Bee Venom Melittin Protects against Cisplatin-Induced Acute Kidney Injury in Mice via the Regulation of M2 Macrophage Activation
Abstract
1. Introduction
2. Results
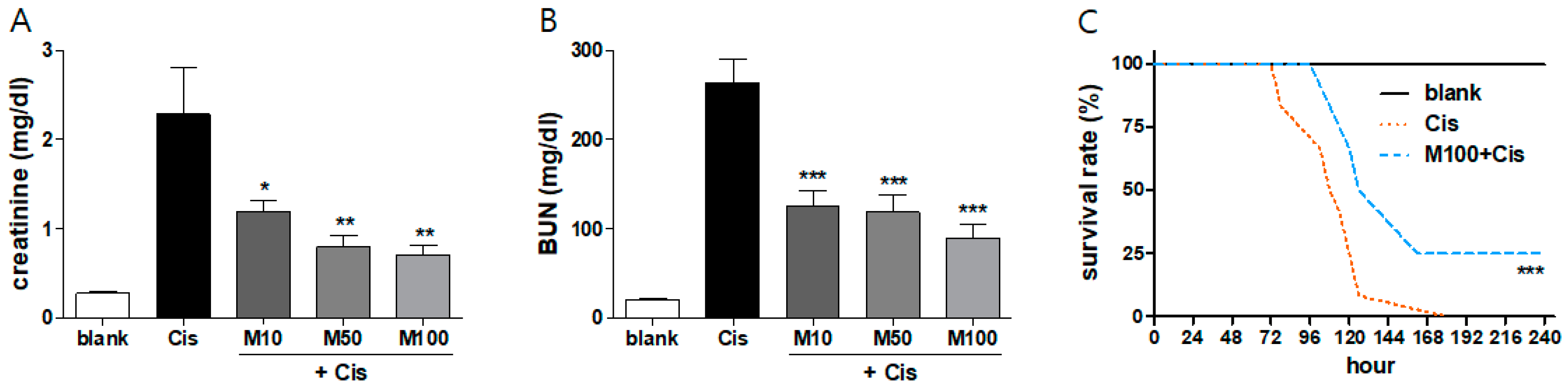
2.1. Protective Effects of Melittin on Cisplatin-Induced AKI in Mice
2.2. Reduction of Cisplatin-Induced Renal Tissue Damage by Melittin Administration
2.3. Effects of Melittin on Pro- and Anti-Inflammatory Cytokine Production in Mice with AKI
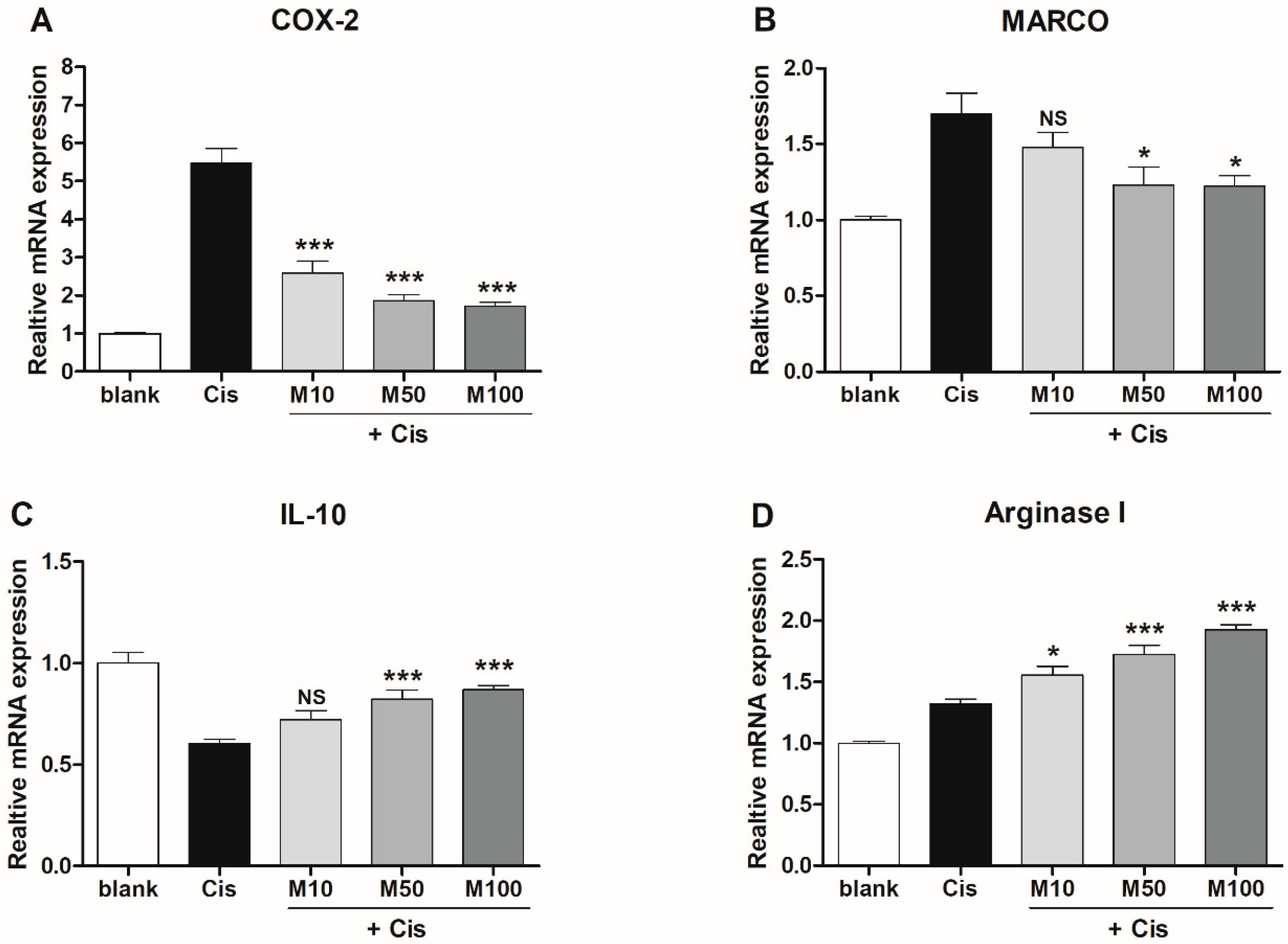
2.4. Effects of Melittin on the Expression of M1 and M2 Macrophage-Associated Genes in Mice with AKI
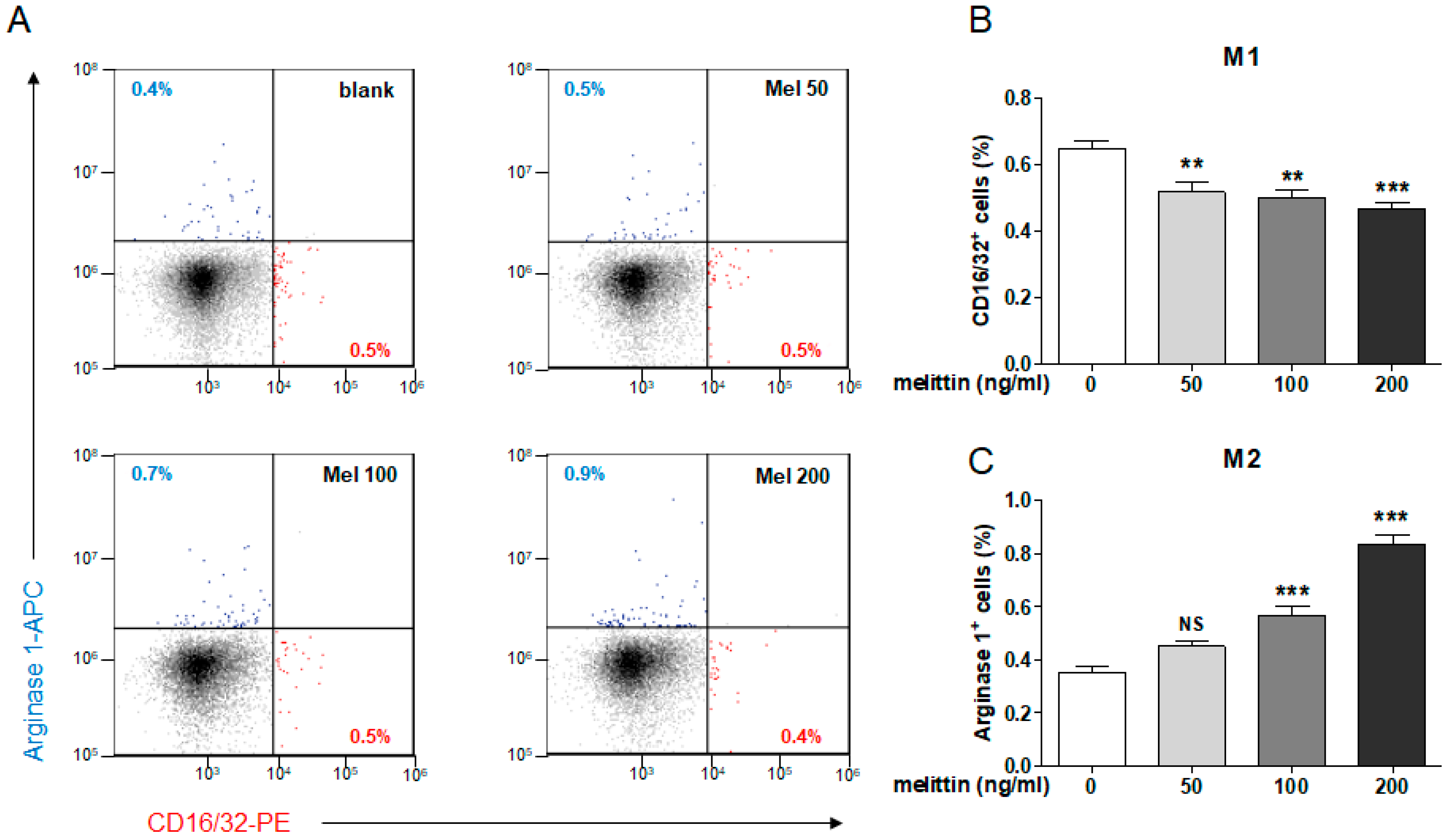
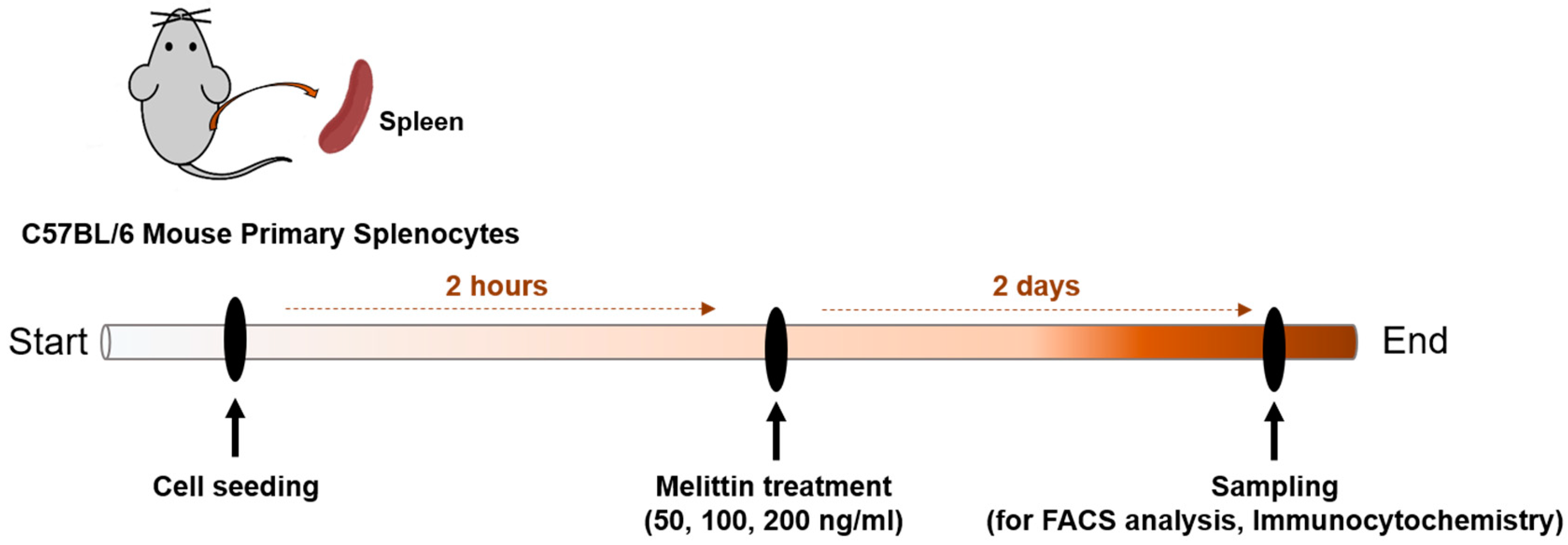
2.5. Effects of Melittin on M1 and M2 Macrophage Populations in Mouse Splenocytes
2.6. Effect of Melittin on M2 Macrophage Infiltration into the Kidney
3. Discussion
4. Materials and Methods
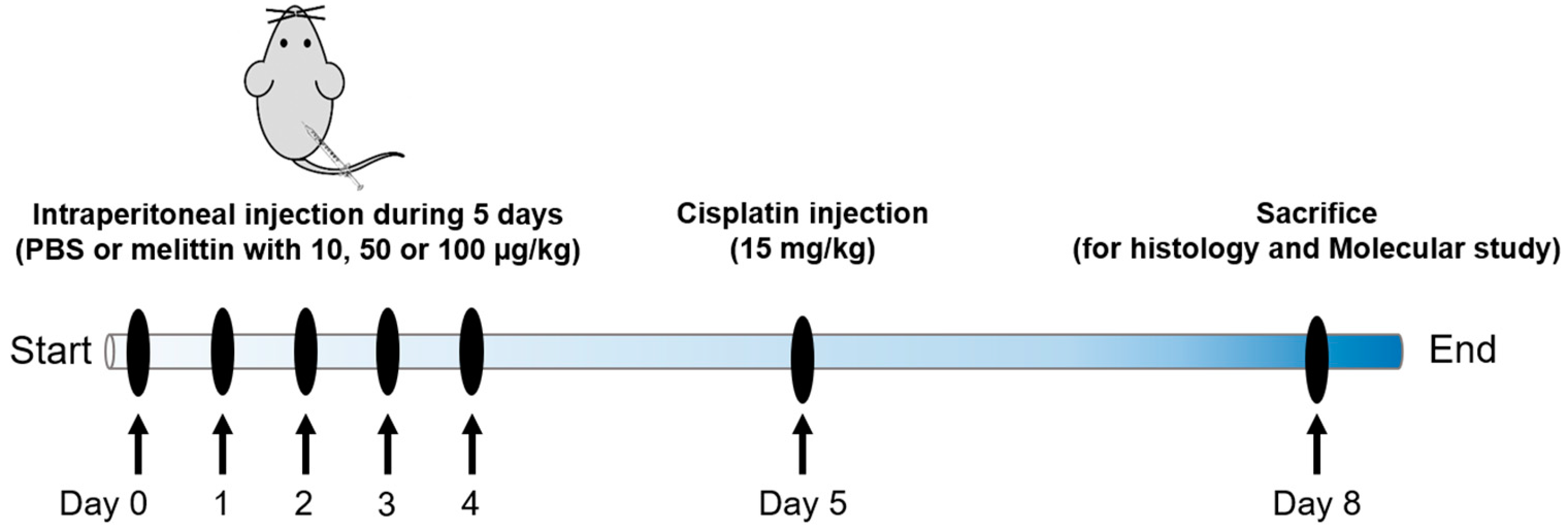
4.1. Animals and Drug Treatments
4.2. Renal Function Analysis
4.3. Morphological Assessment
4.4. ELISA
4.5. Real-Time PCR
4.6. Immunohistochemistry
4.7. Preparation of Murine Splenocytes
4.8. Statistics
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Oh, G.S.; Kim, H.J.; Shen, A.; Bin Lee, S.; Khadka, D.; Pandit, A.; So, H.S. Cisplatin-induced Kidney Dysfunction and Perspectives on Improving Treatment Strategies. Electrolytes Blood Press. 