Abstract
Bee venom (BV) is a rich source of secondary metabolites from honeybees (Apis mellifera L.). It contains a variety of bioactive ingredients including peptides, proteins, enzymes, and volatile metabolites. The compounds contribute to the venom’s observed biological functions as per its anti-inflammatory and anticancer effects. The antimicrobial action of BV has been shown in vitro and in vivo experiments against bacteria, viruses, and fungi. The synergistic therapeutic interactions of BV with antibiotics has been reported. The synergistic effect contributes to a decrease in the loading and maintenance dosage, a decrease in the side effects of chemotherapy, and a decrease in drug resistance. To our knowledge, there have been no reviews on the impact of BV and its antimicrobial constituents thus far. The purpose of this review is to address the antimicrobial properties of BV and its compounds.
Key Contribution:
Here, we critically review the impact of bee venom and its components as antimicrobial agents.
1. Introduction
According to the World Health Organization (WHO), the antimicrobial drug resistance of bacterial pathogens has reached alarming rates in several parts of the world, and few alternatives are available [1]. The discovery of antibiotics served as a promise to eliminate numerous ailments that menaced human life in the past. However, unexpected side effects such as resistance and mutation displayed a new challenge for humankind. The annual deaths attributable to anti-microbial resistance are expected to surpass those of cancer by 2050 [2]. Due to the resulting overuse of antibiotics, microbes have become capable of developing biofilms embedded in an extracellular matrix (ECM) that are more resistant and more difficult to penetrate with antibiotics. The rise of antimicrobial drug resistance calls for a search of new candidates with novel mode of action. Natural products including bee venom (BV), one of many bee products which is rich in bioactive compounds, offer a diversity of activities against variety of diseases causes [3,4,5,6].
Venoms and their peptides from different animals or organisms such as bees, snakes, wasps, and scorpions, represent promising antimicrobial agents against various microbial pathogenesis [7,8,9,10,11,12]. BV is the venomous cocktail secreted by honeybee workers’ poison glands as a protection mechanism [13]. BV is injected into the victim’s skin using stingers, which ultimately leads to the death of the bee itself afterwards. Although BV is toxic to predators, it has acquired medicinal benefits over the years [14]. Therapeutic usage of BV dates back to Ancient Egypt (4000 BC), and was later applied by Hippocrates, Aristotle, and Galen, during the Greek and Roman historical periods [15]. In Traditional Chinese Medicine and other historical practices, BV was introduced for inflammatory diseases such as rheumatoid, arthritis, tendonitis, fibrosis, lupus, and multiple sclerosis [16,17].
It is thought that medical use and subsequent public acceptance of BV is due to the availability of biologically active compounds such as peptides. For instance, melittin is a major compound representing 40–60% of the dry BV weight [18]; it also contains mast cell degranulating peptide (MCDP), secapin, and its isomers (i.e., secapin-1 and -2), adolpanin, tertiapin, apamin [19,20,21,22,23,24,25], and enzymes, i.e., phospholipase A2 (PLA2), hyaluronidases, and acid phosphatase [26,27,28]. Furthermore, dipeptidylpeptidase IV (Api m 5) [29], Api m 6 [30], CUB serine protease (Api m 7) [31], icarapin (Api m 10) [32], major royal jelly proteins (MRJP 8 and 9) [33,34], and volatile compounds (isopentyl acetate and (Z)-I l-eicosen-l-ol) [35] are also present. Attributing to these constituents, BV has been proven to be active as an anti-inflammatory [36,37], radioprotective, [38], and antibacterial agent against several Gram-positive/negative bacteria strains [5,39]. The combination of BV and its constituents with chemotherapy agents (vancomycin, oxacillin, and amikacin) has a synergetic effect against bacteria due to the antibacterial properties [16].
In this review, we discuss the in vitro, in vivo, and in situ therapeutic implications of BV against microbial diseases.
2. Antimicrobial Properties of Bee Venom and Mode of Action for the Venom and its Derived Compounds
2.1. Antibacterial
BV has significant antimicrobial effects [40]. BV, and its major compounds, PLA2 and melittin (Figure 1), were applied against oral pathogens identified as the causative agents of tooth decay. The minimum inhibitory concentration (MIC) for the BV lies between 20 and 40 µg/mL against Streptococcus salivarius, S. sobrinus, S. mutans, S. mitis, S. sanguinis, Lactobacillus casei, and Enterococcus faecalis. Melittin showed MIC values ranging from 4 to 40 µg/mL, whereas the MIC value of PLA2 was above 400 µg/mL (Table 1) [4]. Lyme infection is a tick-borne multi-systemic illness caused by the bacterium Borrelia burgdorferi [41]. Both BV and melittin had impacts on the morphology and size of the biofilms of B. burgdorferi, whereas antibiotics frequently experienced backslide occurrence after discontinuation [6].
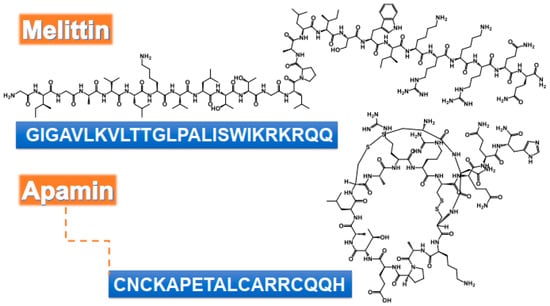
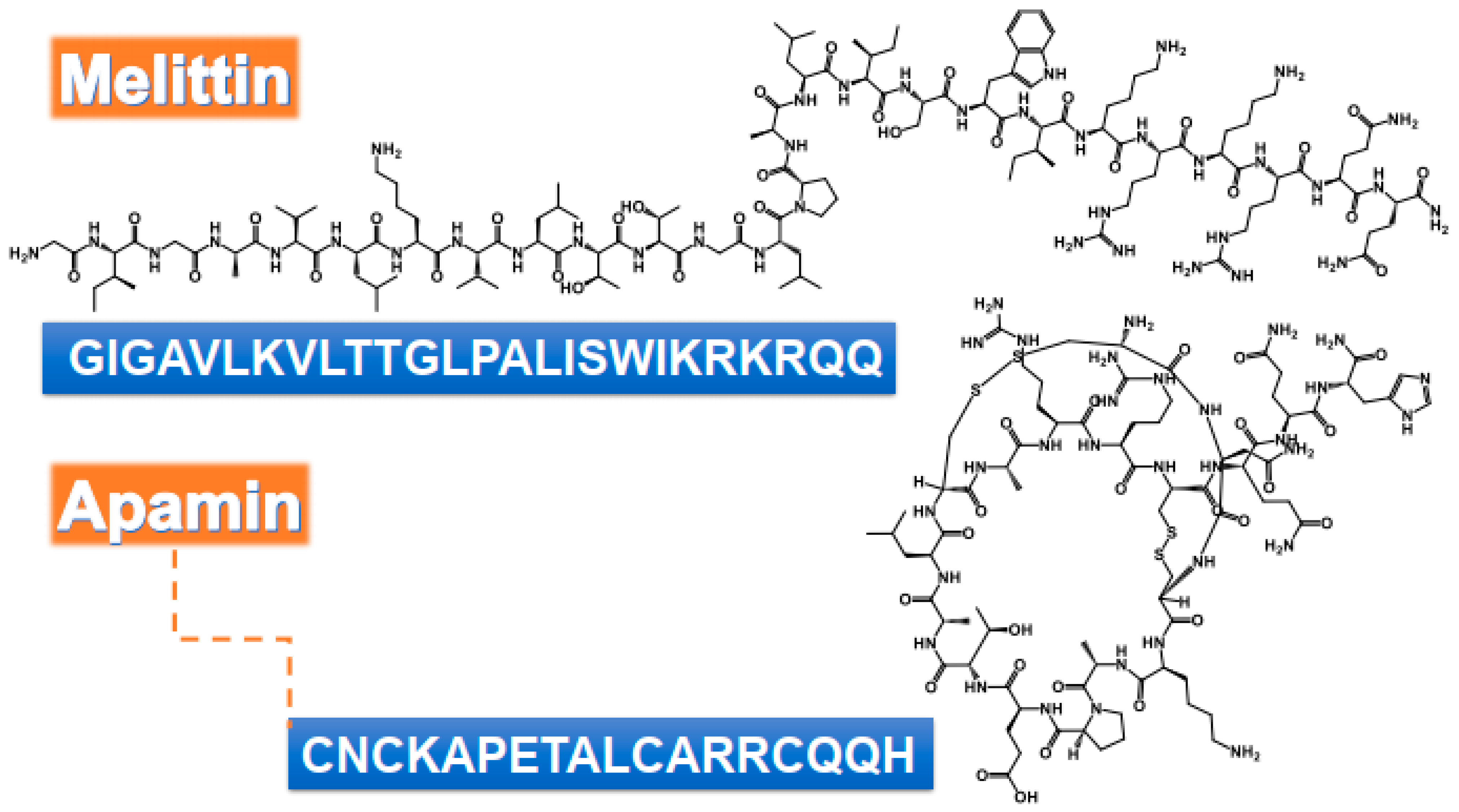
Figure 1.
