Uremia-Associated Ageing of the Thymus and Adaptive Immune Responses
Abstract
:1. Introduction
2. The Immune System and Ageing
2.1. The Adaptive Immune System
2.2. Ageing-Related Decrease in Thymus Function
2.3. Ageing-Related Expansion of Memory T Cells
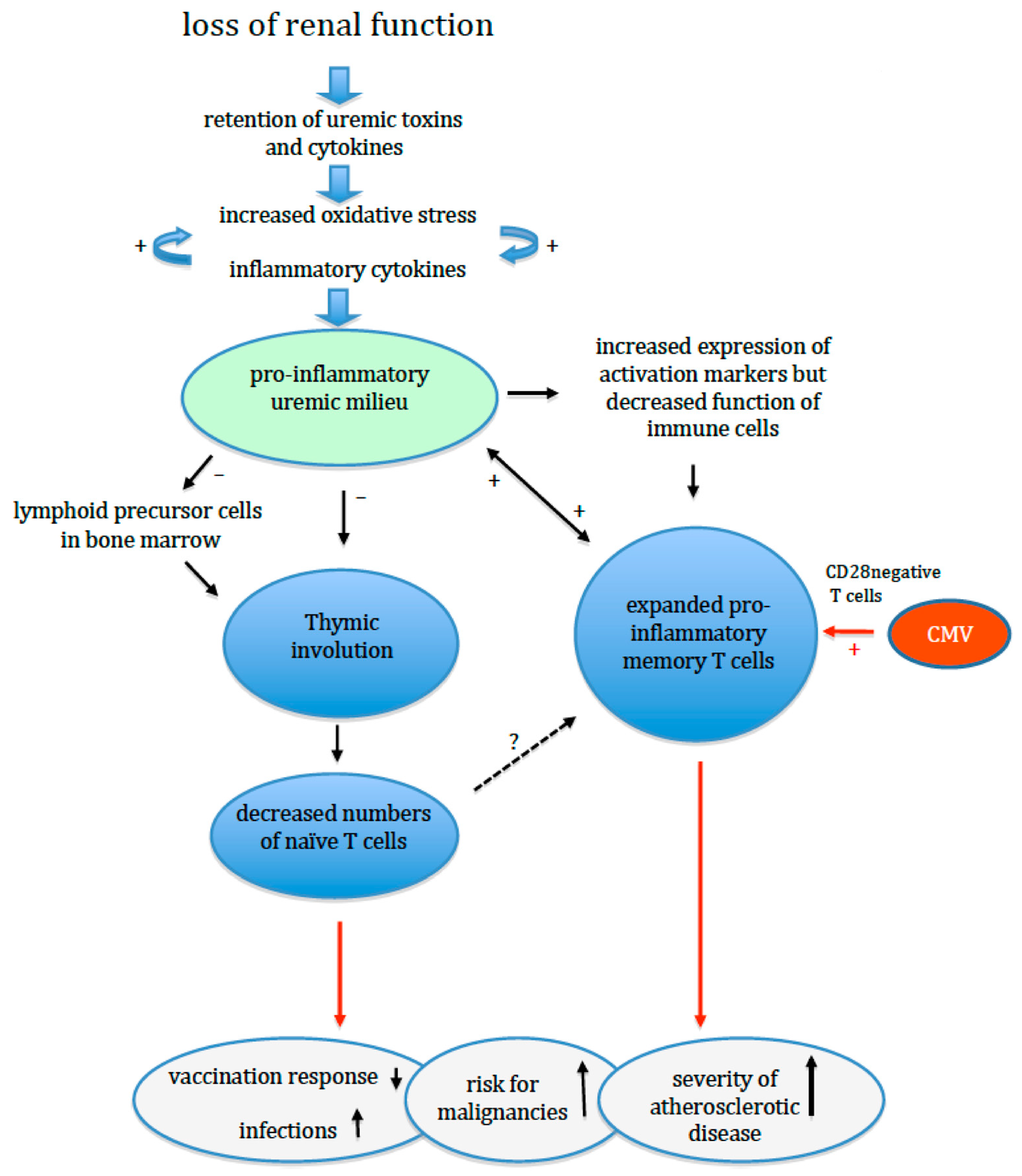
3. Renal Failure and Adaptive Immunity
4. Oxidative Stress, Inflammation and Premature Immunological Ageing in ESRD Patients
5. Premature Ageing of the Adaptive Immune System and Clinical Events
6. Interventions for Premature Immunological Ageing and Loss of Thymic Function
7. Conclusions
Funding
Conflicts of Interest
References
- Betjes, M.G. Immune cell dysfunction and inflammation in end-stage renal disease. Nat. Rev. Nephrol. 2013, 9, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Thapa, P.; Farber, D.L. The Role of the Thymus in the Immune Response. Thorac. Surg. Clin. 2019, 29, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Amigorena, S. The cell biology of antigen presentation in dendritic cells. Curr. Opin. Immunol. 2001, 13, 45–51. [Google Scholar] [CrossRef]
- Meier, P.; Golshayan, D.; Blanc, E.; Pascual, M.; Burnier, M. Oxidized LDL modulates apoptosis of regulatory T cells in patients with ESRD. J. Am. Soc. Nephrol. 2009, 20, 1368–1384. [Google Scholar] [CrossRef] [Green Version]
- Betjes, M.G.; Meijers, R.W.; Litjens, N.H. Loss of renal function causes premature aging of the immune system. Blood Purif. 2013, 36, 173–178. [Google Scholar] [CrossRef]
- Betjes, M.G.; Litjens, N.H. Chronic kidney disease and premature ageing of the adaptive immune response. Curr. Urol. Rep. 2015, 16, 471. [Google Scholar] [CrossRef]
- Betjes, M.G.; Langerak, A.W.; van der Spek, A.; de Wit, E.A.; Litjens, N.H. Premature aging of circulating T cells in patients with end-stage renal disease. Kidney Int. 2011, 80, 208–217. [Google Scholar] [CrossRef] [Green Version]
- Crepin, T.; Legendre, M.; Carron, C.; Vachey, C.; Courivaud, C.; Rebibou, J.M.; Ferrand, C.; Laheurte, C.; Vauchy, C.; Gaiffe, E.; et al. Uraemia-induced immune senescence and clinical outcomes in chronic kidney disease patients. Nephrol. Dial. Transplant. 2018, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Verkade, M.A.; van de Wetering, J.; Klepper, M.; Vaessen, L.M.; Weimar, W.; Betjes, M.G. Peripheral blood dendritic cells and GM-CSF as an adjuvant for hepatitis B vaccination in hemodialysis patients. Kidney Int. 2004, 66, 614–621. [Google Scholar] [CrossRef] [Green Version]
- Stewart, J.H.; Vajdic, C.M.; van Leeuwen, M.T.; Amin, J.; Webster, A.C.; Chapman, J.R.; McDonald, S.P.; Grulich, A.E.; McCredie, M.R. The pattern of excess cancer in dialysis and transplantation. Nephrol. Dial. Transplant. 2009, 24, 3225–3231. [Google Scholar] [CrossRef] [Green Version]
- Litjens, N.H.; Huisman, M.; van den Dorpel, M.; Betjes, M.G. Impaired immune responses and antigen-specific memory CD4+ T cells in hemodialysis patients. J. Am. Soc. Nephrol. 2008, 19, 1483–1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zinger, A.; Cho, W.C.; Ben-Yehuda, A. Cancer and Aging—The Inflammatory Connection. Aging Dis. 2017, 8, 611–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawelec, G.; McElhaney, J. Vaccines for Improved Cellular Immunity to Influenza. EBioMedicine 2018, 30, 12–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawelec, G. Does patient age influence anti-cancer immunity? Semin. Immunopathol. 2019, 41, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, G. Age and immunity: What is “immunosenescence”? Exp. Gerontol. 2018, 105, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Nikolich-Zugich, J. The twilight of immunity: Emerging concepts in aging of the immune system. Nat. Immunol. 2018, 19, 10–19. [Google Scholar] [CrossRef]