2014, 12, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Tbahriti, H.F.; Meknassi, D.; Moussaoui, R.; Messaoudi, A.; Zemour, L.; Kaddous, A.; Bouchenak, M.; Mekki, K. Inflammatory status in chronic renal failure: The role of homocysteinemia and pro-inflammatory cytokines. World J. Nephrol. 2013, 2, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Rabb, H.; Griffin, M.D.; McKay, D.B.; Swaminathan, S.; Pickkers, P.; Rosner, M.H.; Kellum, J.A.; Ronco, C. Inflammation in AKI: Current Understanding, Key Questions, and Knowledge Gaps. J. Am. Soc. Nephrol. 2015, 27, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Domitrović, R.; Cvijanovic, O.; Pernjak-Pugel, E.; Zagorac, G.B.; Mahmutefendic, H.; Škoda, M. Luteolin ameliorates cisplatin-induced nephrotoxicity in mice through inhibition of platinum accumulation, inflammation and apoptosis in the kidney. Toxicology 2013, 310, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.J.; Hou, J.G.; Wang, Z.; Han, Y.; Ren, S.; Hu, J.N.; Chen, C.; Li, W. The protective effects of maltol on cisplatin-induced nephrotoxicity through the AMPK-mediated PI3K/Akt and p53 signaling pathways. Sci. Rep. 2018, 8, 15922. [Google Scholar] [CrossRef]
- Kim, H.; Lee, H.; Lee, G.; Jang, H.; Kim, S.S.; Yoon, H.; Kang, G.H.; Hwang, D.S.; Kim, S.K.; Chung, H.S.; et al. Phospholipase A2 inhibits cisplatin-induced acute kidney injury by modulating regulatory T cells by the CD206 mannose receptor. Kidney Int. 2015, 88, 550–559. [Google Scholar] [CrossRef]
