Using Microcystin Gene Copies to Determine Potentially-Toxic Blooms, Example from a Shallow Eutrophic Lake Peipsi
Abstract
:1. Introduction
2. Results
2.1. Environmental Variables
2.2. The Composition of the Cyanobacterial Community Based on Microscopy
2.3. Abundance of mcyE Genes
2.4. Microcystin- Concentrations and Variants
2.5. Environmental Variables that Favour Potentially Toxic Cyanobacteria Genotypes
3. Discussion
3.1. MC Concentration Versus MC Variants
3.2. Toxin Quota per McyE Gene
4. Materials and Methods
4.1. Study Site and Field Surveys
4.2. Microscopic Analysis
4.3. Detection of Microcystins (MCs)
4.4. Cultivation of the Strains Used as External Standards
4.5. DNA Extraction
4.6. Detection and Quantification of mcyE Genes in Environmental Samples
4.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Characteristic | Peipsi s.s. * | Lämmijärv * | Pihkva ** |
|---|---|---|---|
| Secchi depth, m | 1.8 (1;2.8) | 1 (0.6;1.8) | 0.61 (0.4;0.8) |
| TP, mg/m3 | 41 (21;80) | 69 (40;130) | 133 (57;201) |
| SRP, mg/m3 | 8.6 (2;25) | 11.8 (4;30) | 25.6 (30;110) |
| TN, mg/m3 | 672 (430;1500) | 868 (600;1400) | 1190 (948;1525) |
| NO3-, mg/m3 | 46 (10;240) | 42 (10;300) | 58 (30;110) |
| NO2-, mg/m3 | 1.7 (1;6) | 1.8 (1;4) | 2.4 (1.9;4.4) |
| NH4+, mg/m3 | 1.7 (1;6) | 28 (10;99) | 25 (15;33) |
| Chl a, mg/m3 | 15.8 (5.6;40.5) | 30 (9.6;70.5) | 68 (33.7;123.5) |
| OECD classification | Eutrophic | Eutrophic/hypertrophic | Hypertrophic |
| Target Gene | Primer | Primer Reference | PCR Program |
|---|---|---|---|
| Cyanobacterial 16S rRNA | CYA359F CYA781R | [90] | 95 °C 5 min; 35 cycles: 95 °C 60s; 60 °C 60 s; 72 °C 60 s and 72 °C 10 min |
| Microcystis mcyE | 127F 247R 186P | [76] | 95 °C 15 min; 40 cycles: 95 °C 15 s; 62 °C 60 s; |
| Dolichospermum mcyE | 611F 737R 672P | [76] | 95 °C 15 min; 40 cycles: 95 °C 15 s; 62 °C 60 s; |
| Planktothrix mcyE | 664F 744R 670P | Current study, Table S3 | 95 °C 15 min; 40 cycles: 95 °C 15 s; 60 °C 60 s; |
References
- Chorus, I.; Bartram, J. Introduction. In Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; E & FN Spon: London, UK, 1999; Volume 1, pp. 14–24. [Google Scholar]
- Jeppesen, E.; Meerhoff, M.; Davidson, T.A.; Trolle, D.; SondergaarD, M.; Lauridsen, T.L.; Beklioglu, M.; Brucet Balmaña, S.; Volta, P.; González-Bergonzoni, I.; et al. Climate change impacts on lakes: An integrated ecological perspective based on a multi-faceted approach, with special focus on shallow lakes. J. Limnol. 2014, 73, 88–111. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, C.S. The Ecology of Phytoplankton; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Otten, T.G. Harmful cyanobacterial blooms: Causes, consequences, and controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Jöhnk, K.D.; Huisman, J.; Sharples, J.; Sommeijer, B.; Visser, P.M.; Stroom, J.M. Summer heatwaves promote blooms of harmful cyanobacteria. Glob. Chang. Biol. 2008, 14, 495–512. [Google Scholar] [CrossRef] [Green Version]
- Huber, V.; Wagner, C.; Gerten, D.; Adrian, R. To bloom or not to bloom: Contrasting responses of cyanobacteria to recent heat waves explained by critical thresholds of abiotic drivers. Oecologia 2012, 169, 245–256. [Google Scholar] [CrossRef]
- Paerl, H.W.; Hall, N.S.; Peierls, B.L.; Rossignol, K.L. Evolving Paradigms and Challenges in Estuarine and Coastal Eutrophication Dynamics in a Culturally and Climatically Stressed World. Estuaries Coasts 2014, 37, 243–258. [Google Scholar] [CrossRef]
- Woznicki, S.A.; Nejadhashemi, A.P.; Tang, Y.; Wang, L. Large-scale climate change vulnerability assessment of stream health. Ecol. Indic. 2016, 69, 578–594. [Google Scholar] [CrossRef] [Green Version]
