Ethylenediaminetetraacetic Acid Disodium Salt Acts as an Antifungal Candidate Molecule against Fusarium graminearum by Inhibiting DON Biosynthesis and Chitin Synthase Activity
Abstract
:1. Introduction
2. Results
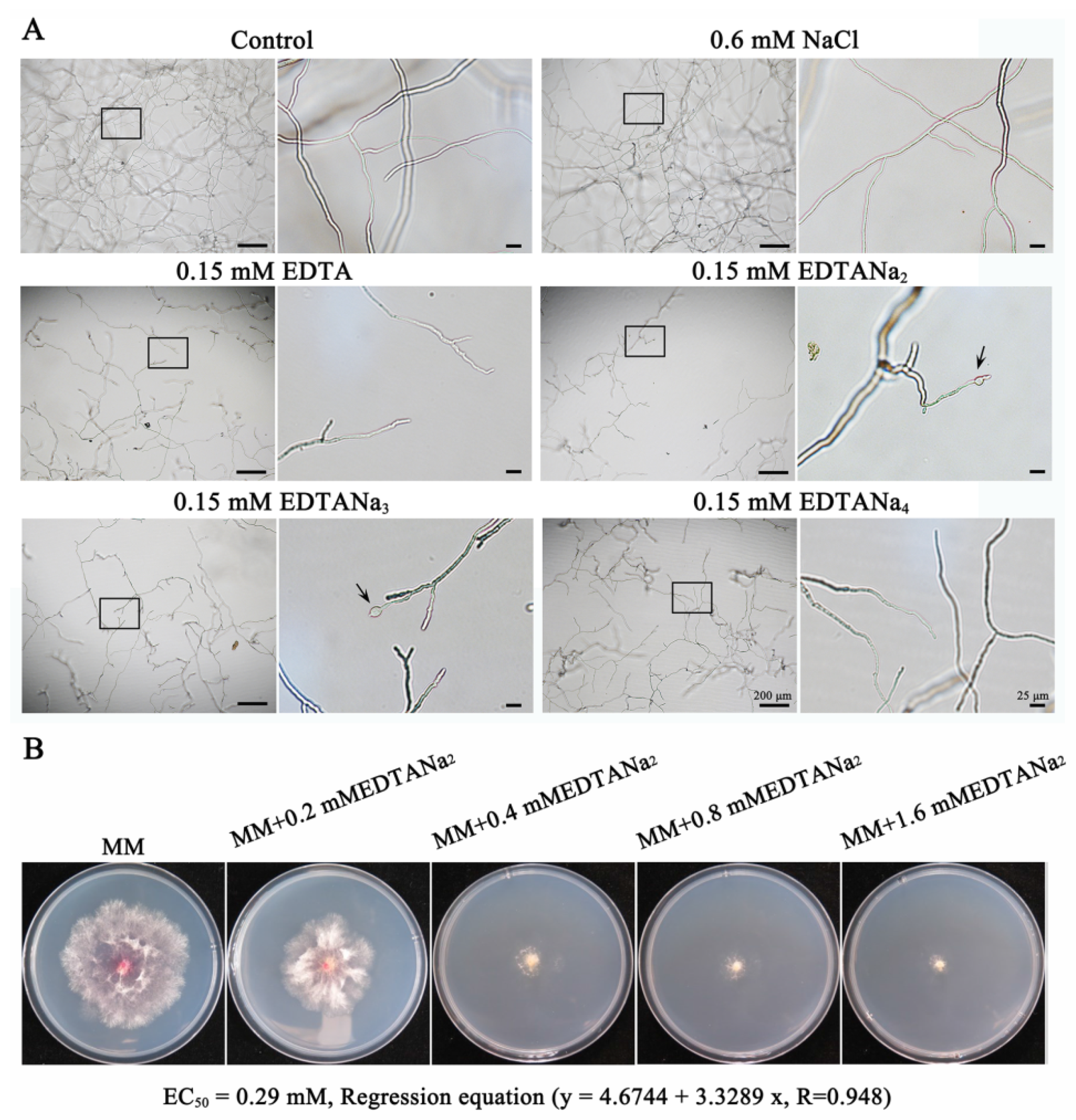
2.1. Ethylenediaminetetraacetic Acid Disodium Salt Exhibits Various Antifungal Activities against Fusarium Species
2.2. The Control Effect and Phytotoxicity Test of Ethylenediaminetetraacetic Acid Disodium Salt