- Kim, H.; Keum, D.J.; Kwak, J.W.; Chung, H.-S.; Bae, H. Bee Venom Phospholipase A2 Protects against Acetaminophen-Induced Acute Liver Injury by Modulating Regulatory T Cells and IL-10 in Mice. PLoS ONE 2014, 9, e114726. [Google Scholar] [CrossRef]
- Kim, H.; Lee, G.; Park, S.; Chung, H.-S.; Lee, H.; Kim, J.-Y.; Nam, S.; Kim, S.K.; Bae, H. Bee Venom Mitigates Cisplatin-Induced Nephrotoxicity by Regulating CD4+CD25+Foxp3+ Regulatory T Cells in Mice. Evidence-Based Complement. Altern. Med. 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Deng, J.; Kohda, Y.; Chiao, H.; Wang, Y.; Hu, X.; Hewitt, S.M.; Miyaji, T.; McLeroy, P.; Nibhanupudy, B.; Li, S.; et al. Interleukin-10 inhibits ischemic and cisplatin-induced acute renal injury. Kidney Int. 2001, 60, 2118–2128. [Google Scholar] [CrossRef]
- Sinuani, I.; Beberashvili, I.; Averbukh, Z.; Sandbank, J. Role of IL-10 in the progression of kidney disease. World J. Transplant. 2013, 3, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Italiani, P.; Boraschi, D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Benakis, C.; García-Bonilla, L.; Iadecola, C.; Anrather, J. The role of microglia and myeloid immune cells in acute cerebral ischemia. Front. Cell. Neurosci. 2015, 8, 461. [Google Scholar] [CrossRef]
- Chã¡vez-Galã¡n, L.; Olleros, M.L.; Vesin, D.; Garcia, I. Much More than M1 and M2 Macrophages, There are also CD169+ and TCR+ Macrophages. Front. Immunol. 2015, 6, 263. [Google Scholar] [CrossRef]