- Le Moal, M.; Gascuel-Odoux, C.; Ménesguen, A.; Souchon, Y.; Étrillard, C.; Levain, A.; Moatar, F.; Pannard, A.; Souchu, P.; Lefebvre, A.; et al. Eutrophication: A new wine in an old bottle? Sci. Total. Environ. 2019, 651, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Kosten, S.; Huszar, V.L.M.; Bécares, E.; Costa, L.S.; Donk, E.; Hansson, L.-A.; Jeppesen, E.; Kruk, C.; Lacerot, G.; Mazzeo, N.; et al. Warmer climates boost cyanobacterial dominance in shallow lakes. Glob. Chang. Biol. 2012, 18, 118–126. [Google Scholar] [CrossRef]
- Sivonen, K.; Jones, G. Cyanobacterial toxins. In Toxic Cyanobacteria in Water: A Guide to their Public Health Consequences, Monitoring and Management; E & FN Spon: London, UK, 1999; Volume 1, pp. 43–112. [Google Scholar]
- Hudnell, H.K. The state of U.S. freshwater harmful algal blooms assessments, policy and legislation. Toxicon 2010, 55, 1024–1034. [Google Scholar] [CrossRef]
- Sieroslawska, A.; Rymuszka, A.; Velisek, J.; Pawlik-Skowrońska, B.; Svobodova, Z.; Skowroński, T. Effects of microcystin-containing cyanobacterial extract on hematological and biochemical parameters of common carp (Cyprinus carpio L.). Fish Physiol. Biochem. 2012, 38, 1159–1167. [Google Scholar] [CrossRef] [Green Version]
- Metcalf, J.S.; Codd, G.A. Cyanobacterial Toxins (Cyanotoxins) in Water; Foundation for Water Research: Marlow, UK, 2014. [Google Scholar]
- Dodds, W.K.; Bouska, W.W.; Eitzmann, J.L.; Pilger, T.J.; Pitts, K.L.; Riley, A.J.; Schloesser, J.T.; Thornbrugh, D.J. Eutrophication of U.S. freshwaters: Analysis of potential economic damages. Environ. Sci. Technol. 2009, 43, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Steffensen, D.A. Economic cost of cyanobacterial blooms. In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Hudnell, H.K., Ed.; Springer: New York, NY, USA, 2008; pp. 855–865. ISBN 978-0-387-75865-7. [Google Scholar]
- Carmichael, W.W. Health Effects of Toxin-Producing Cyanobacteria: “The CyanoHABs”. Hum. Ecol. Risk Assess. Int. J. 2001, 7, 1393–1407. [Google Scholar] [CrossRef]
- Paerl, H.W. Mitigating harmful cyanobacterial blooms in a human- and climatically-impacted world. Life 2014, 4, 988–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freitas, E.C.; Pinheiro, C.; Rocha, O.; Loureiro, S. Can mixtures of cyanotoxins represent a risk to the zooplankton? The case study of Daphnia magna Straus exposed to hepatotoxic and neurotoxic cyanobacterial extracts. Harmful Algae 2014, 31, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Merel, S.; Walker, D.; Chicana, R.; Snyder, S.; Baurès, E.; Thomas, O. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ. Int. 2013, 59, 303–327. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Huisman, J. Climate. Blooms like it hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef] [Green Version]
- Søndergaard, M.; Jensen, J.P.; Jeppesen, E. Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia 2003, 506, 135–145. [Google Scholar] [CrossRef]
- Salmaso, N.; Bernard, C.; Humbert, J.-F.C.; Akçaalan, R.; Albay, M.; Ballot, A.; Catherine, A.; Fastner, J.; Häggqvist, K.; Horecká, M.; et al. Basic guide to detection and monitoring of potentially toxic cyanobacteria. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; John Wiley & Sons: Chichester, UK, 2017; Volume 6, pp. 46–69. [Google Scholar]
- Downing, J.A.; Watson, S.B.; McCauley, E. Predicting Cyanobacteria dominance in lakes. Can. J. Fish Aquat. Sci. 2001, 58, 1905–1908. [Google Scholar] [CrossRef]
- Cózar, A.; Gálvez, J.A.; Hull, V.; García, C.M.; Loiselle, S.A. Sediment resuspension by wind in a shallow lake of Esteros del Ibera (Argentina): A model based on turbidimetry. Ecol. Model. 2005, 186, 63–76. [Google Scholar] [CrossRef]
- Bormans, M.; Maršálek, B.; Jančula, D. Controlling internal phosphorus loading in lakes by physical methods to reduce cyanobacterial blooms: A review. Aquat. Ecol. 2016, 50, 407–422. [Google Scholar] [CrossRef]