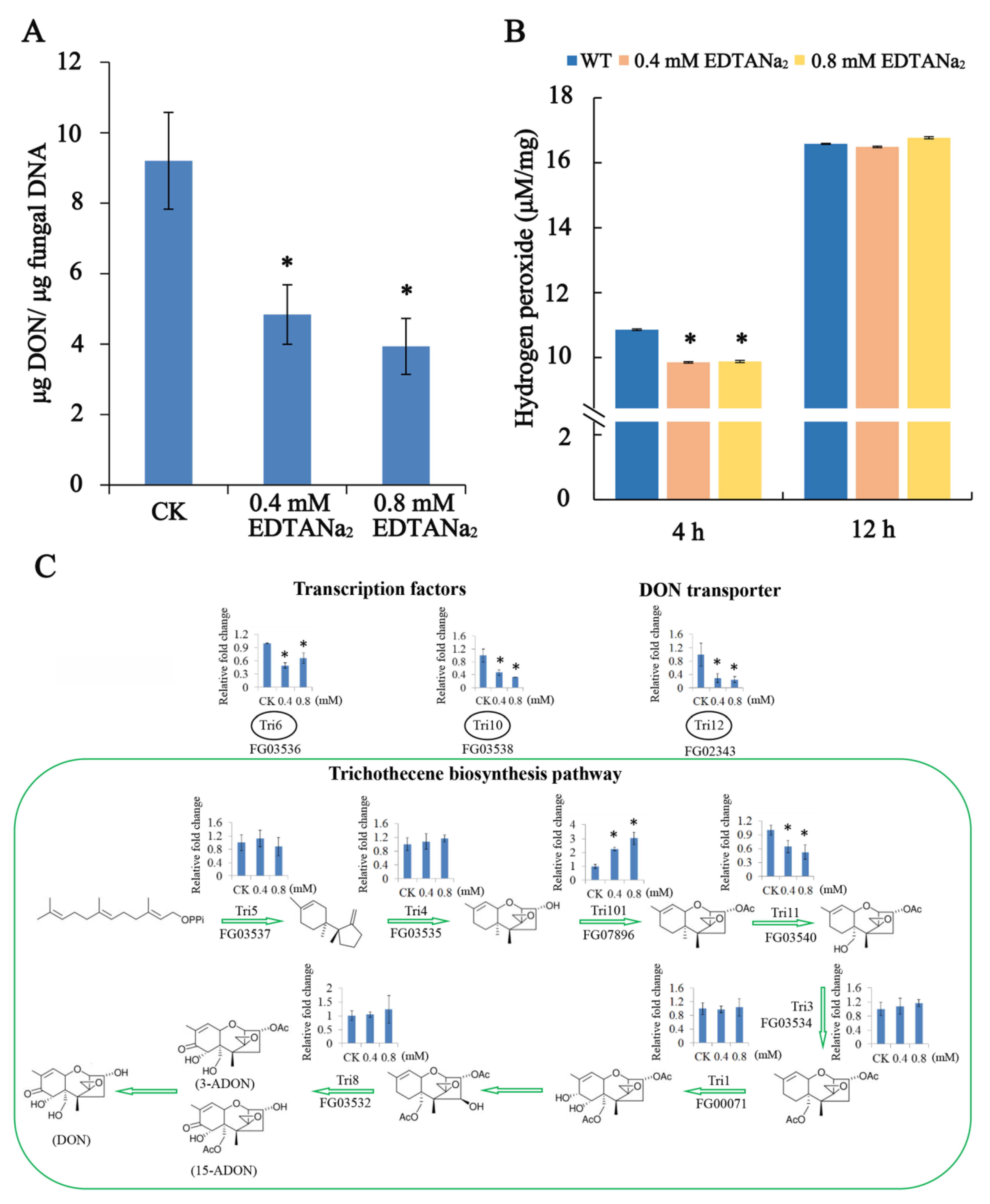
2.3. EDTANa2 Decreases DON Biosynthesis and TRI Gene Expression of Fusarium Graminearum In Vitro
2.4. Ethylenediaminetetraacetic Acid Disodium Salt Affected Cell Wall Formation and Cell Permeability
2.5. The Inhibitory Effects of Ethylenediaminetetraacetic Acid Disodium Salt against Fusarium are Ameliorated by Mn2+ but not Mg2+ and Ca2+