Chemical structure and amino acids sequence of bioactive peptides from bee venom as antimicrobial agents.

Table 1.
Anti-bacterial properties of bee venom and its compounds.
BV antimicrobial and antibiofilm activity was identified in 16 poultry-isolated Salmonella strains. BV MIC ranged 256–1024 μg/mL. Sub-inhibitory BV concentrations significantly reduced the development of biofilm in 14 of the 16 Salmonella strains studied, with substantial motility increases. BV did not show any influence on the motility of Salmonella isangi IG1 and S. infantis Lhica I17. The percentage of biofilm reduction observed ranged from 27.66% (S. Infantis Lhica I17) to 68.22% (Salmonella enterica subsp. salamae SA3), with significant variability among the different Salmonella strains tested [42].
BV was proven effective, synergistic, and safe when combined with some conventional drugs against certain types of microbes; however, there is a slow and careful consideration towards its investigations in pre-clinical and clinical applications. For example, BV and melittin exhibited a broad-spectrum antibacterial activity against both Gram-positive (MIC values between 10 and 100 μg/mL) and Gram-negative bacteria (MIC values between 30 and >500 μg/mL). Combination of BV and melittin with other antibiotic drugs, i.e., oxacillin, vancomycin, and amikacin, using checkerboard dilution gave fractional inhibitory concentration (FIC) indices ranging between 0.24 and 0.5; the FIC index is determined by the MIC of the test material in combination with an antibiotic medication divided by the MIC of the test material individually [16]. BV increased the antibody production against formalin-killed S. gallinarum in broiler chicks [43]. BV and melittin exhibited a broad spectrum antibacterial activity against Methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci at MIC values of 6–800 μg/mL, compared to vancomycin’s (reference drug) MIC value of 1.6–25 μg/mL [16]. The combination of BV and melittin with oxacillin showed a bactericidal effect on MRSA ATCC 33591. The treatment with both BV and melittin led to changes in the bacterial cell membrane caused by the loss of membrane integrity and exhibition of changes in the cell morphology including cell distortion and loss of cytoplasm content [11].
The antibacterial efficiency of melittin was studied against a variety of bacteria, such as Escherichia coli, S. aureus, and B. burgdorferi [39,44,45,46,47]. Gram-positive bacteria have sensitivity to melittin, compared to Gram-negative ones, due to the nature of the organism’s cell membrane [39,46,48,49]. Melittin can penetrate the peptideoglycan layer of the Gram-positive cell membrane more easily than the Gram-negative cells, which have a layer of lipopolysaccharides protecting their membrane. The presence of proline residue in position 14 has been shown to play a central role in the antimicrobial activity of melittin. Its absence in a melittin analog significantly reduced antimicrobial activity compared to the native peptide [50]. Similarly, two synthetic melittin, serine-substituted melittin (Mel-S) and asparagine-substituted melittin (Mel-N), were capable of penetrating E. coli cell membrane. Mel-S was more efficient than Mel-N [51]. Melittin, in general, possesses a greater ability to destroy biofilms formed by S. aureus compared to E. coli (biofilm production was 56% vs. 37%, respectively) [46]. MDP1: GIGAVLKVLTTGLPALIKRKRQQ and MDP2: GIGAVLKWLPALIKRKRQQ displayed strong antibacterial activity against reference strains of S. aureus, E. coli, and Pseudomonas aeruginosa compared to the native melittin. The antibacterial effects of MDP1 and MDP2 were explained by the changes in the bacterial membrane and the destruction of the bacterial cell membrane. Furthermore, the hemolytic activity of melittin (93.5%) at the dose of 3.84 µg/mL with average MIC values showed significant reduction in MDP1 (1.46% at geometric mean (GM) of 3.01 µg/mL) and MDP2 (5.15% at GM of MICs 2.18 µg/mL) [52]. The antibacterial activity of native melittin and its two mutants, namely melittin I17K (GIGAVLKVLTTGLPALKSWIKRKRQQ) with a higher charge and lower hydrophobicity and mutant G1I (IIGAVLKVLTTGLPALISWIKRKRQQ) of higher hydrophobicity, were investigated against different strains of Listeria, as mentioned in Table 1 [53].
The increased frequency of multi-drug resistant (MDR) bacteria is a major challenge to antimicrobial treatment. Melittin shows broad antibacterial activity toward different types of bacteria such as methicillin-susceptible S. aureus (MSSA), MRSA, and Enterococcus spp at MICs 0.5–4, 0.5–4, and 1–8 µg/mL, respectively. Furthermore, synergetic action between melittin and some antibiotics, i.e., daptomycin, vancomycin, linezolid, ampicillin, and erythromycin, against the previously mentioned bacteria were investigated by Dosler et al. [54]. Melittin‘s antibacterial and synergistic effects with β-lactam antibiotics to Acinetobacter baumannii was reported using broth microdilution method. The MIC values of melittin, ciprofloxacin, co-amoxiclav, imipenem, netilmicin, ceftazidime, and piperacillin are 4, 8, 16, 16, 16, 32, and 128 µg/mL, respectively. However, FIC indices for combinations of melittin with the same antibacterial drugs are 0.750, 0.312, 0.250, 1.25, 0.187, and 0.375 µg/mL, respectively [55]. The application of melittin–doripenem has resulted in a significant decrease in the MIC of MDR baumannii strains. When the combinations of melittin–doripenem and melittin–ceftazidime were administrated to strains of MDR P. aeruginosa, the dose of melittin was significantly reduced. The combination of melittin with doripenem and ceftazidime against MDR microbial pathogens could be of great therapeutic value [56].