- Gensel, J.C.; Zhang, B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 2015, 1619, 1–11. [Google Scholar] [CrossRef]
- Gensel, J.C.; Kopper, T.J.; Zhang, B.; Orr, M.B.; Bailey, W.M. Predictive screening of M1 and M2 macrophages reveals the immunomodulatory effectiveness of post spinal cord injury azithromycin treatment. Sci. Rep. 2017, 7, 40144. [Google Scholar] [CrossRef]
- Ozkok, A.; Edelstein, C.L. Pathophysiology of Cisplatin-Induced Acute Kidney Injury. BioMed Res. Int. 2014, 2014, 1–17. [Google Scholar] [CrossRef]
- Pabla, N.; Dong, Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008, 73, 994–1007. [Google Scholar] [CrossRef]
- Baek, Y.H.; Huh, J.E.; Lee, J.D.; Choi, D.Y.; Park, D.S. Antinociceptive effect and the mechanism of bee venom acupuncture (Apipuncture) on inflammatory pain in the rat model of collagen-induced arthritis: Mediation by α2-Adrenoceptors. Brain Res. 2006, 305–310. [Google Scholar] [CrossRef]
- Gajski, G.; Garaj-Vrhovac, V. Radioprotective Effects of Honeybee Venom (Apis mellifera) Against 915-MHz Microwave Radiation–Induced DNA Damage in Wistar Rat Lymphocytes: In Vitro Study. Int. J. Toxicol. 2009, 28, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.E.; Baek, Y.H.; Lee, M.H.; Choi, -Y.; Park, D.S.; Lee, J.D. Bee venom inhibits tumor angiogenesis and metastasis by inhibiting tyrosine phosphorylation of VEGFR-2 in LLC-tumor-bearing mice. Cancer Lett. 2010, 292, 98–110. [Google Scholar] [CrossRef]
