takayama, F.; Taki, K.N.T. Bifidobacterium in gastro-resistant seamless capsule reduces serum levels of indoxyl sulfate in patients on hemodialysis. Am. J. Kidney Dis. 2003, 41, S142–S145. [Google Scholar] [CrossRef]
- Eidi, F.; Poor-Reza Gholi, F.; Ostadrahimi, A.; Dalili, N.; Samadian, F.B.A. Effect of Lactobacillus Rhamnosus on serum uremic toxins (phenol and P-Cresol) in hemodialysis patients: A double blind randomized clinical trial. Clin. Nutr. ESPEN 2018, 28, 158–164. [Google Scholar] [CrossRef]
- Borges, N.A.; Carmo, F.L.; Stockler-Pinto, M.B.; de Brito, J.S.; Dolenga, C.J.; Ferreira, D.C.; Nakao, L.S.; Rosado, A.; Fouque, D.; Mafra, D. Probiotic Supplementation in Chronic Kidney Disease: A Double-blind, Randomized, Placebo-controlled Trial. J. Ren. Nutr. 2018, 28, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Meijers, B.K.I.; De Preter, V.; Verbeke, K.; Vanrenterghem, Y.E.P. P-Cresyl sulfate serum concentrations in haemodialysis patients are reduced by the prebiotic oligofructose-enriched inulin. Nephrol. Dial. Transplant. 2010, 25, 219–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esgalhado, M.; Kemp, J.A.; Azevedo, R.; Paiva, B.R.; Stockler-Pinto, M.B.; Dolenga, C.J.; Borges, N.A.; Nakao, L.S.M.D. Could resistant starch supplementation improve inflammatory and oxidative stress biomarkers and uremic toxins levels in hemodialysis patients? A pilot randomized controlled trial. Food Funct. 2018, 9, 6508–6516. [Google Scholar] [CrossRef]
- Nakabayashi, I.; Nakamura, M.; Kawakami, K.; Ohta, T.; Kato, I.; Uchida, K.; Yoshida, M. Effects of synbiotic treatment on serum level of p-cresol in haemodialysis patients: A preliminary study. Nephrol. Dial. Transplant. 2011, 26, 1094–1098. [Google Scholar] [CrossRef] [Green Version]
- Donati, G.; La Manna, G.; Cianciolo, G. Extracorporeal detoxification for hepatic failure using molecular adsorbent recirculating system: Depurative efficiency and clinical results in a long-term follow-up. Artif. Organs 2014, 38, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Bonello, M.; Bordoni, V.R.Z.; D’Intini, V.; Bellomo, R.L.N. Extracorporeal therapies in non-renal disease: Treatment of sepsis and the peak concentration hypothesis. Blood Purif. 