Furthermore, the combination between melittin and PLA2 (0.5 mg of each compound) has been investigated against oral pathogens S. salivarius, S. sobrinus, S. mutans, S. mitis, S. sanguinis, Lactobacillus casei, and E. faecalis. The MIC was studied for each one individually (melittin with MIC from 4 to 40 µg /mL and PLA2 with MIC values of >400 µg/mL) and in combination with each other (MIC values ranging 6–80 µg/mL) [4]. The combination of BV with ampicillin or penicillin yielded an index of inhibitory concentrations ranging from 0.631 to 1.002, indicating a partial synergistic effect. The two MRSA strains were more susceptible to the combination of BV with gentamicin or vancomycin compared to combination of BV with ampicillin or penicillin [5].
2.2. Anti-Viral
During the last decade, viral diseases such as hepatitis C, smallpox, polio, rubella, and AIDS have threatened the lives of millions worldwide, especially immunocompromised patients [71]. Water contamination (waterborne diseases) represents a major health problem in regards to the spread of many viral diseases like hepatitis viral disease, poliomyelitis, gastroenteritis, diarrhea, etc. [72,73,74]. Searching for anti-viral substitutes that are low or completely free of diverse effects is an urgent need. In this context, natural products, in particular BV, embody a variable of exotic constituents, suggesting an immeasurable source of anti-viral agents [75].
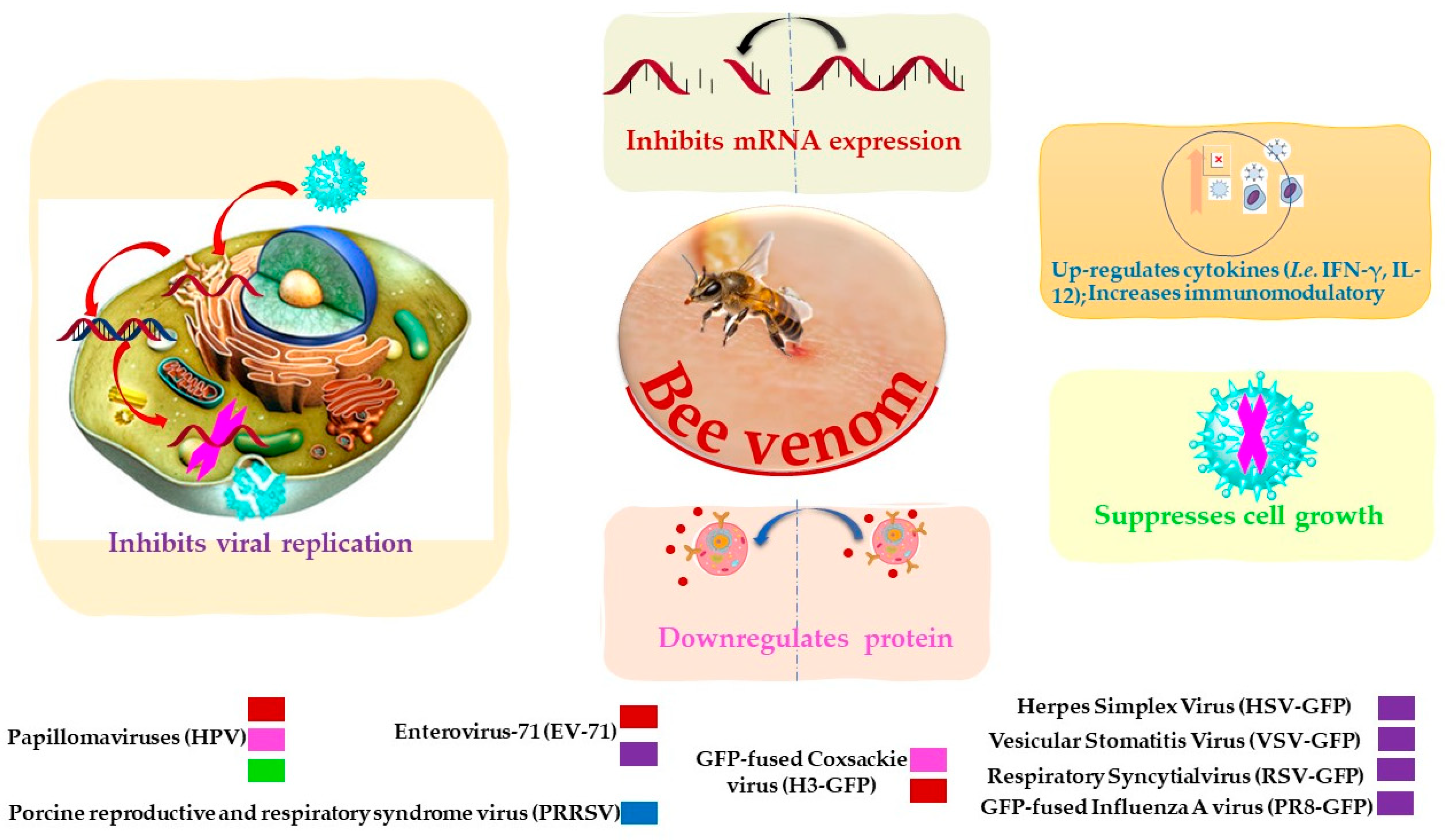
BV and its constituents show prominent anti-viral activities against various enveloped and non-enveloped viruses such as Vesicular Stomatitis (VSV), Herpes Simplex (HSV), Enterovirus-71 (EV-71), Coxsackie (H3), Respiratory Syncytial Influenza A (A/PuertoRico/8/34) (in vitro study), and influenza A subtype (H1N1) (in vivo study) (Table 2 and Figure 2) [76]. Papillomaviruses (HPVs) are considered the most common agents responsible for cervical carcinoma. BV was able to inhibit the growth of cervical cancer cells by the downregulation of E6/E7 proteins of HPV viruses (Table 2) [77]. BV and its constituent melittin (Figure 1) can induce the immunity against porcine reproductive and respiratory syndrome viruses (PRRSV) via significant up-regulation of Th1 cytokines (IFN-γ and IL-12) and several types of immune cells, including CD3+CD8+, CD4+CD8+, and γδ T cells, leading to reduction of the viral load and decrease of the severity of interstitial pneumonia in PRRSV-infected pigs [78].

Table 2.
Bee venom and its compounds as antiviral agents.
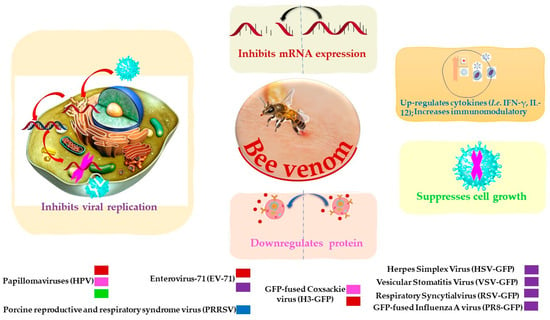
Figure 2.