- Vasto, S.; Malavolta, M.; Pawelec, G. Age and immunity. Immun. Ageing 2006, 3, 2. [Google Scholar] [CrossRef] [Green Version]
- Pawelec, G.; Koch, S.; Griesemann, H.; Rehbein, A.; Hahnel, K.; Gouttefangeas, C. Immunosenescence, suppression and tumour progression. Cancer Immunol. Immunother. 2006, 55, 981–986. [Google Scholar] [CrossRef]
- Koelman, L.; Pivovarova-Ramich, O.; Pfeiffer, A.F.H.; Grune, T.; Aleksandrova, K. Cytokines for evaluation of chronic inflammatory status in ageing research: Reliability and phenotypic characterisation. Immun. Ageing 2019, 16, 11. [Google Scholar] [CrossRef] [Green Version]
- Franceschi, C.; Capri, M.; Monti, D.; Giunta, S.; Olivieri, F.; Sevini, F.; Panourgia, M.P.; Invidia, L.; Celani, L.; Scurti, M.; et al. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 2007, 128, 92–105. [Google Scholar] [CrossRef]
- Gallegos, A.M.; Bevan, M.J. Central tolerance: Good but imperfect. Immunol. Rev. 2006, 209, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Cebula, A.; Seweryn, M.; Rempala, G.A.; Pabla, S.S.; McIndoe, R.A.; Denning, T.L.; Bry, L.; Kraj, P.; Kisielow, P.; Ignatowicz, L. Thymus-derived regulatory T cells contribute to tolerance to commensal microbiota. Nature 2013, 497, 258–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohler, S.; Thiel, A. Life after the thymus: CD31+ and CD31- human naive CD4+ T-cell subsets. Blood 2009, 113, 769–774. [Google Scholar] [CrossRef] [Green Version]
- Ravkov, E.; Slev, P.; Heikal, N. Thymic output: Assessment of CD4(+) recent thymic emigrants and T-Cell receptor excision circles in infants. Cytom. B Clin. Cytom. 2017, 92, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Dedeoglu, B.; de Weerd, A.E.; Huang, L.; Langerak, A.W.; Dor, F.J.; Klepper, M.; Verschoor, W.; Reijerkerk, D.; Baan, C.C.; Litjens, N.H.; et al. Lymph node and circulating T cell characteristics are strongly correlated in end-stage renal disease patients, but highly differentiated T cells reside within the circulation. Clin. Exp. Immunol. 2017, 188, 299–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litjens, N.H.; van Druningen, C.J.; Betjes, M.G. Progressive loss of renal function is associated with activation and depletion of naive T lymphocytes. Clin. Immunol. 2006, 118, 83–91. [Google Scholar] [CrossRef]
- Nace, G.; Evankovich, J.; Eid, R.; Tsung, A. Dendritic cells and damage-associated molecular patterns: Endogenous danger signals linking innate and adaptive immunity. J. Innate. Immun. 2012, 4, 6–15. [Google Scholar] [CrossRef]
- McHeyzer-Williams, L.J.; McHeyzer-Williams, M.G. Antigen-specific memory B cell development. Annu. Rev. Immunol. 2005, 23, 487–513. [Google Scholar] [CrossRef]
- Thomas, R.; Wang, W.; Su, D.M. Contributions of Age-Related Thymic Involution to Immunosenescence and Inflammaging. Immun. Ageing 2020, 17, 2. [Google Scholar] [CrossRef] [Green Version]
- Ou, X.L.; Gao, J.; Wang, H.; Wang, H.S.; Lu, H.L.; Sun, H.Y. Predicting human age with bloodstains by sjTREC quantification. PLoS ONE 2012, 7, e42412. [Google Scholar] [CrossRef] [Green Version]