- Paerl, H.W.; Xu, H.; McCarthy, M.J.; Zhu, G.; Qin, B.; Li, Y.; Gardner, W.S. Controlling harmful cyanobacterial blooms in a hyper-eutrophic lake (Lake Taihu, China): The need for a dual nutrient (N & P) management strategy. Water Res. 2011, 45, 1973–1983. [Google Scholar] [PubMed]
- Molot, L.A.; Watson, S.B.; Creed, I.F.; Trick, C.G.; Mccabe, S.K.; Verschoor, M.J.; Sorichetti, R.J.; Powe, C.; Venkiteswaran, J.J.; Schiff, S.L. A novel model for cyanobacteria bloom formation: The critical role of anoxia and ferrous iron. Freshw. Biol. 2014, 59, 1323–1340. [Google Scholar] [CrossRef]
- Paerl, H.W.; Gardner, W.S.; Havens, K.E.; Joyner, A.R.; McCarthy, M.J.; Newell, S.E.; Qin, B.; Scott, J.T. Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae 2016, 54, 213–222. [Google Scholar] [CrossRef] [Green Version]
- Codd, G.A.; Meriluoto, J.; Metcalf, J.S. Introduction: Cyanobacteria, Cyanotoxins, Their Human Impact, and Risk Management. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Meriluoto, J., Spoof, L., Codd, G.A., Eds.; John Wiley & Sons, Ltd: Chichester, UK, 2016; Volume 51, pp. 1–8. [Google Scholar]
- Utermöhl, H. Zur vervollkommnung der quantitativen phytoplankton-methodik: Mit 1 Tabelle und 15 abbildungen im Text und auf 1 Tafel. Mitt. Int. Ver. Angew. Limnol. 1958, 9, 1–38. [Google Scholar] [CrossRef]
- Pacheco, A.B.F.; Guedes, I.A.; Azevedo, S.M.F.O. Is qPCR a reliable indicator of cyanotoxin risk in freshwater? Toxins 2016, 8, 172. [Google Scholar] [CrossRef] [Green Version]
- Tamm, M.; Ligi, M.; Panksep, K.; Teeveer, K.; Freiberg, R.; Laas, P.; Paavel, B.; Kutser, T.; Reinart, A.; Tõnno, I.; et al. Boosting the monitoring of phytoplankton in optically complex coastal waters by combining pigment-based chemotaxonomy and in situ radiometry. Ecol. Indic. 2019, 97, 329–340. [Google Scholar] [CrossRef]
- Hisbergues, M.; Christiansen, G.; Rouhiainen, L.; Sivonen, K.; Börner, T. PCR-based identification of microcystin-producing genotypes of different cyanobacterial genera. Arch. Microbiol. 2003, 180, 402–410. [Google Scholar] [CrossRef]
- Rantala, A.; Rajaniemi-Wacklin, P.; Lyra, C.; Lepistö, L.; Rintala, J.; Mankiewicz-Boczek, J.; Sivonen, K. Detection of microcystin-producing cyanobacteria in Finnish lakes with genus-specific microcystin synthetase gene E (mcyE) PCR and associations with environmental factors. Appl. Environ. Microbiol 2006, 72, 6101–6110. [Google Scholar] [CrossRef] [Green Version]
- Baxa, D.V.; Kurobe, T.; Ger, K.A.; Lehman, P.W.; Teh, S.J. Estimating the abundance of toxic Microcystis in the San Francisco Estuary using quantitative real-time PCR. Harmful Algae 2010, 9, 342–349. [Google Scholar] [CrossRef]
- Jaani, A. The location, size and general characterization of Lake Peipsi and its catchment area. In Lake Peipsi: Meteorology, Hydrology, Hydrochemistry; Nõges, T., Ed.; Sulemees Publishers: Tartu, Estonia, 2001; pp. 10–17. [Google Scholar]
- Kangur, K.; Möls, T. Changes in spatial distribution of phosphorus and nitrogen in the large north-temperate lowland Lake Peipsi (Estonia/Russia). Hydrobiologia 2008, 599, 31–39. [Google Scholar] [CrossRef]
- Noges, T.; Tuvikene, L.; Noges, P. Contemporary trends of temperature, nutrient loading, and water quality in large Lakes Peipsi and Vortsjärv, Estonia. Aquat Ecosyst Health Manag 2010, 13, 143–153. [Google Scholar] [CrossRef]
- Loigu, E.; Leisk, Ü.; Iital, A.; Pachel, K. Pollution load and water quality of the Lake Peipsi basin. In Peipsi; Eesti Loodusfoto: Tartu, Estonia, 2008; Volume 2008, pp. 179–199. [Google Scholar]
- Hydrobiological Monitoring of Lake Peipsi. Available online: https://kese.envir.ee/kese/listPublicReport.action (accessed on 15 January 2020).