2004, 22, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, H.; Nakamoto, H.; Okada, H. Newly developed immobilized polymyxin B fibers improve the survival of patients with sepsis. Blood Purif. 2001, 19, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Winchester, J.F.; Ronco, C. Sorbent Augmented Hemodialysis Systems: Are We There Yet? J. Am. Soc. Nephrol. 2010, 21, 209–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandeman, S.R.; Howell, C.A.; Phillips, G.J.; Zheng, Y.; Standen, G.; Pletzenauer, R.; Davenport, A.; Basnayake, K.; Boyd, O.; Holt, S.; et al. An adsorbent monolith device to augment the removal of uraemic toxins during haemodialysis. J. Mater. Sci. Mater. Med. 2014, 25, 1589–1597. [Google Scholar] [CrossRef] [Green Version]
- Pavlenko, D.; Giasaf, D.; Charalambopoul, G.; van Geffen, E.; Gerritsen, K.G.F. Carbon Adsorbents With Dual Porosity for Efficient Removal of Uremic Toxins and Cytokines from Human Plasma. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.W.; Peattie, J.W.; Miller, J.D.; Dinh, D.C.; Recht, N.S.; Walther, J.L.H.T. Increasing the clearance of protein-bound solutes by addition of a sorbent to the dialysate. J. Am. Soc. Nephrol. 2007, 18, 868–874. [Google Scholar] [CrossRef]
- Eloot, S.; Schneditz, D.; Cornelis, T.; Van Biesen, W.; Glorieux, G.; Dhondt, A.; Kooman, J.; Vanholder, R. Protein-bound uremic toxin profiling as a tool to optimize hemodialysis. PLoS ONE 2016, 11, e0147159. [Google Scholar] [CrossRef] [Green Version]
- Di Iorio, B.R.; Rocchetti, M.T.; De Angelis, M.; Cosola, C.; Marzocco, S.; Di Micco, L.; di Bari, I.; Accetturo, M.; Vacca, M.; Gobbetti, M.; et al. Nutritional Therapy Modulates Intestinal Microbiota and Reduces Serum Levels of Total and Free Indoxyl Sulfate and P-Cresyl Sulfate in Chronic Kidney Disease (Medika Study). J. Clin. Med. 2019, 8, 1424. [Google Scholar] [CrossRef] [Green Version]
- Wang, I.K.; Wu, Y.Y.; Yang, Y.F.; Ting, I.W.; Lin, C.C.; Yen, T.H.; Chen, J.H.; Wang, C.H.; Huang, C.C.; Lin, H.C. The effect of probotics on serum levels of cytokine and endotoxin in peritoneal dialysis patients: A randomised, double-blind, placebo-controlled trial. Benef. Microbes 2015, 6, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Mora, J.; Martínez-Hernández, N.E.; Martín del Campo-López, F.; Viramontes-Hörner, D.; Vizmanos-Lamotte, B.; Muñoz-Valle, J.F.; García-García, G.; Parra-Rojas, I.C.