- Jang, M.-H.; Shin, M.-C.; Lim, S.; Han, S.-M.; Park, H.-J.; Shin, I.; Lee, J.-S.; Kim, K.-A.; Kim, E.-H.; Kim, C.-J. Bee Venom Induces Apoptosis and Inhibits Expression of Cyclooxygenase-2 mRNA in Human Lung Cancer Cell Line NCI-H1299. J. Pharmacol. Sci. 2003, 91, 95–104. [Google Scholar] [CrossRef]
- Lee, K.-G.; Cho, H.-J.; Bae, Y.-S.; Park, K.-K.; Choe, J.-Y.; Chung, I.-K.; Kim, M.; Yeo, J.-H.; Park, K.-H.; Lee, Y.-S.; et al. Bee venom suppresses LPS-mediated NO/iNOS induction through inhibition of PKC-α expression. J. Ethnopharmacol. 2009, 123, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Rady, I.; Siddiqui, I.A.; Rady, M.I.; Mukhtar, H. Melittin, a major peptide component of bee venom, and its conjugates in cancer therapy. Cancer Lett. 2017, 402, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.; McClean, S. Melittin exhibits necrotic cytotoxicity in gastrointestinal cells which is attenuated by cholesterol. Biochem. Pharmacol. 2008, 75, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, K.H.; Lee, W.R.; Han, S.M.; Park, K.K. Protective effect of melittin on inflammation and apoptosis in acute liver failure. Apoptosis 2011, 17, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.R.; Kim, K.H.; An, H.J.; Kim, J.Y.; Chang, Y.C.; Chung, H.; Park, Y.Y.; Lee, M.L.; Park, K.K. The Protective Effects of Melittin on Propionibacterium acnes –Induced Inflammatory Responses In Vitro and In Vivo. J. Investig. Dermatol. 2014, 134, 1922–1930. [Google Scholar] [CrossRef] [PubMed]