- Laugaste, R.; Panksep, K.; Haldna, M. Dominant cyanobacterial genera in Lake Peipsi (Estonia/Russia): Effect of weather and nutrients in summer months. Est. J. Ecol. 2013, 62, 229–243. [Google Scholar] [CrossRef]
- Laugaste, R.; Nõges, T.; Tõnno, I. Vetikad. In Peipsi; Eesti Loodusfoto: Tartu, Estonia, 2008; pp. 251–270. [Google Scholar]
- Laugaste, R.; Haberman, J.; Krause, T.; Salujõe, J. Significant changes in phyto-and zooplankton in L. Peipsi in recent years: What is the underlying reason. Proc. Est. Acad. Sci. Biol. Ecol. 2007, 56, 106–123. [Google Scholar]
- Tammeorg, O.; Niemistö, J.; Möls, T.; Laugaste, R.; Panksep, K.; Kangur, K. Wind-induced sediment resuspension as a potential factor sustaining eutrophication in large and shallow Lake Peipsi. Aquat. Sci. 2013, 75, 559–570. [Google Scholar] [CrossRef]
- Tammeorg, O.; Horppila, J.; Tammeorg, P.; Haldna, M.; Niemistö, J. Internal phosphorus loading across a cascade of three eutrophic basins: A synthesis of short- and long-term studies. Sci. Total. Environ. 2016, 572, 943–954. [Google Scholar] [CrossRef]
- Tanner, R.; Kangur, K.; Spoof, L.; Meriluoto, J. Hepatotoxic cyanobacterial peptides in Estonian freshwater bodies and inshore marine water. Proc. Eston. Acad. Sci. Biol. Ecol. 2005, 54, 40–52. [Google Scholar]
- Merel, S.; Villarín, M.C.; Chung, K.; Snyder, S. Spatial and thematic distribution of research on cyanotoxins. Toxicon 2013, 76, 118–131. [Google Scholar] [CrossRef]
- Meriluoto, J.; Blaha, L.; Bojadzija, G.; Bormans, M.; Brient, L.; Codd, G.A.; Drobac, D.; Faassen, E.J.; Fastner, J.; Hiskia, A.; et al. Toxic cyanobacteria and cyanotoxins in European waters—recent progress achieved through the CYANOCOST Action and challenges for further research. Adv. Oceanogr. Limnol. 2017, 8, 161–178. [Google Scholar] [CrossRef] [Green Version]
- Guedes, I.A.; Pacheco, A.B.F.; Vilar, M.C.P.; Mello, M.M.; Marinho, M.M.; Lurling, M.; Azevedo, S.M.F.O. Intraspecific variability in response to phosphorus depleted conditions in the cyanobacteria Microcystis aeruginosa and Raphidiopsis raciborskii. Harmful Algae 2019, 86, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Halstvedt, C.B.; Rohrlack, T.; Andersen, T.; Skulberg, O.; Edvardsen, B. Seasonal dynamics and depth distribution of Planktothrix spp. in Lake Steinsfjorden (Norway) related to environmental factors. J. Plankton Res. 2007, 29, 471–482. [Google Scholar] [CrossRef] [Green Version]
- Oberhaus, L.; Briand, J.F.; Leboulanger, C.; Jacquet, S.; Humbert, J.F. Comparative effects of the quality and quantity of light and temperature on the growth of Planktothrix agardhii and P. rubescens. J. Phycol. 2007, 43, 1191–1199. [Google Scholar] [CrossRef]
- Istvánovics, V.; Osztoics, A.; Honti, M. Dynamics and ecological significance of daily internal load of phosphorus in shallow Lake Balaton, Hungary. Freshw. Biol. 2004, 49, 232–252. [Google Scholar] [CrossRef]