- van den Broek, T.; Borghans, J.A.M.; van Wijk, F. The full spectrum of human naive T cells. Nat. Rev. Immunol. 2018, 18, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, R.D.; Rickabaugh, T.; Hultin, L.E.; Hultin, P.; Hausner, M.A.; Detels, R.; Phair, J.; Jamieson, B.D. Homeostasis of the naive CD4+ T cell compartment during aging. J. Immunol. 2008, 180, 1499–1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fagnoni, F.F.; Vescovini, R.; Passeri, G.; Bologna, G.; Pedrazzoni, M.; Lavagetto, G.; Casti, A.; Franceschi, C.; Passeri, M.; Sansoni, P. Shortage of circulating naive CD8(+) T cells provides new insights on immunodeficiency in aging. Blood 2000, 95, 2860–2868. [Google Scholar] [CrossRef] [PubMed]
- Cicin-Sain, L.; Smyk-Pearson, S.; Currier, N.; Byrd, L.; Koudelka, C.; Robinson, T.; Swarbrick, G.; Tackitt, S.; Legasse, A.; Fischer, M.; et al. Loss of naive T cells and repertoire constriction predict poor response to vaccination in old primates. J. Immunol. 2010, 184, 6739–6745. [Google Scholar] [CrossRef] [PubMed]
- Cho, R.H.; Sieburg, H.B.; Muller-Sieburg, C.E. A new mechanism for the aging of hematopoietic stem cells: Aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood 2008, 111, 5553–5561. [Google Scholar] [CrossRef] [Green Version]
- Muller-Sieburg, C.E.; Sieburg, H.B.; Bernitz, J.M.; Cattarossi, G. Stem cell heterogeneity: Implications for aging and regenerative medicine. Blood 2012, 119, 3900–3907. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Brown, R.; Chen, S.; Zhuge, Q.; Su, D.M. Aging induced decline in T-lymphopoiesis is primarily dependent on status of progenitor niches in the bone marrow and thymus. Aging (Albany NY) 2012, 4, 606–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovtonyuk, L.V.; Fritsch, K.; Feng, X.; Manz, M.G.; Takizawa, H. Inflamm-Aging of Hematopoiesis, Hematopoietic Stem Cells, and the Bone Marrow Microenvironment. Front. Immunol. 2016, 7, 502. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Gui, J.; Dohkan, J.; Cheng, L.; Barnes, P.F.; Su, D.M. Lymphohematopoietic progenitors do not have a synchronized defect with age-related thymic involution. Aging Cell 2007, 6, 663–672. [Google Scholar] [CrossRef]
- Gui, J.; Zhu, X.; Dohkan, J.; Cheng, L.; Barnes, P.F.; Su, D.M. The aged thymus shows normal recruitment of lymphohematopoietic progenitors but has defects in thymic epithelial cells. Int. Immunol. 2007, 19, 1201–1211. [Google Scholar] [CrossRef] [Green Version]
- Saule, P.; Trauet, J.; Dutriez, V.; Lekeux, V.; Dessaint, J.P.; Labalette, M. Accumulation of memory T cells from childhood to old age: Central and effector memory cells in CD4(+) versus effector memory and terminally differentiated memory cells in CD8(+) compartment. Mech. Ageing Dev. 2006, 127, 274–281. [Google Scholar] [CrossRef]
- Fagnoni, F.F.; Vescovini, R.; Mazzola, M.; Bologna, G.; Nigro, E.; Lavagetto, G.; Franceschi, C.; Passeri, M.; Sansoni, P. Expansion of cytotoxic CD8+ CD28- T cells in healthy ageing people, including centenarians. Immunology 1996, 88, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Betjes, M.G. Clinical consequences of circulating CD28-negative T cells for solid organ transplantation. Transpl. Int. 2016, 29, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Hadrup, S.R.; Strindhall, J.; Kollgaard, T.; Seremet, T.; Johansson, B.; Pawelec, G.; Thor Straten, P.; Wikby, A. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J. Immunol. 2006, 176, 2645–2653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naismith, E.; Pangrazzi, L.; Grasse, M.; Keller, M.; Miggitsch, C.; Weinberger, B.; Trieb, K.; Grubeck-Loebenstein, B. Peripheral antibody concentrations are associated with highly differentiated T cells and inflammatory processes in the human bone marrow. Immun. & Ageing 2019, 16, 21. [Google Scholar]