Possible inhibitory mechanisms of bee venom against a variety of viruses.
Based on the HIV tropism, honeybee PLA2 and its derivatives p3bv (containing 21–35 amino acids of PLA2) possess potent anti-human immunodeficiency virus (HIV) activity. The p3bv peptide showed anti-HIV activity via the prevention of the cell–cell fusion process and inhibition of the replication of T-tropic viruses in contrast to PLA2 that inhibited both M- and T-tropic HIV viruses but was unable to inhibit cell–cell fusion under the same condition. The authors illustrated that the mechanism behind the inhibition of HIV replication is different for the two enzymes. PLA2 is presumably linked to a high-affinity binding receptor of the host cells but P3bv peptide is linked with a CXCR4 chemokine receptor [79,80]. Another type of BV phospholipases A2 called sPLA2 was evaluated using plaque assay and proven to suppress the activity of Japanese encephalitis virus (JEV), Hepatitis C virus (HCV), and Dengue virus (DENV) with IC50 values of 49, 117, and 183 ng/mL, respectively [81].
Furthermore, melittin represents an agent against Arenavirus Junin (JV) and Herpes Simplex Types 1 (HSV-1) and 2 (HSV-2) via inhibition of virus multiplication, adsorption, and penetration, as well as Na+ and K+ pumps of the host cell. Utilizing plaque and viral penetration assays, melittin at a multiplicity of infections (m.i.o) of 0.02 and 0.05 µM inhibited plaque formation giving 37 plaque formation units (PFU) compared to 220 PFU observed in the absence of melittin [82,83]. In another in vitro study, melittin was evaluated using the plaque assay against different viruses, namely Respiratory syncytial virus (RSV), EV-71, HSV, H3, Fused Influenza A virus (PR8), and VSV, with EC50 values of 0.35 ± 0.08, 0.76 ± 0.03, 0.94 ± 0.07, 0.99 ± 0.09, 1.15 ± 0.09, and 1.18 ± 0.09 μg/mL, respectively [76]. Furthermore, melittin could also be used against HIV-1, as it can inhibit the replication of HIV-1 by interfering with host cell-directed viral gene expression [84]. In an in vivo study, melittin was examined against lethal doses of the pathogenic H1N1 virus in mice; the results show that melittin can inhibit the replication of the virus, as the Log10 50% tissue culture infectious dose of a virus (TCID50) was 1.53 ± 0.25, compared to phosphate-buffered saline (PBS) at Log10 TCID50 4.22 ± 0.2 [76]. Taken together, these results suggest that BV and its constituents have the potential to become therapeutic agents to combat infectious viral diseases.
2.3. Anti-fungal
Fungal related diseases cause colonization, superficial skin infections, and allergies, representing a devastating health problem worldwide. Additionally, the toxicity and resistance to antifungal drugs are major challenges. Natural products from plants, marine life, microorganisms, and bee products could be considered promising antifungal agents with fewer side effects [88].
Recently, BV was reported as an effective agent against many of the fungal related diseases, as mentioned in Table 3. BV can inhibit dermatophytosis, which occurs via Trichophyton mentagrophytes and Trichophyton rubrum fungi. BV reduced all populations of T. mentagrophytes at 15 and 30 ppm within 5 min, while, at the same dose of BV, T. rubrum growth inhibition was observed within 5 min. On the other hand, fluconazole did not prevent the development of the same pathogens. The study proved that the BV was more potent than fluconazole (commercial antifungal drugs) [89]. The anti-fungal action of BV on 10 clinical isolates of Candida albicans was studied, with MIC values ranging from 62.5 to 125 μg/mL [88]. In another study, melittin showed antimicrobial activity against various strains of fungi with MIC values between 30 and 300 μg/mL [16]. Melittin produced oxygen free radicals (OH). that could induce apoptosis of C. albicans. The fungal cell death was explained by the disrupted mitochondrial membrane via the Ca2+ release [90,91].

Table 3.
Anti-fungi properties of bee venom and its compounds.
Alternaria alternate sp. and Aspergillus pillows are common pathogens that grow in the nasal cavity. The irritation/inflammation caused by the fungus induces the production of chemical mediators from nasal epithelial cells and fibroblasts. Melittin and apamin (Figure 1) were able to inhibit the growth of A. alternate sp. and A. pillows causing upper airway inflammatory diseases. The mechanism of action was shown to be via the inhibition of chemical mediators production, i.e., interleukin (IL)-6, IL-8, and ECM, as well as induction of the phosphorylation of Smad 2/3 and p38 MAPK [3].
3. Concluding Remarks
Microbial diseases are problematic, particularly with the emergence of drug resistance; therefore, researchers are looking for new sources of bioactive candidates. Natural products are considered a renewable source with fewer complications that could provide a wide number of active compounds. BV is a complex mixture of proteins, peptides, and low molecular weight components including melittin, PLA2, apamin, adolapin, and MCDP. BV contains a variety of bioactive components including melittin, apamin, and PLA2, which play a vital role as antimicrobials through various mechanisms against bacteria, viruses, and fungi. The synergistic effect of BV and melittin through the combination of chemotherapy drugs leads to a reduction in dosage, side effects, and greater efficacy of the treatment strategy against microbial ailments. BV established its role as antimicrobial.
BV and its constituents in combination with antibiotic drugs emerge as a plausible approach to overcome drug resistance of current antibiotics treatment in a controlled manner. Another promising and feasible implication is to test BV to combat microbes causing skin diseases. Interestingly, BV can be useful as a topical agent for encouraging skin regeneration or treatment of certain epidermal conditions [5,94]. Therefore, BV has contributed to some formulations against bacteria that cause acne [95,96].
Therefore, BV and melittin are attractive therapeutic candidates for microbial diseases. However, using BV and melittin induces extensive hemolysis and toxicity of the cells, a severe side effect that limits their future development and clinical application. Ongoing research is addressing practical issues including standardization, toxicity, and stability [97,98,99,100].
Funding
This work was supported by the Swedish Research Council Vetenskapsrådet (grants 2015-05468 and 2016-05885).