- Steinman, A.; Chu, X.; Ogdahl, M. Spatial and temporal variability of internal and external phosphorus loads in Mona Lake, Michigan. Aquat. Ecol. 2009, 43, 1–18. [Google Scholar] [CrossRef]
- Nürnberg, G.K. Assessing internal phosphorus load—Problems to be solved. Lake Reserv. Manag. 2009, 25, 419–432. [Google Scholar] [CrossRef]
- Nürnberg, G.K.; LaZerte, B.D. More than 20 years of estimated internal phosphorus loading in polymictic, eutrophic Lake Winnipeg, Manitoba. J. Gt. Lakes Res. 2016, 42, 18–27. [Google Scholar] [CrossRef]
- Tammeorg, O.; Horppila, J.; Möls, T.; Haldna, M.; Laugaste, R.; Niemistö, J. Studies of legacy internal phosphorus load in Lake Peipsi (Estonia/Russia). In Internal Phosphorus Loading in Lakes: Causes, Case Studies and Management; Steinman, A., Spears, B., Eds.; J. Ross Publishing: Plantation, FL, USA, 2019; pp. 187–209. [Google Scholar]
- Hyenstrand, P.; Blomqvist, P.; Pettersson, A. Factors determining cyanobacterial success in aquatic systems: A literature review. Adv. Limnol. 1998, 51, 41–62. [Google Scholar]
- Haldna, M.; Milius, A.; Laugaste, R.; Kangur, K. Nutrients and phytoplankton in Lake Peipsi during two periods that differed in water level and temperature. Hydrobiologia 2008, 599, 3–11. [Google Scholar] [CrossRef]
- Tammeorg, O.; Möls, T.; Kangur, K. Weather conditions influencing phosphorus concentration in the growing period in the large shallow Lake Peipsi (Estonia/Russia). J. Limnol. 2014, 73, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Ye, W.; Liu, X.; Tan, J.; Li, D.; Yang, H. Diversity and dynamics of microcystin—Producing cyanobacteria in China’s third largest lake, Lake Taihu. Harmful Algae 2009, 8, 637–644. [Google Scholar] [CrossRef]
- Otten, T.G.; Xu, H.; Qin, B.; Zhu, G.; Paerl, H.W. Spatiotemporal patterns and ecophysiology of toxigenic microcystis blooms in Lake Taihu, China: Implications for water quality management. Environ. Sci. Technol. 2012, 46, 3480–3488. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Kong, F.; Zhang, M.; Yang, Z.; Shi, X.; Du, M. The dynamics of microcystis genotypes and microcystin production and associations with environmental factors during blooms in Lake Chaohu, China. Toxins 2014, 6, 3238–3257. [Google Scholar] [CrossRef]
- Bartlett, S.L.; Brunner, S.L.; Klump, J.V.; Houghton, E.M.; Miller, T.R. Spatial analysis of toxic or otherwise bioactive cyanobacterial peptides in Green Bay, Lake Michigan. J. Gt. Lakes Res. 2018, 44, 924–933. [Google Scholar] [CrossRef]
- Rinta-Kanto, J.M.; Ouellette, A.J.A.; Boyer, G.L.; Twiss, M.R.; Bridgeman, T.B.; Wilhelm, S.W. Quantification of toxic Microcystis spp. during the 2003 and 2004 blooms in western Lake Erie using quantitative real-time PCR. Environ. Sci. Technol. 2005, 39, 4198–4205. [Google Scholar] [CrossRef]