- van de Berg, P.J.; van Stijn, A.; Ten Berge, I.J.; van Lier, R.A. A fingerprint left by cytomegalovirus infection in the human T cell compartment. J. Clin. Virol. 2008, 41, 213–217. [Google Scholar] [CrossRef]
- Betjes, M.G.; Huisman, M.; Weimar, W.; Litjens, N.H. Expansion of cytolytic CD4+CD28- T cells in end-stage renal disease. Kidney Int. 2008, 74, 760–767. [Google Scholar] [CrossRef] [Green Version]
- van de Berg, P.J.; Heutinck, K.M.; Raabe, R.; Minnee, R.C.; Young, S.L.; van Donselaar-van der Pant, K.A.; Bemelman, F.J.; van Lier, R.A.; ten Berge, I.J. Human cytomegalovirus induces systemic immune activation characterized by a type 1 cytokine signature. J. Infect. Dis. 2010, 202, 690–699. [Google Scholar] [CrossRef] [Green Version]
- Betjes, M.G.; Meijers, R.W.; de Wit, L.E.; Litjens, N.H. A killer on the road: Circulating CD4(+)CD28null T cells as cardiovascular risk factor in ESRD patients. J. Nephrol. 2012, 25, 183–191. [Google Scholar] [CrossRef]
- Betjes, M.G.; Weimar, W.; Litjens, N.H. Circulating CD4(+)CD28null T Cells May Increase the Risk of an Atherosclerotic Vascular Event Shortly after Kidney Transplantation. J. Transplant. 2013, 2013, 841430. [Google Scholar] [CrossRef] [Green Version]
- Betjes, M.G.; de Wit, E.E.; Weimar, W.; Litjens, N.H. Circulating pro-inflammatory CD4posCD28null T cells are independently associated with cardiovascular disease in ESRD patients. Nephrol. Dial. Transplant. 2010, 25, 3640–3646. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.W.; Gollapudi, S.; Pahl, M.V.; Vaziri, N.D. Naive and central memory T-cell lymphopenia in end-stage renal disease. Kidney Int. 2006, 70, 371–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, Y.L.; Shu, K.H.; Yang, F.J.; Chou, T.Y.; Chen, P.M.; Lay, F.Y.; Pan, S.Y.; Lin, C.J.; Litjens, N.H.R.; Betjes, M.G.H.; et al. A comprehensive characterization of aggravated aging-related changes in T lymphocytes and monocytes in end-stage renal disease: The iESRD study. Immun. Ageing 2018, 15, 27. [Google Scholar] [CrossRef] [PubMed]
- Betjes, M.G.H.; Langerak, A.W.; Klepper, M.; Litjens, N.H.R. A very low thymus function identifies patients with substantial increased risk for long-term mortality after kidney transplantation. Immun. & Ageing 2020, 17, 4. [Google Scholar]
- Gruver, A.L.; Sempowski, G.D. Cytokines, leptin, and stress-induced thymic atrophy. J. Leukoc. Biol. 2008, 84, 915–923. [Google Scholar] [CrossRef]
- Yadav, A.K.; Jha, V. CD4+CD28null cells are expanded and exhibit a cytolytic profile in end-stage renal disease patients on peritoneal dialysis. Nephrol. Dial. Transplant. 2011, 26, 1689–1694. [Google Scholar] [CrossRef] [Green Version]
- Gamadia, L.E.; Remmerswaal, E.B.; Weel, J.F.; Bemelman, F.; van Lier, R.A.; Ten Berge, I.J. Primary immune responses to human CMV: A critical role for IFN-gamma-producing CD4+ T cells in protection against CMV disease. Blood 2003, 101, 2686–2692. [Google Scholar] [CrossRef]
- Kuijpers, T.W.; Vossen, M.T.; Gent, M.R.; Davin, J.C.; Roos, M.T.; Wertheim-van Dillen, P.M.; Weel, J.F.; Baars, P.A.; van Lier, R.A. Frequencies of circulating cytolytic, CD45RA+CD27-, CD8+ T lymphocytes depend on infection with CMV. J. Immunol. 2003, 170, 4342–4348. [Google Scholar] [CrossRef] [Green Version]