- An, H.J.; Kim, J.Y.; Kim, W.H.; Han, S.M.; Park, K.K. The Protective Effect of Melittin on Renal Fibrosis in an Animal Model of Unilateral Ureteral Obstruction. Molecules 2016, 21, 1137. [Google Scholar] [CrossRef] [PubMed]
- Chawla, L.S.; Bellomo, R.; Bihorac, A.; Goldstein, S.L.; Siew, E.D.; Bagshaw, S.M.; Bittleman, D.; Cruz, D.; Endre, Z.H.; Fitzgerald, R.L.; et al. Acute kidney disease and renal recovery: Consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat. Rev. Nephrol. 2017, 13, 241–257. [Google Scholar] [CrossRef]
- Koumanov, K.; Momchilova, A.; Wolf, C. Bimodal regulatory effect of melittin and phospholipase A2-activating protein on human type II secretory phospholipase A2. Cell Boil. Int. 2003, 27, 871–877. [Google Scholar] [CrossRef]
- Asthana, N.; Yadav, S.P.; Ghosh, J.K. Dissection of Antibacterial and Toxic Activity of Melittin. J. Boil. Chem. 2004, 279, 55042–55050. [Google Scholar] [CrossRef] [PubMed]
- Rayahin, J.E.; Buhrman, J.S.; Gemeinhart, R.A. Melittin-glutathione S-transferase fusion protein exhibits anti-inflammatory properties and minimal toxicity. Eur. J. Pharm. Sci. 2014, 65, 112–121. [Google Scholar] [CrossRef] [PubMed]



| Gene | 5′–3′ | Primer Sequence |
|---|---|---|
| COX2 | Forward | ACTACGTGCAACACCTGAGC |
| Reverse | GAGTGAGTCCATGTTCCAGG | |