Acknowledgments
H. El-Seedi is very grateful to the Swedish Research links grant 2016-05885 (VR for the years 2017–2019) and the Department of Molecular Biosciences, Wenner-Grens Institute, Stockholm University, Sweden, for the financial support and S. Al-Mousawi and M. Moustafa are grateful to the Kuwait Foundation for the Advancement of Science, project number PR1714SC02.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rehman, K.; Fiayyaz, F.; Khurshid, M.; Sabir, S. Antibiotics and Antimicrobial Resistance: Temporal and Global Trends in the Environment; Elsevier Inc.: Amsterdam, The Netherlands, 2020; Chapter 2; ISBN 9780128188828. [Google Scholar]
- Memariani, H.; Memariani, M.; Shahidi-Dadras, M.; Nasiri, S.; Akhavan, M.M.; Moravvej, H. Melittin: From honeybees to superbugs. Appl. Microbiol. Biotechnol. 2019, 103, 3265–3276. [Google Scholar] [CrossRef]
- Shin, S.; Ye, M.; Choi, S.; Park, K. The effects of melittin and apamin on airborne Fungi-Induced chemical mediator and extracellular matrix production from nasal polyp fibroblasts. Toxicon 2017, 9, 384. [Google Scholar] [CrossRef]
- Leandro, L.F.; Mendes, C.A.; Casemiro, L.A. Antimicrobial activity of apitoxin, melittin and phospholipase A 2 of honey bee (Apis mellifera) venom against oral pathogens. An. Acad. Bras. Cienc. 2015, 87, 147–155. [Google Scholar] [CrossRef]
- Han, S.M.; Kim, J.M.; Hong, I.P.; Woo, S.O.; Kim, S.G.; Jang, H.R.; Pak, S.C. Antibacterial activity and antibiotic-enhancing effects of honeybee venom against methicillin-resistant Staphylococcus aureus. Molecules 2016, 21, 79. [Google Scholar] [CrossRef]
- Socarras, K.M.; Theophilus, P.A.S.; Torres, J.P.; Gupta, K.; Sapi, E. Antimicrobial activity of bee venom and melittin against borrelia burgdorferi. Antibiotics 2017, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, T.; Rima, M.; Karam, M.; Fajloun, J.M.S. Antimicrobials from venomous animals: An oeriew. Molecules 2020, 25, 2402. [Google Scholar] [CrossRef]
- De Barros, E.; Gonçalves, R.M.; Cardoso, M.H.; Santos, N.C.; Franco, O.L.; Cândido, E.S. Snake venom cathelicidins as natural antimicrobial peptides. Front. Pharmacol. 2019, 10, 1415–1427. [Google Scholar] [CrossRef]
- Das Neves, R.C.; Mortari, M.R.; Schwartz, E.F.; Kipnis, A.; Junqueira-Kipnis, A.P. Antimicrobial and antibiofilm effects of peptides from venom of social wasp and scorpion on multidrug-resistant Acinetobacter baumannii. Toxins 2019, 11, 216. [Google Scholar] [CrossRef]
- Samy, R.P.; Gopalakrishnakone, P.; Thwin, M.M.; Chow, T.K.V.; Bow, H.; Yap, E.H.; Thong, T.W.J. Antibacterial activity of snake, scorpion and bee venoms: A comparison with purified venom phospholipase A2 enzymes. J. Appl. Microbiol. 2007, 102, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Flávia, A.; Pereira, M.; Albano, M.; Cristina, F.; Alves, B.; Fernanda, B.; Teles, M.; Furlanetto, A.; Mores, V.L. Influence of apitoxin and melittin from Apis mellifera bee on Staphylococcus aureus strains. Microb. Pathog. 2020, 141, 104011. [Google Scholar]
- Wang, L.; Zhao, X.; Zhu, C.; Zhao, Y.; Liu, S.; Xia, X.; Liu, X.; Zhang, H.; Xu, Y.; Hang, B.; et al. The antimicrobial peptide MPX kills Actinobacillus pleuropneumoniae and reduces its pathogenicity in mice. Vet. Microbiol. 2020, 243, 108634. [Google Scholar] [CrossRef] [PubMed]
- Baracchi, D.; Francese, S.; Turillazzi, S. Beyond the antipredatory defence: Honey bee venom function as a component of social immunity. Toxicon 2011, 58, 550–557. [Google Scholar] [CrossRef]
- Pak, S.C. An introduction to the Toxins special issue on “Bee and wasp venoms: Biological characteristics and therapeutic application”. Toxins 2016, 8, 315. [Google Scholar] [CrossRef] [PubMed]
- Bellik, Y. Bee Venom: Its potential use in alternative medicine. Anti-Infect. Agents 2015, 13, 3–16. [Google Scholar] [CrossRef]
- AL-Ani, I.; Zimmermann, S.; Reichling, J.; Wink, M. Pharmacological synergism of bee venom and melittin with antibiotics and plant secondary metabolites against multi-drug resistant microbial pathogens. Phytomedicine 2015, 22, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Janik, J.E.; Wania-Galicia, L.; Kalauokalani, D. Bee stings—A remedy for postherpetic neuralgia? A case report. Reg. Anesth. Pain Med. 2007, 32, 533–535. [Google Scholar] [CrossRef]
- Dong, J.; Ying, B.; Huang, S.; Ma, S.; Long, P.; Tu, X.; Yang, W.; Wu, Z.; Chen, W.; Miao, X. High-Performance liquid chromatography combined with intrinsic fluorescence detection to analyse melittin in individual honeybee (Apis mellifera) venom sac. J. Chromatogr. B 2015, 1002, 139–143. [Google Scholar] [CrossRef]
- Huh, J.; Kang, J.W.; Nam, D.; Baek, Y.; Choi, D. Melittin suppresses VEGFA-induced tumor growth by blocking VEGFR-2 and the COX-2-mediated MAPK signaling pathway. J. Nat. Prod. 2012, 75, 1922–1929. [Google Scholar] [CrossRef]
- Buku, A.; Reibman, J.; Pistelli, A.; Blandina, P.; Gazis, D. Mast cell degranulating (MCD) peptide analogs with reduced ring structure. J. Protein Chem. 1992, 11, 275–280. [Google Scholar] [CrossRef]
- Mourelle, D.; Brigatte, P.; Bringanti, L.D.B.; De Souza, B.M.; Arcuri, H.A.; Gomes, P.C.; Baptista-Saidemberg, N.B.; Ruggiero Neto, J.; Palma, M.S. Hyperalgesic and edematogenic effects of Secapin-2, a peptide isolated from Africanized honeybee (Apis mellifera) venom. Peptides 2014, 59, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Yang, X.X.; Sheng, Y.X.; Zhang, J.L.; Yu, D.Q. A novel peptide from Apis mellifera and solid-phase synthesis of its analogue. Chin. Chem. Lett. 2012, 23, 1161–1164. [Google Scholar] [CrossRef]
- Gauldie, J.; Hanson, J.M.; Shipolini, R.A.; Vernon, C.A. The structures of some peptides from bee venom. Eur. J. Biochem. 1978, 83, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Shkenderov, S.; Koburova, K. Adolapin—A newly isolated analgetic and anti-inflammatory polypeptide from bee venom. Toxicon 1982, 20, 317–321. [Google Scholar] [CrossRef]
- Drici, M.D.; Diochot, S.; Terrenoire, C.; Romey, G.; Lazdunski, M. The bee venom peptide tertiapin underlines the role of I(KACh) in acetylcholine-induced atrioventricular blocks. Br. J. Pharmacol. 2000, 131, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, D.; Greunke, K.; Genov, N.; Betzel, C. 3-D Model of the bee venom acid phosphatase: Insights into allergenicity. Biochem. Biophys. Res. Commun. 2009, 378, 711–715. [Google Scholar] [CrossRef]