- Litjens, N.H.R.; Huang, L.; Dedeoglu, B.; Meijers, R.W.J.; Kwekkeboom, J.; Betjes, M.G.H. Protective Cytomegalovirus (CMV)-Specific T-Cell Immunity Is Frequent in Kidney Transplant Patients without Serum Anti-CMV Antibodies. Front. Immunol. 2017, 8, 1137. [Google Scholar] [CrossRef] [Green Version]
- Meijers, R.W.; Litjens, N.H.; Hesselink, D.A.; Langerak, A.W.; Baan, C.C.; Betjes, M.G. Primary Cytomegalovirus Infection Significantly Impacts Circulating T Cells in Kidney Transplant Recipients. Am. J. Transplant. 2015, 15, 3143–3156. [Google Scholar] [CrossRef]
- Pardieck, I.N.; Beyrend, G.; Redeker, A.; Arens, R. Cytomegalovirus infection and progressive differentiation of effector-memory T cells. F1000Research 2018, 7, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redeker, A.; Remmerswaal, E.B.M.; van der Gracht, E.T.I.; Welten, S.P.M.; Hollt, T.; Koning, F.; Cicin-Sain, L.; Nikolich-Zugich, J.; Ten Berge, I.J.M.; van Lier, R.A.W.; et al. The Contribution of Cytomegalovirus Infection to Immune Senescence Is Set by the Infectious Dose. Front. Immunol. 2017, 8, 1953. [Google Scholar] [CrossRef] [PubMed]
- Litjens, N.H.; de Wit, E.A.; Betjes, M.G. Differential effects of age, cytomegalovirus-seropositivity and end-stage renal disease (ESRD) on circulating T lymphocyte subsets. Immun. Ageing 2011, 8, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meijers, R.W.; Betjes, M.G.; Baan, C.C.; Litjens, N.H. T-cell ageing in end-stage renal disease patients: Assessment and clinical relevance. World J. Nephrol. 2014, 3, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Meijers, R.W.; Litjens, N.H.; de Wit, E.A.; Langerak, A.W.; van der Spek, A.; Baan, C.C.; Weimar, W.; Betjes, M.G. Uremia causes premature ageing of the T cell compartment in end-stage renal disease patients. Immun. Ageing 2012, 9, 19. [Google Scholar] [CrossRef] [Green Version]
- Meijers, R.W.; Litjens, N.H.; de Wit, E.A.; Langerak, A.W.; van der Spek, A.; Baan, C.C.; Weimar, W.; Betjes, M.G. Cytomegalovirus contributes partly to uraemia-associated premature immunological ageing of the T cell compartment. Clin. Exp. Immunol. 2013, 174, 424–432. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Betjes, M.G.H.; Klepper, M.; Langerak, A.W.; Baan, C.C.; Litjens, N.H.R. End-Stage Renal Disease Causes Skewing in the TCR Vbeta-Repertoire Primarily within CD8(+) T Cell Subsets. Front. Immunol. 2017, 8, 1826. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Langerak, A.W.; Wolvers-Tettero, I.L.; Meijers, R.W.; Baan, C.C.; Litjens, N.H.; Betjes, M.G. End stage renal disease patients have a skewed T cell receptor Vbeta repertoire. Immun. Ageing 2015, 12, 28. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Litjens, N.H.R.; Kannegieter, N.M.; Klepper, M.; Baan, C.C.; Betjes, M.G.H. pERK-dependent defective TCR-mediated activation of CD4(+) T cells in end-stage renal disease patients. Immun. Ageing 2017, 14, 14. [Google Scholar] [CrossRef] [Green Version]
- Pahl, M.V.; Gollapudi, S.; Sepassi, L.; Gollapudi, P.; Elahimehr, R.; Vaziri, N.D. Effect of end-stage renal disease on B-lymphocyte subpopulations, IL-7, BAFF and BAFF receptor expression. Nephrol. Dial. Transplant. 2010, 25, 205–212. [Google Scholar] [CrossRef]