- Rinta-Kanto, J.M.; Konopko, E.A.; DeBruyn, J.M.; Bourbonniere, R.A.; Boyer, G.L.; Wilhelm, S.W. Lake Erie Microcystis: Relationship between microcystin production, dynamics of genotypes and environmental. Harmful Algae 2009, 8, 665–673. [Google Scholar] [CrossRef]
- Wood, S.A.; Maier, M.Y.; Puddick, J.; Pochon, X.; Zaiko, A.; Dietrich, D.R.; Hamilton, D.P. Trophic state and geographic gradients influence planktonic cyanobacterial diversity and distribution in New Zealand lakes. FEMS Microbiol. Ecol. 2017, 93, fiw234. [Google Scholar] [CrossRef]
- Panksep, K.; Agasild, H.; Tõnno, I.; Nõges, T. Cyanobacteria and Cyanotoxins in a Diet of Zooplankton in Shallow Eutrophic Lakes. (Unpublished; manuscript in preparation).
- Arnaud, C.; Bernard, C.; Spoof, L.; Bruno, M. Microcystins and Nodularins. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Meriluoto, J., Spoof, L., Codd, G.A., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2016; Volume 1, pp. 107–126. [Google Scholar]
- Sivonen, K.; Namikoshi, M.; Luukkainen, R.; Färdig, M.; Rouhiainen, L.; Evans, W.R.; Carmichael, W.W.; Rinehart, K.L.; Niemelä, S.I. Variation of Cyanobacterial Hepatotoxins in Finland. In Detection Methods for Cynobacterial Toxins; Codd, G.A., Jefferies, T.M., Keevil, C.W., Potter, E., Eds.; Woodhead Publishing: Sawston, UK, 1994; pp. 152–154. ISBN 978-1-85573-802-7. [Google Scholar]
- Mantzouki, E.; Lürling, M.; Fastner, J.; de Senerpont Domis, L.; Wilk-Woźniak, E.; Koreivienė, J.; Seelen, L.; Teurlincx, S.; Verstijnen, Y.; Krztoń, W.; et al. Temperature Effects Explain Continental Scale Distribution of Cyanobacterial Toxins. Toxins 2018, 10, 156. [Google Scholar] [CrossRef] [Green Version]
- Mantzouki, E.; Ibelings, B.W. The Principle and Value of the European Multi Lake Survey. Limnol. Ocean. Bull. 2018, 27, 82–86. [Google Scholar] [CrossRef]
- Panksep, K.; Mantzouki, E.; Lurling, M.; Fastner, J.; Visser, P.; Tammert, H.; Ibelings, B.W. A continental scale multilake survey of cyanobacteria, toxin synthetase genes and toxins during a heatwave summer. In Proceedings of the 19th International Conference on harmful Algae (ICHA 2018), Nantes, France, 21–26 October 2018; p. 176. [Google Scholar]
- Sipari, H.; Rantala-Ylinen, A.; Jokela, J.; Oksanen, I.; Sivonen, K. Development of a chip assay and quantitative PCR for detecting microcystin synthetase E gene expression. Appl. Environ. Microbiol. 2010, 76, 3797–3805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christiansen, G.; Molitor, C.; Philmus, B.; Kurmayer, R. Nontoxic strains of cyanobacteria are the result of major gene deletion events induced by a transposable element. Mol. Biol. Evol. 2008, 25, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Rantala, A.; Fewer, D.P.; Hisbergues, M.; Rouhiainen, L.; Vaitomaa, J.; Börner, T.; Sivonen, K. Phylogenetic evidence for the early evolution of microcystin synthesis. Proc. Natl. Acad. Sci. USA 2004, 101, 568–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humbert, J.