-A.N. Effects of a symbiotic on gut microbiota in Mexican patients with end-stage renal disease. J. Ren. Nutr. 2014, 24, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, A.; Zarrati Mojarrad, M.; Bahmani, F.; Taghizadeh, M.; Ramezani, M.; Tajabadi-Ebrahimi, M.; Jafari, P.; Esmaillzadeh, A.A.Z. Probiotic supplementation in diabetic hemodialysis patients has beneficial metabolic effects. Kidney Int. 2017, 91, 435–442. [Google Scholar] [CrossRef]
- Clark, W.R.; La, G.; Ronco, C. Extracorporeal Sorbent Technologies: Basic Concepts and Clinical Application. Contrib. Nephrol. 2017, 190, 43–57. [Google Scholar] [PubMed]
- Gruda, M.C.; Ruggeberg, K.G.; O’Sullivan, P.; Guliashvili, T.; Scheirer, A.R.; Golobish, T.D.; Capponi, V.J.; Chan, P.P. Broad adsorption of sepsis-related PAMP and DAMP molecules, mycotoxins, and cytokines from whole blood using CytoSorb® sorbent porous polymer beads. PLoS ONE 2018, 13, e0191676. [Google Scholar] [CrossRef] [PubMed]
- Schädler, D.; Pausch, C.; Heise, D.; Meier-Hellmann, A.; Brederlau, J.; Weiler, N.; Marx, G.; Putensen, C.; Spies, C.; Jörres, A.; et al. The effect of a novel extracorporeal cytokine hemoadsorption device on IL-6 elimination in septic patients: A randomized controlled trial. PLoS ONE 2017, 12, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deltombe, O.; Van Biesen, W.; Glorieux, G.; Massy, Z.; Dhondt, A.; Eloot, S. Exploring protein binding of uremic toxins in patients with different stages of chronic kidney disease and during hemodialysis. Toxins 2015, 7, 3933–3946. [Google Scholar] [CrossRef] [PubMed] [Green Version]

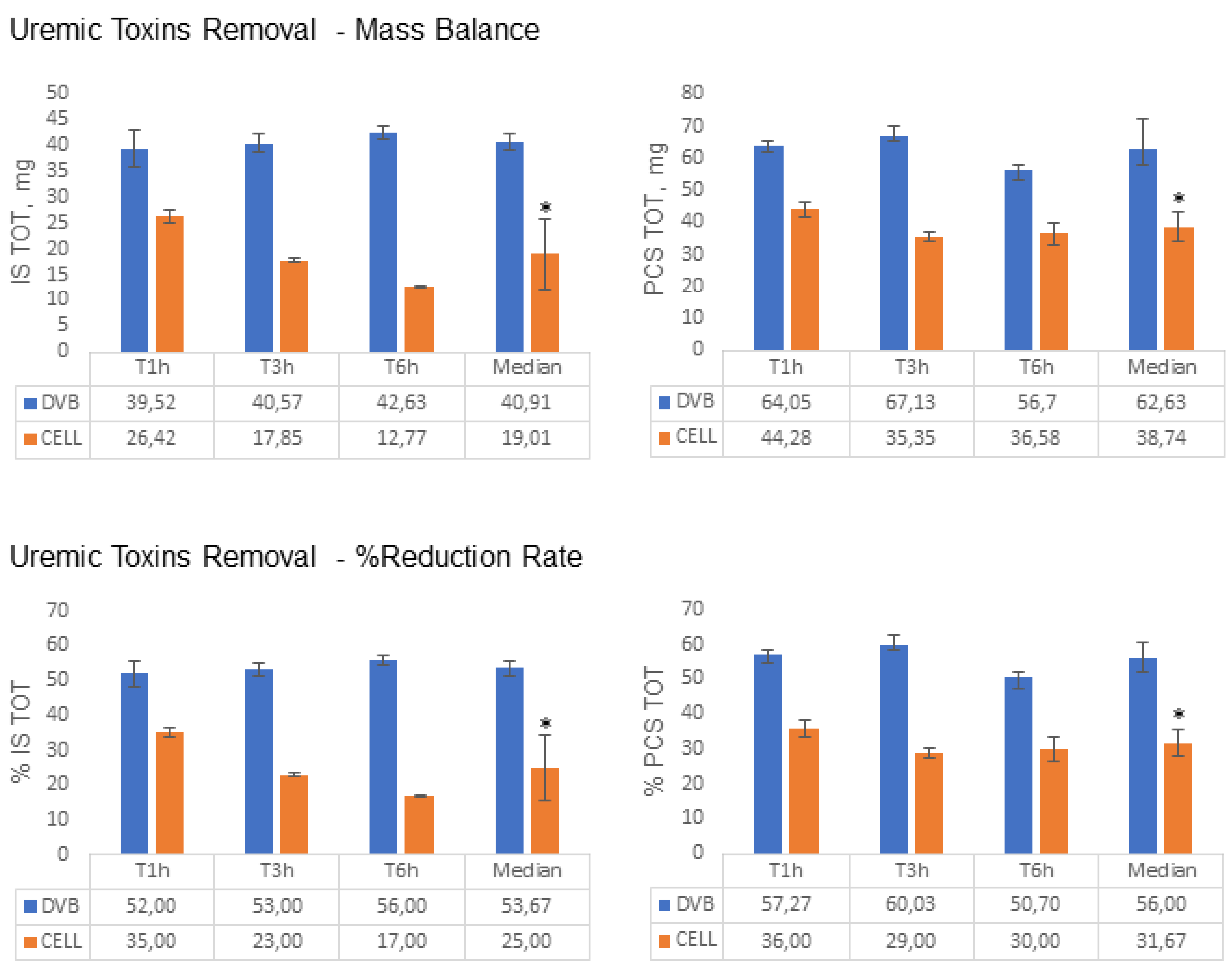



| Solutes | Total Amount | |||
|---|---|---|---|---|
| At start | Removed by DVB-PVP | Removed by Cellulose | ||
| Indoxyl sulfate MB, mg-(RR%) | Total | 75.4 | 40.9-(54%) | 19.1-(25%) |
| Free | 11.8 | 8.2-(66%) | 1.7-(15%) | |
| p-cresyl sulfate MB, mg-(RR%) | Total | 115.7 | 62.6-(56%) | 38.7-(31%) |
| Free | 14.7 | 10.4-(70%) | 2.3-(15%) | |
| BSA MB, g-(RR%) | 126 | 17.7-(14%) | 10.6-(8%) | |
| Patients Data | Study Population | Controls | ||||
|---|---|---|---|---|---|---|
| Baseline (T0) | BHD (T7w) | DVB-PVP HD (T8w) | Baseline (T0) | BHD (T7w) | DVB-PVP HD (T8w) | |