- Lim, W.H.; Kireta, S.; Thomson, A.W.; Russ, G.R.; Coates, P.T. Renal transplantation reverses functional deficiencies in circulating dendritic cell subsets in chronic renal failure patients. Transplantation 2006, 81, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Hesselink, D.A.; Betjes, M.G.; Verkade, M.A.; Athanassopoulos, P.; Baan, C.C.; Weimar, W. The effects of chronic kidney disease and renal replacement therapy on circulating dendritic cells. Nephrol. Dial. Transplant. 2005, 20, 1868–1873. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Gollapudi, P.; Elahimehr, R.; Pahl, M.V.; Vaziri, N.D. Effects of end-stage renal disease and haemodialysis on dendritic cell subsets and basal and LPS-stimulated cytokine production. Nephrol. Dial. Transplant. 2010, 25, 737–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verkade, M.A.; van Druningen, C.J.; Vaessen, L.M.; Hesselink, D.A.; Weimar, W.; Betjes, M.G. Functional impairment of monocyte-derived dendritic cells in patients with severe chronic kidney disease. Nephrol. Dial. Transplant. 2007, 22, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Witko-Sarsat, V.; Friedlander, M.; Nguyen Khoa, T.; Capeillere-Blandin, C.; Nguyen, A.T.; Canteloup, S.; Dayer, J.M.; Jungers, P.; Drueke, T.; Descamps-Latscha, B. Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure. J. Immunol. 1998, 161, 2524–2532. [Google Scholar]
- Vaziri, N.D. Oxidative stress in uremia: Nature, mechanisms, and potential consequences. Semin. Nephrol. 2004, 24, 469–473. [Google Scholar] [CrossRef]
- Akar, H.; Akar, G.C.; Carrero, J.J.; Stenvinkel, P.; Lindholm, B. Systemic consequences of poor oral health in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 218–226. [Google Scholar] [CrossRef]
- Anders, H.J.; Andersen, K.; Stecher, B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int. 2013, 83, 1010–1016. [Google Scholar] [CrossRef] [Green Version]
- Cepeda, S.; Griffith, A.V. Thymic stromal cells: Roles in atrophy and age-associated dysfunction of the thymus. Exp. Gerontol. 2018, 105, 113–117. [Google Scholar] [CrossRef]
- Majumdar, S.; Nandi, D. Thymic Atrophy: Experimental Studies and Therapeutic Interventions. Scand. J. Immunol. 2018, 87, 4–14. [Google Scholar] [CrossRef]
- Paz-Miguel, J.E.; Flores, R.; Sanchez-Velasco, P.; Ocejo-Vinyals, G.; Escribano de Diego, J.; Lopez de Rego, J.; Leyva-Cobian, F. Reactive oxygen intermediates during programmed cell death induced in the thymus of the Ts(1716)65Dn mouse, a murine model for human Down’s syndrome. J. Immunol. 1999, 163, 5399–5410. [Google Scholar] [PubMed]
- Venet, F.; Monneret, G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat. Rev. Nephrol. 2018, 14, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Venet, F.; Rimmele, T.; Monneret, G. Management of Sepsis-Induced Immunosuppression. Crit. Care Clin. 2018, 34, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Adiga, V.; Raghavan, A.; Rananaware, S.R.; Nandi, D. Comparative analysis of thymic subpopulations during different modes of atrophy identifies the reactive oxygen species scavenger, N-acetyl cysteine, to increase the survival of thymocytes during infection-induced and lipopolysaccharide-induced thymic atrophy. Immunology 2019, 157, 21–36. [Google Scholar]
- Raskova, J.; Czerwinski, D.K.; Shea, S.M.; Raska, K., Jr. Cellular immunity and lymphocyte populations in developing uremia in the rat. J. Exp. Pathol. 1986, 2, 229–245. [Google Scholar] [PubMed]
- Sato, K.; Kato, A.; Sekai, M.; Hamazaki, Y.; Minato, N. Physiologic Thymic Involution Underlies Age-Dependent Accumulation of Senescence-Associated CD4(+) T Cells. J. Immunol. 2017, 199, 138–148. [Google Scholar] [CrossRef] [Green Version]