- Rybak-Chmielewska, H.; Szczęsna, T. HPLC study of chemical composition of honeybee (Apis mellifera L.) venom. J. Apic. Sci. 2004, 48, 103–109. [Google Scholar]
- Shipolini, R.A.; Callewaert, G.L.; Cottrell, R.C.; Doonan, S.; Vernon, C.A.; Banks, B.E. Phospholipase A from bee venom. Eur. J. Biochem. 1971, 20, 459–468. [Google Scholar] [CrossRef]
- Blank, S.; Seismann, H.; Bockisch, B.; Braren, I.; Cifuentes, L.; McIntyre, M.; Rühl, D.; Ring, J.; Bredehorst, R.; Ollert, M.W.; et al. Identification, recombinant expression, and characterization of the 100 kDa high molecular weight Hymenoptera venom allergens Api m 5 and Ves v 3. J. Immunol. 2010, 184, 5403–5413. [Google Scholar]
- Kettner, A.; Hughes, G.J.; Frutiger, S.; Astori, M.; Roggero, M.; Spertini, F.; Corradin, G. Api m 6: A new bee venom allergen. J. Allergy Clin. Immunol. 2001, 107, 914–920. [Google Scholar] [CrossRef]
- Georgieva, D.; Greunke, K.; Betzel, C. Three-dimensional model of the honeybee venom allergen Api m 7: Structural and functional insights. Mol. Biosyst. 2010, 6, 1056–1060. [Google Scholar] [CrossRef]
- Blank, S.; Seismann, H.; Michel, Y.; Mcintyre, M.; Cifuentes, L.; Braren, I.; Grunwald, T.; Darsow, U.; Ring, J.; Bredehorst, R.; et al. Api m 10, a genuine A. mellifera venom allergen, is clinically relevant but underrepresented in therapeutic extracts. Eur. J. Allergy Clin. Immunol. 2011, 66, 1322–1329. [Google Scholar] [CrossRef] [PubMed]
- Blank, S.; Bantleon, F.I.; McIntyre, M.; Ollert, M.; Spillner, E. The major royal jelly proteins 8 and 9 (Api m 11) are glycosylated components of Apis mellifera venom with allergenic potential beyond carbohydrate-based reactivity. Clin. Exp. Allergy 2012, 42, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Peiren, N.; de Graaf, D.C.; Vanrobaeys, F.; Danneels, E.L.; Devreese, B.; Van Beeumen, J.; Jacobs, F.J. Proteomic analysis of the honey bee worker venom gland focusing on the mechanisms of protection against tissue damage. Toxicon 2008, 52, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Pickets, J.A.; Williams, I.H.; Martin, A.P. (Z)-11-Eicosen-1-ol, an important new pheromonal component from the sting of the honey bee, Apis mellifera L. (Hymenoptera, Apidae). J. Chem. Ecol. 1982, 8, 163–175. [Google Scholar] [CrossRef]
- Kocyigit, A.; Guler, E.M.; Kaleli, S. Anti-inflammatory and antioxidative properties of honey bee venom on freund’s complete adjuvant-induced arthritis model in rats. Toxicon 2019, 161, 4–11. [Google Scholar] [CrossRef]
- Sobral, F.; Sampaio, A.; Falcão, S.; João, M.; Queiroz, R.P.; Ricardo, C. Chemical characterization, antioxidant, anti-inflammatory and cytotoxic properties of bee venom collected in Northeast Portugal Filipa. Food Chem. Toxicol. 2016, 94, 172–177. [Google Scholar] [CrossRef]
- Varanda, E.A.; Tavares, D.C. Radioprotection: Mechanisms and radioprotective agents including honeybee venom. J. Venom. Anim. Toxins 1998, 4, 5–21. [Google Scholar] [CrossRef]
- Han, S.; Yeo, J.; Baek, H.; Lin, S.M.; Meyer, S.; Molan, P. Postantibiotic effect of purified melittin from honeybee (Apis mellifera) venom against Escherichia coli and Staphylococcus aureus. J. Asian Nat. Prod. Res. 2009, 11, 796–804. [Google Scholar] [CrossRef]
- Han, S.M.; Lee, K.G.; Yeo, J.H.; Baek, H.J.; Park, K. Antibacterial and anti-inflammatory effects of honeybee (Apis mellifera) venom against acne-inducing bacteria. J. Med. Plants Res. 2010, 4, 459–464. [Google Scholar]
- McGhee, S.; Visovksy, C.; Zambroski, C.; Finnegan, A. Lyme disease: Recognition and management for emergency nurses. Emerg. Nurse 2018, 28. [Google Scholar] [CrossRef]
- Arteaga, V.; Lamas, A.; Regal, P.; Vázquez, B.; Manuel, J.; Cepeda, A.; Manuel, C. Antimicrobial activity of apitoxin from Apis mellifera in Salmonella enterica strains isolated from poultry and its effects on motility, biofilm formation and gene expression. Microb. Pthogenesis 2019, 137, 103771–103776. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.; Lee, J.; Park, S.; Hyun, P.; Park, J.; Suh, G. Immunoprophylactic effects of administering honeybee (Apis melifera ) venom spray against salmonella gallinarum in broiler chicks. J. Vet. Sci. 2013, 75, 1287–1295. [Google Scholar] [CrossRef]
- Choi, J.H.; Jang, A.Y.; Lin, S.; Lim, S.; Kim, D.; Park, K.; Han, S.M.; Yeo, J.H.; Seo, H.S. Melittin, a honeybee venom-derived antimicrobial peptide, may target methicillin-resistant Staphylococcus aureus. Mol. Med. Rep. 2015, 12, 6483–6490. [Google Scholar] [CrossRef] [PubMed]
- Jamasbi, E.; Mularski, A.; Separovic, F. Model membrane and cell studies of antimicrobial activity of melittin analogues. Curr. Top. Med. Chem. 2016, 16, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Picoli, T.; Peter, C.M.; Zani, J.L.; Waller, S.B.; Lopes, M.G.; Boesche, K.N.; D’Ávila Vargas, G.; de Oliveira Hübner, S.; Fischer, G. Melittin and its potential in the destruction and inhibition of the biofilm formation by Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa isolated from bovine milk. Microb. Pathog. 2017, 112, 57–62. [Google Scholar] [CrossRef]
- Lubke, L.L.; Garon, C.F. The antimicrobial agent melittin exhibits powerful in vitro inhibitory effects on the lyme disease spirochete. Clin. Infect. Dis. 1997, 25, S48–S51. [Google Scholar] [CrossRef] [PubMed]
- Navarre, W.W.; Schneewind, O. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol. Mol. Biol. Rev. 1999, 63, 174–229. [Google Scholar] [CrossRef]
- Lüderitz, O.; Freudenberg, M.A.; Galanos, C.; Lehmann, V.; Rietschel, E.T.; Shaw, D.H. Lipopolysaccharides of gram-negative bacteria. Curr. Top. Membr. Transp. 1982, 17, 79–151. [Google Scholar]
- Jamasbi, E.; Batinovic, S.; Sharples, R.A.; Sani, M.A.; Robins-Browne, R.M.; Wade, J.D.; Separovic, F.; Hossain, M.A. Melittin peptides exhibit different activity on different cells and model membranes. Amino Acids 2014, 46, 2759–2766. [Google Scholar] [CrossRef]