| Patients treated with Synbiotic (n = 6) | Patients treated with Placebo (n = 5) | |||||
| Age, years | 56 ± 7 | 52 ± 5 | ||||
| Male, n(%) | 5 (83%) | 4 (80%) | ||||
| SCr, mg/dL | 8.5 ± 1.2 | 8.1 ± 1.5 | 8.4 ± 1.1 | 9.4 ± 1.5 | 8.5 ± 2.4 | 7.5 ± 2.7§ |
| Albumin, g/dL | 3.6 ± 0.4 | 3.7 ± 0.2 | 3.3 ± 0.4* | 3.4 ± 0.4 | 3.3 ± 0.4 | 3.0 ± 0.3*,§ |
| Azotemia mg/dL | 132 ± 44 | 125 ± 20 | 114 ± 19 | 120 ± 33 | 114 ± 42 | 100 ± 54 |
| Glycemia, mg/dL | 51 ± 19 | 48 ± 30 | 79 ± 16*,§ | 72 ± 12 | 80 ± 20 | 84 ± 23 |
| CPR mg/L | 6.4 ± 5.1 | 3.1 ± 0.4 | 5.8 ± 3.9 | 10 ± 14 | 10 ± 9 | 10 ± 7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocchetti, M.T.; Cosola, C.; di Bari, I.; Magnani, S.; Galleggiante, V.; Scandiffio, L.; Dalfino, G.; Netti, G.S.; Atti, M.; Corciulo, R.; et al. Efficacy of Divinylbenzenic Resin in Removing Indoxyl Sulfate and P-cresol Sulfate in Hemodialysis Patients: Results from an In Vitro Study and an In Vivo Pilot Trial (xuanro4-Nature 3.2). Toxins 2020, 12, 170. https://doi.org/10.3390/toxins12030170
Rocchetti MT, Cosola C, di Bari I, Magnani S, Galleggiante V, Scandiffio L, Dalfino G, Netti GS, Atti M, Corciulo R, et al. Efficacy of Divinylbenzenic Resin in Removing Indoxyl Sulfate and P-cresol Sulfate in Hemodialysis Patients: Results from an In Vitro Study and an In Vivo Pilot Trial (xuanro4-Nature 3.2). Toxins. 2020; 12(3):170. https://doi.org/10.3390/toxins12030170
Chicago/Turabian StyleRocchetti, Maria Teresa, Carmela Cosola, Ighli di Bari, Stefania Magnani, Vanessa Galleggiante, Letizia Scandiffio, Giuseppe Dalfino, Giuseppe Stefano Netti, Mauro Atti, Roberto Corciulo, and et al. 2020. "Efficacy of Divinylbenzenic Resin in Removing Indoxyl Sulfate and P-cresol Sulfate in Hemodialysis Patients: Results from an In Vitro Study and an In Vivo Pilot Trial (xuanro4-Nature 3.2)" Toxins 12, no. 3: 170. https://doi.org/10.3390/toxins12030170
APA StyleRocchetti, M. T., Cosola, C., di Bari, I., Magnani, S., Galleggiante, V., Scandiffio, L., Dalfino, G., Netti, G. S., Atti, M., Corciulo, R., & Gesualdo, L. (2020). Efficacy of Divinylbenzenic Resin in Removing Indoxyl Sulfate and P-cresol Sulfate in Hemodialysis Patients: Results from an In Vitro Study and an In Vivo Pilot Trial (xuanro4-Nature 3.2). Toxins, 12(3), 170. https://doi.org/10.3390/toxins12030170