- Sauce, D.; Larsen, M.; Fastenackels, S.; Duperrier, A.; Keller, M.; Grubeck-Loebenstein, B.; Ferrand, C.; Debre, P.; Sidi, D.; Appay, V. Evidence of premature immune aging in patients thymectomized during early childhood. J. Clin. Investig. 2009, 119, 3070–3078. [Google Scholar] [CrossRef] [Green Version]
- Liuzzo, G.; Goronzy, J.J.; Yang, H.; Kopecky, S.L.; Holmes, D.R.; Frye, R.L.; Weyand, C.M. Monoclonal T-Cell Proliferation and Plaque Instability in Acute Coronary Syndromes. Circulation 2000, 101, 2883–2888. [Google Scholar] [CrossRef] [Green Version]
- Liuzzo, G.; Kopecky, S.L.; Frye, R.L.; O’Fallon, W.M.; Maseri, A.; Goronzy, J.J.; Weyand, C.M. Perturbation of the T-cell repertoire in patients with unstable angina. Circulation 1999, 100, 2135–2139. [Google Scholar] [CrossRef] [Green Version]
- Panayiotou, A.G.; Nicolaides, A.N.; Griffin, M.; Tyllis, T.; Georgiou, N.; Bond, D.; Martin, R.M.; Hoppensteadt, D.; Fareed, J.; Humphries, S.E. Leukocyte telomere length is associated with measures of subclinical atherosclerosis. Atherosclerosis 2010, 211, 176–181. [Google Scholar] [CrossRef]
- Spyridopoulos, I.; Hoffmann, J.; Aicher, A.; Brummendorf, T.H.; Doerr, H.W.; Zeiher, A.M.; Dimmeler, S. Accelerated Telomere Shortening in Leukocyte Subpopulations of Patients With Coronary Heart Disease: Role of Cytomegalovirus Seropositivity. Circulation 2009, 120, 1364–1372. [Google Scholar] [CrossRef] [Green Version]
- Bottomley, M.J.; Harden, P.N.; Wood, K.J. CD8+ Immunosenescence Predicts Post-Transplant Cutaneous Squamous Cell Carcinoma in High-Risk Patients. J. Am. Soc. Nephrol. 2016, 27, 1505–1515. [Google Scholar] [CrossRef] [Green Version]
- Dedeoglu, B.; Meijers, R.W.; Klepper, M.; Hesselink, D.A.; Baan, C.C.; Litjens, N.H.; Betjes, M.G. Loss of CD28 on Peripheral T Cells Decreases the Risk for Early Acute Rejection after Kidney Transplantation. PLoS ONE 2016, 11, e0150826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betjes, M.G.; Meijers, R.W.; de Wit, E.A.; Weimar, W.; Litjens, N.H. Terminally differentiated CD8+ Temra cells are associated with the risk for acute kidney allograft rejection. Transplantation 2012, 94, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Betjes, M.G.H.; Litjens, N.H.R. High numbers of differentiated CD28null CD8+ T cells are associated with a lowered risk for late rejection and graft loss after kidney transplantation. PLoS ONE 2020, 15, e0228096. [Google Scholar] [CrossRef]
- Dedeoglu, B.; Meijers, R.W.; Klepper, M.; Hesselink, D.A.; Baan, C.C.; Litjens, N.H.; Betjes, M.G. Uremia-Associated Premature Aging of T Cells Does Not Predict Infectious Complications After Renal Transplantation. Am. J. Transplant. 2016, 16, 2324–2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Struijk, G.H.; Gijsen, A.F.; Yong, S.L.; Zwinderman, A.H.; Geerlings, S.E.; Lettinga, K.D.; van Donselaar-van der Pant, K.A.; ten Berge, I.J.; Bemelman, F.J. Risk of Pneumocystis jiroveci pneumonia in patients long after renal transplantation. Nephrol. Dial. Transplant. 2011, 26, 3391–3398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ducloux, D.; Courivaud, C.; Bamoulid, J.; Vivet, B.; Chabroux, A.; Deschamps, M.; Rebibou, J.M.; Ferrand, C.; Chalopin, J.M.; Tiberghien, P.; et al. Prolonged CD4 T cell lymphopenia increases morbidity and mortality after renal transplantation. J. Am. Soc. Nephrol. 