- Park, D.; Jung, J.W.; Lee, M.O.; Lee, S.Y.; Kim, B.; Jin, H.J.; Kim, J.; Ahn, Y.J.; Lee, K.W.; Song, Y.S.; et al. Functional characterization of naturally occurring melittin peptide isoforms in two honey bee species, Apis mellifera and Apis cerana. Peptides 2014, 53, 185–193. [Google Scholar] [CrossRef]
- Akbari, R.; Hakemi, M.; Ali, V.; Hossein, H.; Jean, A.; Sabatier, M. Action mechanism of melittin—derived antimicrobial peptides, MDP1 and MDP2, de novo designed against multidrug resistant bacteria. Amino Acids 2018, 50, 1231–1243. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Singh, A.K.; Wu, X.; Lyu, Y.; Bhunia, A.K.; Narsimhan, G. Characterization of antimicrobial activity against Listeria and cytotoxicity of native melittin and its mutant variants. Colloids Surf. B Biointerfaces 2016, 143, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Dosler, S.; Gerceker, A.A. In vitro activities of antimicrobial cationic peptides; melittin and nisin, alone or in combination with antibiotics against Gram-positive bacteria. J. Chemother. 2012, 24, 137–143. [Google Scholar] [CrossRef]
- Giacometti, A.; Cirioni, O.; Kamysz, W.; D’Amato, G.; Silvestri, C.; Del Prete, M.S.; Łukasiak, J.; Scalise, G. Comparative activities of cecropin A, melittin, and cecropin A–melittin peptide CA (1–7) M (2–9) NH2 against multidrug-resistant nosocomial isolates of Acinetobacter baumannii. Peptides 2003, 24, 1315–1318. [Google Scholar] [CrossRef]
- Akbari, R.; Hakemi-vala, M.; Pashaie, F.; Bevalian, P.; Hashemi, A.; Bagheri, K.P. Highly synergistic effects of melittin with conventional antibiotics against multidrug-resistant isolates of acinetobacter baumannii and pseudomonas aeruginosa. Microb. Drug Resist. 2018, 25, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-T.; Hwang, J.-Y.; Sung, M.-S.; Je, S.-Y.; Bae, D.-R.; Han, S.-M.; Lee, S.-H. The minimum inhibitory concentration (MIC) of bee venom against bacteria isolated from pigs and chickens. Korean J. Vet. Serv 2006, 29, 19–26. [Google Scholar]
- Fernández, N.J.; Porrini, M.P.; Podaza, E.A.; Damiani, N.; Gende, L.B.; Eguaras, M.J. A scientific note on the first report of honeybee venom inhibiting Paenibacillus larvae growth. Apidologie 2014, 45, 719–721. [Google Scholar] [CrossRef]
- Zolfagharian, H.; Mohajeri, M.; Babaie, M. Bee venom (Apis mellifera) an effective potential alternative to gentamicin for specific bacteria strains: Bee venom an effective potential for bacteria. J. Pharmacopunct. 2016, 19, 225–230. [Google Scholar] [CrossRef]
- Frangieh, J.; Salma, Y.; Haddad, K.; Mattei, C.; Legros, C.; Fajloun, Z.; El Obeid, D. First characterization of the venom from Apis mellifera syriaca, a honeybee from the middle east region. Toxins 2019, 11, 191. [Google Scholar] [CrossRef]
- Vila-Farres, X.; Garcia de la Maria, C.; López-Rojas, R.; Pachón, J.; Giralt, E.; Vila, J. In vitro activity of several antimicrobial peptides against colistin-susceptible and colistin-resistant Acinetobacter baumannii. Clin. Microbiol. Infect. 2012, 18, 383–387. [Google Scholar] [CrossRef]
- Pashaei, F.; Bevalian, P.; Akbari, R.; Bagheri, K.P. Single dose eradication of extensively drug resistant Acinetobacter spp. in a mouse model of burn infection by melittin antimicrobial peptide. Microb. Pathog. 2019, 127, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Yokota, H.; Nagashima, Y.; Shiomi, K. Interaction of grammistins with lipids and their antibacterial activity. Fish. Sci. 2001, 67, 928–933. [Google Scholar] [CrossRef]
- Karyne, R.; Lechuga, G.C.; Souza, A.L.A.; das Silva Carvalho, J.P.R.; Bôas, M.H.S.V.; De Simone, S.G. Pan-drug resistant acinetobacter baumannii, but not other strains, are resistant to the bee venom peptide mellitin. Antibiotics 2020, 9, 178. [Google Scholar]
- Jamasbi, E.; Lucky, S.S.; Li, W.; Akhter, M.; Gopalakrishnakone, P.; Separovic, F. Effect of dimerized melittin on gastric cancer cells and antibacterial activity. Amino Acids 2018, 50, 1101–1110. [Google Scholar] [CrossRef]
- Asthana, N.; Yadav, S.P.; Ghosh, J.K. Dissection of antibacterial and toxic activity of melittin: A leucine zipper motif plays a crucial role in determining its hemolytic activity but not antibacterial activity. J. Biol. Chem. 2004, 279, 55042–55050. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Li, W.; Zhang, L.; Zhang, Y.; Cao, B. Cecropin A—melittin mutant with improved proteolytic stability and enhanced antimicrobial activity against bacteria and fungi associated with gastroenteritis in vitro. Biochem. Biophys. Res. Commun. 2014, 451, 650–655. [Google Scholar] [CrossRef]
- Saugar, J.M.; Alarcón, T.; López-Hernández, S.; López-Brea, M.; Andreu, D.; Rivas, L. Activities of polymyxin B and cecropin A-melittin peptide CA (1–8) M (1–18) against a multiresistant strain of acinetobacter baumannii. Antimicrob. Agents Chemother. 2002, 46, 875–878. [Google Scholar] [CrossRef]
- Saravanan, R.; Bhunia, A.; Bhattacharjya, S. Micelle-bound structures and dynamics of the hinge deleted analog of melittin and its diastereomer: Implications in cell selective lysis by d-amino acid containing antimicrobial peptides. Biochim. Biophys. Acta-Biomembr. 2010, 1798, 128–139. [Google Scholar] [CrossRef]
- Sun, X.; Chen, S.; Li, S.; Yan, H.; Fan, Y.; Mi, H. Deletion of two C-terminal Gln residues of 12–26-residue fragment of melittin improves its antimicrobial activity. Peptides 2005, 26, 369–375. [Google Scholar] [CrossRef]
- Da Silva, C.M.; da Silva, D.L.; Modolo, L.V.; Alves, R.B.; de Resende, M.A.; Martins, C.V.B.; de Fátima, Â. Schiff bases: A short review of their antimicrobial activities. J. Adv. Res. 2011, 2, 1–8. [Google Scholar] [CrossRef]
- Soboksa, N.E.; Gari, S.R.; Hailu, A.B.; Alemu, B.M. Association between microbial water quality, sanitation and hygiene practices and childhood diarrhea in Kersa and Omo Nada districts of Jimma Zone, Ethiopia. PLoS ONE 2020, 15, e0229303. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Izopet, J.; Pavio, N.; Aggarwal, R.; Labrique, A.; Wedemeyer, H.; Dalton, H.R. Hepatitis E virus infection. Nat. Rev. Dis. Prim. 2017, 3, 17086–17101. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.K. Water-Associated Infectious Diseases; Springer Nature Singapore Pte Ltd.: Singapore, 2019; ISBN 9789811391972. [Google Scholar]