-F. Molecular tools for the detection of toxigenic Cyanobacteria in natural ecosystems. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Meriluoto, J., Spoof, L., Codd, G.A., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2016; Volume 37, pp. 280–283. ISBN 978-1-119-06876-1. [Google Scholar]
- Horst, G.P.; Sarnelle, O.; White, J.D.; Hamilton, S.K.; Kaul, R.B.; Bressie, J.D. Nitrogen availability increases the toxin quota of a harmful cyanobacterium, Microcystis aeruginosa. Water Res. 2014, 54, 188–198. [Google Scholar] [CrossRef]
- Wood, S.A.; Borges, H.; Puddick, J.; Biessy, L.; Atalah, J.; Hawes, I.; Dietrich, D.R.; Hamilton, D.P. Contrasting cyanobacterial communities and microcystin concentrations in summers with extreme weather events: Insights into potential effects of climate change. Hydrobiologia 2017, 785, 71–89. [Google Scholar] [CrossRef] [Green Version]
- Visser, P.M.; Verspagen, J.M.H.; Sandrini, G.; Stal, L.J.; Matthijs, H.C.P.; Davis, T.W.; Paerl, H.W.; Huisman, J. How rising CO2 and global warming may stimulate harmful cyanobacterial blooms. Harmful Algae 2016, 54, 145–159. [Google Scholar] [CrossRef] [Green Version]
- Cremona, F.; Tuvikene, L.; Haberman, J.; Nõges, P.; Nõges, T. Factors controlling the three-decade long rise in cyanobacteria biomass in a eutrophic shallow lake. Sci. Total. Environ. 2018, 621, 352–359. [Google Scholar] [CrossRef]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Solomon, S.; Qin, D.; Manning, M.; Chen, Z.; Marquis, M.; Averyt, K.B.; Tignor, M.; Miller, H.L. The physical science basis. In Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007; Volume 996, p. 2007. [Google Scholar]
- Komarek, J. Cyanoprokaryota 3. Teil: Heterocystous Genera. In Süsswasserflora von Mitteleuropa; Springer: Berlin/Heidelberg, Germany, 2013; Volume 19/3. [Google Scholar]
- Komarek, J.; Anagnostidis, K. Cyanoprokaryota 1. Teil: Chroococcales. In Süsswasserflora von Mitteleuropa; Springer: Berlin/Heidelberg, Germany, 1999; Volume 19/1. [Google Scholar]
- Komarek, J. Anagnostidis Cyanoprokaryota 2. Teil: Oscillatoriales. In Süsswasserflora von Mitteleuropa; Springer: Berlin/Heidelberg, Germany, 2005; Volume 19/31. [Google Scholar]
- Edler, L. Recommendations on methods for marine biological studies in the Baltic Sea. Phytoplankton and chlorophyll. Publ. Balt. Mar. Biol. Bmb Swed. 1979, 9, 38. [Google Scholar]
- Nubel, U.; Muyzer, G.; Garcia-pichel, F.; Muyzer, G. PCR primers to amplify 16S rRNA genes from cyanobacteria PCR Primers to Amplify 16S rRNA Genes from Cyanobacteria. Microbiology 1997, 63, 3327–3332. [Google Scholar]