2010, 21, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Wikby, A.; Mansson, I.A.; Johansson, B.; Strindhall, J.; Nilsson, S.E. The immune risk profile is associated with age and gender: Findings from three Swedish population studies of individuals 20–100 years of age. Biogerontology 2008, 9, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Guo, J.; Sun, L.; Fu, J.; Barnes, P.F.; Metzger, D.; Chambon, P.; Oshima, R.G.; Amagai, T.; Su, D.M. Postnatal tissue-specific disruption of transcription factor FoxN1 triggers acute thymic atrophy. J. Biol. Chem. 2010, 285, 5836–5847. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Guo, J.; Brown, R.; Amagai, T.; Zhao, Y.; Su, D.M. Declining expression of a single epithelial cell-autonomous gene accelerates age-related thymic involution. Aging Cell 2010, 9, 347–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zook, E.C.; Krishack, P.A.; Zhang, S.; Zeleznik-Le, N.J.; Firulli, A.B.; Witte, P.L.; Le, P.T. Overexpression of Foxn1 attenuates age-associated thymic involution and prevents the expansion of peripheral CD4 memory T cells. Blood 2011, 118, 5723–5731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, Y.W.; Memon, S.A.; Sharrow, S.O.; Hakim, F.T.; Eckhaus, M.; Lucas, P.J.; Gress, R.E. Exogenous IL-7 increases recent thymic emigrants in peripheral lymphoid tissue without enhanced thymic function. Blood 2004, 104, 1110–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sportes, C.; Hakim, F.T.; Memon, S.A.; Zhang, H.; Chua, K.S.; Brown, M.R.; Fleisher, T.A.; Krumlauf, M.C.; Babb, R.R.; Chow, C.K.; et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J. Exp. Med. 2008, 205, 1701–1714. [Google Scholar] [CrossRef] [PubMed]
- Henson, S.M.; Snelgrove, R.; Hussell, T.; Wells, D.J.; Aspinall, R. An IL-7 fusion protein that shows increased thymopoietic ability. J. Immunol. 2005, 175, 4112–4118. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, S.; Pergola, P.E.; Zager, R.A.; Vaziri, N.D. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013, 83, 1029–1041. [Google Scholar] [CrossRef] [Green Version]
- Meijers, R.W.; Litjens, N.H.; de Wit, E.A.; Langerak, A.W.; Baan, C.C.; Betjes, M.G. Uremia-associated immunological aging is stably imprinted in the T-cell system and not reversed by kidney transplantation. Transpl. Int. 2014, 27, 1272–1284. [Google Scholar] [CrossRef]
- Yang, H.; Youm, Y.H.; Vandanmagsar, B.; Rood, J.; Kumar, K.G.; Butler, A.A.; Dixit, V.D. Obesity accelerates thymic aging. Blood 2009, 114, 3803–3812. [Google Scholar] [CrossRef] [Green Version]
- Duggal, N.A.; Niemiro, G.; Harridge, S.D.R.; Simpson, R.J.; Lord, J.M. Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity? Nat. Rev. Immunol. 2019, 19, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Duggal, N.A.; Pollock, R.D.; Lazarus, N.R.; Harridge, S.; Lord, J.M. Major features of immunesenescence, including reduced thymic output, are ameliorated by high levels of physical activity in adulthood. Aging Cell 2018, 17, 1–3. [Google Scholar] [CrossRef] [Green Version]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Betjes, M.G. Uremia-Associated Ageing of the Thymus and Adaptive Immune Responses. Toxins 2020, 12, 224. https://doi.org/10.3390/toxins12040224
Betjes MG. Uremia-Associated Ageing of the Thymus and Adaptive Immune Responses. Toxins. 2020; 12(4):224. https://doi.org/10.3390/toxins12040224
Chicago/Turabian StyleBetjes, Michiel GH. 2020. "Uremia-Associated Ageing of the Thymus and Adaptive Immune Responses" Toxins 12, no. 4: 224. https://doi.org/10.3390/toxins12040224
APA StyleBetjes, M. G. (2020). Uremia-Associated Ageing of the Thymus and Adaptive Immune Responses. Toxins, 12(4), 224. https://doi.org/10.3390/toxins12040224