- Memariani, H.; Memariani, M.; Moravvej, H.; Shahidi-Dadras, M. Melittin: A venom-derived peptide with promising anti-viral properties. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.B.; Lee, B.-H.; Nikapitiya, C.; Kim, J.-H.; Kim, T.-H.; Lee, H.-C.; Kim, C.G.; Lee, J.-S.; Kim, C.-J. Inhibitory effects of bee venom and its components against viruses in vitro and in vivo. J. Microbiol. 2016, 54, 853–866. [Google Scholar] [CrossRef]
- Kim, Y.-W.; Chaturvedi, P.; Chun, S.; Lee, Y.; Ahn, W. Honeybee venom possesses anticancer and antiviral effects by differential inhibition of HPV E6 and�E7 expression on cervical cancer cell line. Oncol. Rep. 2015, 33, 1675–1682. [Google Scholar] [CrossRef]
- Lee, J.-A.; Kim, Y.-M.; Hyun, P.-M.; Jeon, J.-W.; Park, J.-K.; Suh, G.-H.; Jung, B.-G.; Lee, B.-J. Honeybee (Apis mellifera) venom reinforces viral clearance during the early stage of infection with porcine reproductive and respiratory syndrome virus through the up-regulation of Th1-specific immune responses. Toxins 2015, 7, 1837–1853. [Google Scholar] [CrossRef]
- Fenard, D.; Lambeau, G.; Maurin, T.; Lefebvre, J.-C.; Doglio, A. A peptide derived from bee venom-secreted phospholipase A2 inhibits replication of T-cell tropic HIV-1 strains via interaction with the CXCR4 chemokine receptor. Mol. Pharmacol. 2001, 60, 341–347. [Google Scholar] [CrossRef]
- Fenard, D.; Lambeau, G.; Valentin, E.; Lefebvre, J.-C.; Lazdunski, M.; Doglio, A. Secreted phospholipases A 2, a new class of HIV inhibitors that block virus entry into host cells. J. Clin. Investig. 1999, 104, 611–618. [Google Scholar] [CrossRef]
- Chen, M.; Aoki-Utsubo, C.; Kameoka, M.; Deng, L.; Terada, Y.; Kamitani, W.; Sato, K.; Koyanagi, Y.; Hijikata, M.; Shindo, K. Broad-spectrum antiviral agents: Secreted phospholipase A 2 targets viral envelope lipid bilayers derived from the endoplasmic reticulum membrane. Sci. Rep. 2017, 7, 15931. [Google Scholar] [CrossRef]
- Albiol Matanic, V.C.; Castilla, V. Antiviral activity of antimicrobial cationic peptides against Junin virus and herpes simplex virus. Int. J. Antimicrob. Agents 2004, 23, 382–389. [Google Scholar] [CrossRef]
- Baghian, A.; Kousoulas, K.G. Role of the Na+, K+ pump in herpes simplex type 1-induced cell fusion: Melittin causes specific reversion of syncytial mutants with the syn1 mutation to Syn+ (wild-type) phenotype. Virology 1993, 196, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Wachinger, M.; Kleinschmidt, A.; Winder, D.; von Pechmann, N.; Ludvigsen, A.; Neumann, M.; Holle, R.; Salmons, B.; Erfle, V.; Brack-Werner, R. Antimicrobial peptides melittin and cecropin inhibit replication of human immunodeficiency virus 1 by suppressing viral gene expression. J. Gen. Virol. 1998, 79, 731–740. [Google Scholar] [CrossRef]
- Kamal, S.A. In vitro study on the effect of bee venom on some cell lines and lumpy skin disease virus. J. Agric. Sci. Technol. A 2016, 6, 124–135. [Google Scholar]
- Fujii, G.; Horvath, S.; Woodward, S.; Eiserling, F.; Eisenberg, D. A molecular model for membrane fusion based on solution studies of an amphiphilic peptide from HIV gp41. Protein Sci. 1992, 1, 1454–1464. [Google Scholar] [CrossRef]
- Yasin, B.; Pang, M.; Turner, J.S.; Cho, Y.; Dinh, N.N.; Waring, A.J.; Lehrer, R.I.; Wagar, E.A. Evaluation of the inactivation of infectious herpes simplex virus by host-defense peptides. Eur. J. Clin. Microbiol. Infect. Dis. 2000, 19, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-B. Antifungal activity of bee venom and sweet bee venom against clinically isolated candida albicans. J. Pharmacopunct. 2016, 19, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.R.; Kim, J.J.; Park, G.S.; Oh, S.M.; Han, C.S.; Lee, M.Y. The antifungal activity of bee venom against dermatophytes. J. Appl. Biol. Chem. 2012, 55, 7–11. [Google Scholar] [CrossRef]
- Lee, J.; Lee, D.G. Melittin triggers apoptosis in Candida albicans through the reactive oxygen species-mediated mitochondria/caspase-dependent pathway. FEMS Microbiol. Lett. 2014, 355, 36–42. [Google Scholar] [CrossRef]
- Park, C.; Lee, D.G. Melittin induces apoptotic features in Candida albicans. Biochem. Biophys. Res. Commun. 2010, 394, 170–172. [Google Scholar] [CrossRef]
- Ali, E.M. Contributions of some biological activities of honey bee venom. J. Apic. Res. 2014, 53, 441–451. [Google Scholar] [CrossRef]
- Lee, J.; Lee, D.G. Influence of the hydrophobic amino acids in the N- and C-terminal regions of pleurocidin on antifungal activity. J. Microbiol. Biotechnol. 2010, 20, 1192–1195. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Gupta, A.; Karne, S.P.; Kamble, S.; Shinde, B. Anti-inflammatory activity of sting protein from Apis mellifera. Int. J. Life Sci. Sci. Res. 2017, 3, 914–919. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, W.R.; Kim, K.H.; An, H.J.; Chang, Y.C.; Han, S.M.; Park, Y.Y.; Pak, S.C.; Park, K.K. Effects of bee venom against Propionibacterium acnes-induced inflammation in human keratinocytes and monocytes. Int. J. Mol. Med. 2015, 35, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Han, S.M.; Pak, S.C.; Nicholls, Y.M.; Macfarlane, N. Evaluation of anti-acne property of purified bee venom serum in humans. J. Cosmet. Dermatol. 2016, 15, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Diwakar, L.; Ewan, P.; Huber, P.A.J.; Clark, A.; Nasser, S.; Krishna, M.T. The impact of national guidelines on venom immunotherapy practice in the United Kingdom. Clin. Exp. Allergy 2016, 46, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Safaeinejad, Z.; Nabiuni, M.; Nazari, Z. Potentiation of a novel palladium (II) complex lethality with bee venom on the human T-cell acute lymphoblastic leukemia cell line (MOLT-4). J. Venom. Anim. Toxins Incl. Trop. Dis. 2013, 19, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Alizadehnohi, M.; Nabiuni, M.; Nazari, Z.; Safaeinejad, Z.; Irian, S. The synergistic cytotoxic effect of cisplatin and honey bee venom on human ovarian cancer cell line A2780cp. J. Venom Res. 2012, 3, 22–27. [Google Scholar]
- Trindade, R.A.; Kiyohara, P.K.; De Araujo, P.S.; da Costa Bueno, M.H. PLGA microspheres containing bee venom proteins for preventive immunotherapy. Int. J. Pharm. 2012, 423, 124–133. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).