- Vaitomaa, J.; Rantala, A.; Halinen, K.; Rouhiainen, L.; Tallberg, P.; Mokelke, L.; Sivonen, K. Quantitative Real-Time PCR for Determination of Microcystin Synthetase E Copy Numbers for Microcystis and Anabaena in Lakes. Appl. Environ. Microbiol. 2003, 69, 7289–7297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Legendre, P.; Legendre, L. Developments in Environmental Modelling; Elsevier Science & Technology Books: Amsterdam, The Netherlands, 1998; Volume 20, ISBN 978-0-08-052317-0. [Google Scholar]
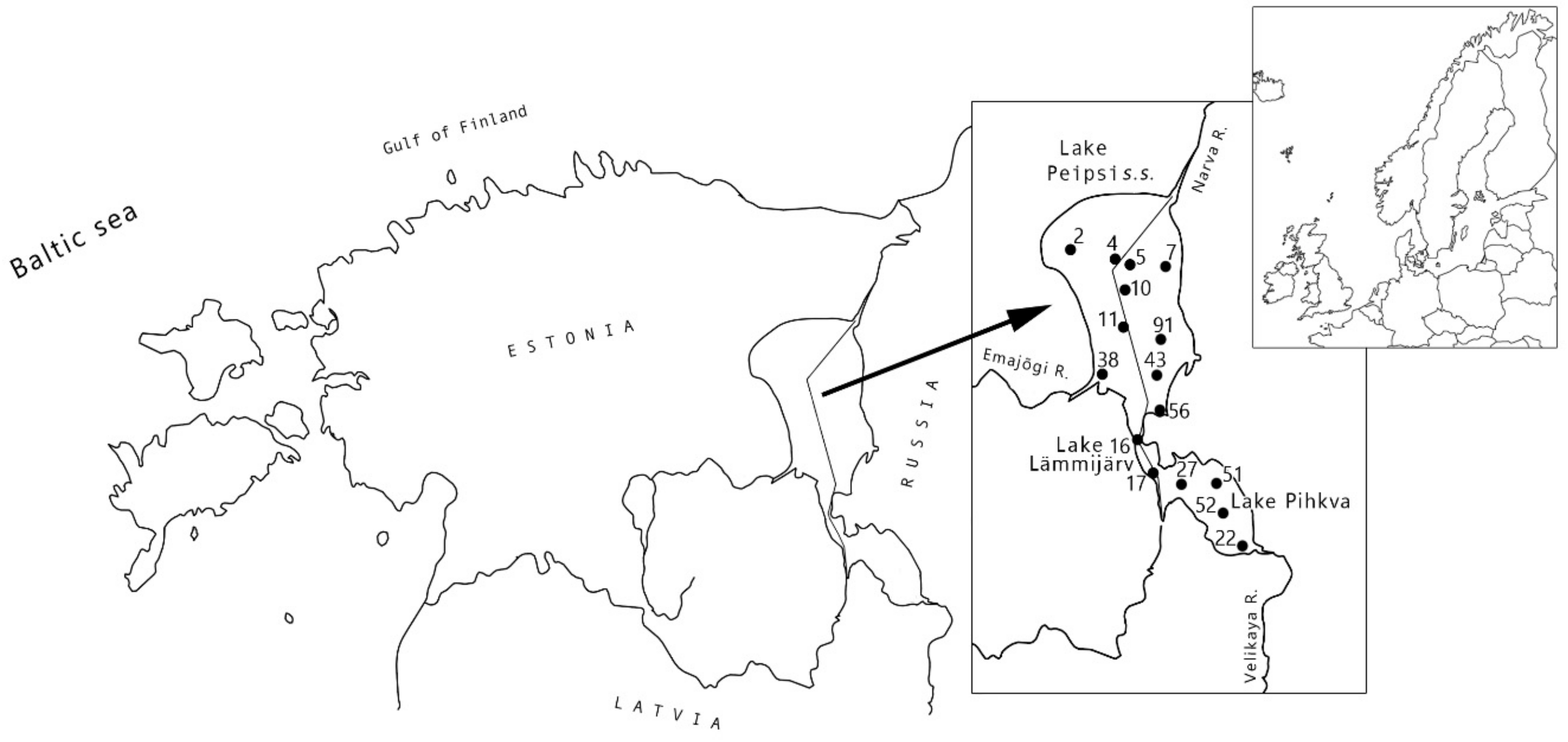
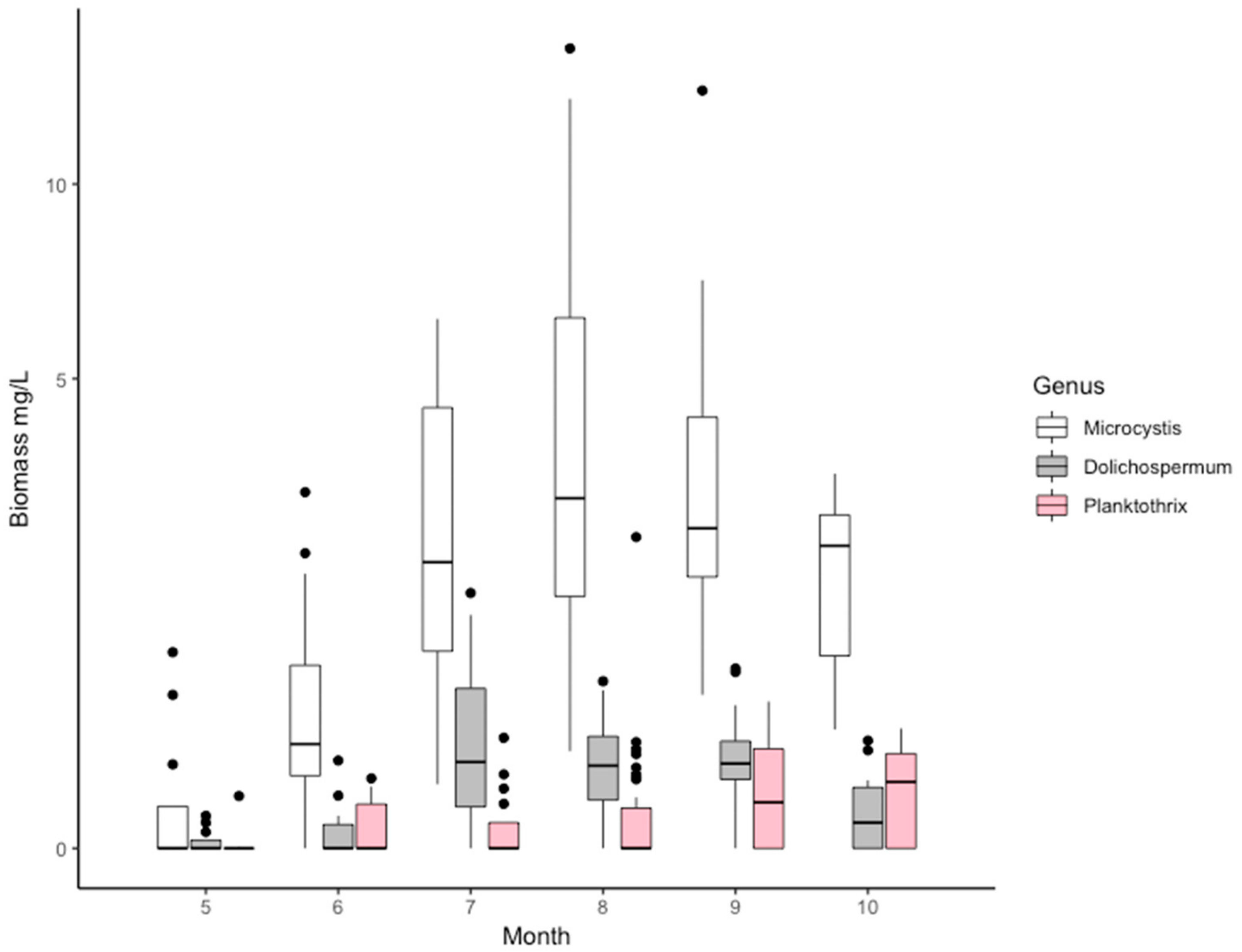





| Characteristic | Peipsi s.s. * | Lämmijärv * | Pihkva ** | |||
|---|---|---|---|---|---|---|
| Mean | Range | Mean | Range | Mean | Range | |
| Number of Samples | 91 | 38 | 12 | |||
| Area, km2 | 2611 | 236 | 708 | |||
| Mean depth, m | 8.3 | 2.5 | 3.8 | |||
| Max depth, m | 12.9 | 15.3 | 5.3 | |||
| Volume, km3 | 21.79 | 0.6 | 2.68 | |||
| TP, mg/m3 | 41 | 15–70 | 75 | 36–110 | 116 | 88–170 |
| SRP, mg/m3 | 12 | 2–49 | 13 | 3–25 | 28 | 13–79 |
| TN, mg/m3 | 701 | 460–1500 | 1001 | 410–1500 | 1147 | 950–1400 |
| NO3−, mg/m3 | 91 | 15–930 | 115 | 30–820 | 91 | 30–220 |
| NO2−, mg/m3 | 2 | 2–9 | 3 | 2–15 | 3 | 2–5 |
| NH4+, mg/m3 | 28 | 10–162 | 25 | 10–120 | 24 | 10–58 |
| chl-a, mg/m3 | 23.3 | 6.9–52.4 | 49.1 | 20.5–79 | 61.2 | 41.4–78.3 |
| pH | 8.5 | 8–8.9 | 8.6 | 8.3–9 | 8.9 | 8.4–9.2 |
| Water temp, °C | 18.2 | 5–23.9 | 17.9 | 10.3–24.7 | 22 | 19.7–25.6 |
| Secchi depth, m | 1.64 | 0.9–3.5 | 0.87 | 0.6–1.3 | 0.67 | 0.4–0.9 |
| OECD classification | Eutrophic | Eutrophic/hypertrophic | Hypertrophic | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panksep, K.; Tamm, M.; Mantzouki, E.; Rantala-Ylinen, A.; Laugaste, R.; Sivonen, K.; Tammeorg, O.; Kisand, V. Using Microcystin Gene Copies to Determine Potentially-Toxic Blooms, Example from a Shallow Eutrophic Lake Peipsi. Toxins 2020, 12, 211. https://doi.org/10.3390/toxins12040211
Panksep K, Tamm M, Mantzouki E, Rantala-Ylinen A, Laugaste R, Sivonen K, Tammeorg O, Kisand V. Using Microcystin Gene Copies to Determine Potentially-Toxic Blooms, Example from a Shallow Eutrophic Lake Peipsi. Toxins. 2020; 12(4):211. https://doi.org/10.3390/toxins12040211
Chicago/Turabian StylePanksep, Kristel, Marju Tamm, Evanthia Mantzouki, Anne Rantala-Ylinen, Reet Laugaste, Kaarina Sivonen, Olga Tammeorg, and Veljo Kisand. 2020. "Using Microcystin Gene Copies to Determine Potentially-Toxic Blooms, Example from a Shallow Eutrophic Lake Peipsi" Toxins 12, no. 4: 211. https://doi.org/10.3390/toxins12040211
APA StylePanksep, K., Tamm, M., Mantzouki, E., Rantala-Ylinen, A., Laugaste, R., Sivonen, K., Tammeorg, O., & Kisand, V. (2020). Using Microcystin Gene Copies to Determine Potentially-Toxic Blooms, Example from a Shallow Eutrophic Lake Peipsi. Toxins, 12(4), 211. https://doi.org/10.3390/toxins12040211