| IL-10 | Forward | TAACTGCACCCACTTCCCAG |
| Reverse | AGGCTTGGCAACCCAAGTAA | |
| MARCO | Forward | GCACAGAAGACAGAGCCGAT |
| Reverse | AGTGATCCATTGCCACAGCA | |
| Arginase 1 | Forward | GACAGGGCTCCTTTCAGGAC |
| Reverse | GCCAAGGTTAAAGCCACTGC | |
| β-actin | Forward | GTGATGGTGGGAATGGGTCAG |
| Reverse | TCACGGTTGGCCTTAGGGTTC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Hong, J.Y.; Jeon, W.-J.; Baek, S.H.; Ha, I.-H. Bee Venom Melittin Protects against Cisplatin-Induced Acute Kidney Injury in Mice via the Regulation of M2 Macrophage Activation. Toxins 2020, 12, 574. https://doi.org/10.3390/toxins12090574
Kim H, Hong JY, Jeon W-J, Baek SH, Ha I-H. Bee Venom Melittin Protects against Cisplatin-Induced Acute Kidney Injury in Mice via the Regulation of M2 Macrophage Activation. Toxins. 2020; 12(9):574. https://doi.org/10.3390/toxins12090574
Chicago/Turabian StyleKim, Hyunseong, Jin Young Hong, Wan-Jin Jeon, Seung Ho Baek, and In-Hyuk Ha. 2020. "Bee Venom Melittin Protects against Cisplatin-Induced Acute Kidney Injury in Mice via the Regulation of M2 Macrophage Activation" Toxins 12, no. 9: 574. https://doi.org/10.3390/toxins12090574
APA StyleKim, H., Hong, J. Y., Jeon, W.-J., Baek, S. H., & Ha, I.-H. (2020). Bee Venom Melittin Protects against Cisplatin-Induced Acute Kidney Injury in Mice via the Regulation of M2 Macrophage Activation. Toxins, 12(9), 574. https://doi.org/10.3390/toxins12090574