2.6. Chitin Synthases are More Active in F. graminearum When Mn2+ Is Used as a Cofactor
3. Discussion
4. Materials and Methods
4.1. Fungi, Plants, and Culture Conditions
4.2. Control Effect Measurement on Wheat Seedling Blight
4.3. Control Effect Measurement on Fusarium Head Blight and Phytotoxicity Field Test
4.4. Mycelial Growth Inhibition by EDTANa2
4.5. Optical, Scanning Electron Microscopy and Transmission Electron Microscopy Observation
4.6. Measurement of H2O2 and DON
4.7. Chitin Content, Chitin Synthase Activity and Cell Membrane Permeability Measurement
4.8. Fungicidal Activity of EDTANa(n) against Fusarium spp.
4.9. Effect of Metal Ions on EDTANa2 Activity
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ma, L.J.; Geiser, D.M.; Proctor, R.H.; Rooney, A.P.; O’Donnell, K.; Trail, F.; Gardiner, D.M.; Manners, J.M.; Kazan, K. Fusarium pathogenomics. Annu. Rev. Microbiol. 2013, 67, 399–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Summerell, B.A. Resolving Fusarium: Current status of the genus. Annu. Rev. Phytopathol. 2019, 25, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.E.; Dignani, M.C.; Anaissie, E.J. Taxonomy, biology, and clinical aspects of Fusarium species. Clin. Microbiol. Rev. 1994, 7, 479–504. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.H.; Shaner, G. Management and resistance in wheat and barley to Fusarium head blight. Annu. Rev. Phytopathol. 2004, 42, 135–161. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.R. Fusarium oxysporum and the Fusarium Wilt Syndrome. Annu. Rev. Phytopathol. 2017, 55, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Kazan, K.; Gardiner, D.M.; Manners, J.M. On the trail of a cereal killer: Recent advances in Fusarium graminearum pathogenomics and host resistance. Mol. Plant. Pathol. 2012, 13, 399–413. [Google Scholar] [CrossRef]
- Loffler, M.; Kessel, B.; Ouzunova, M.; Miedaner, T. Population parameters for resistance to Fusarium graminearum and Fusarium verticillioides ear rot among large sets of early, mid-late and late maturing European maize (Zea mays L.) inbred lines. Theor. Appl. Genet. 2010, 120, 1053–1062. [Google Scholar] [CrossRef]
- Xu, X.; Nicholson, P. Community ecology of fungal pathogens causing wheat head blight. Annu. Rev. Phytopathol. 2009, 47, 83–103. [Google Scholar] [CrossRef]
- Edwards, J.E., Jr.; Bodey, G.P.; Bowden, R.A.; Buchner, T.; de Pauw, B.E.; Filler, S.G.; Ghannoum, M.A.; Glauser, M.; Herbrecht, R.; Kauffman, C.A.; et al. International conference for the development of a consensus on the management and prevention of severe candidal infections. Clin. Infect Dis. 1997, 25, 43–59. [Google Scholar] [CrossRef]
- Treikale, O.; Priekule, I.; Javoisha, B.; Lazareva, L. Fusarium head blight: Distribution in wheat in Latvia. Commun. Agric. Appl. Biol. Sci. 2010, 75, 627–634. [Google Scholar]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Woloshuk, C.P.; Shim, W.B. Aflatoxins, fumonisins, and trichothecenes: A convergence of knowledge. Fems. Microbiol. Rev. 2013, 37, 94–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brauer, E.K.; Balcerzak, M.; Rocheleau, H.; Leung, W.; Schernthaner, J.; Subramaniam, R.; Ouellet, T. Genome editing of a deoxynivalenol-induced transcription factor confers resistance to Fusarium graminearum in wheat. Mol. Plant Microbe Interact. 2020, 33, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Dill-Macky, R.; Jones, R.K. The effect of previous crop residues and tillage on Fusarium head blight of wheat. Plant Dis. 2000, 84, 71–76. [Google Scholar] [CrossRef] [Green Version]
- McMullen, M.; Bergstrom, G.; De Wolf, E.; Dill-Macky, R.; Hershman, D.; Shaner, G.; Van Sanford, D. A unified effort to fight an enemy of wheat and barley: Fusarium head blight. Plant Dis. 2012, 96, 1712–1728. [Google Scholar] [CrossRef] [Green Version]
- D’Angelo, D.L.; Bradley, C.A.; Ames, K.A.; Willyerd, K.T.; Madden, L.V.; Paul, P.A. Efficacy of fungicide applications during and after anthesis against Fusarium head blight and deoxynivalenol in soft red winter wheat. Plant Dis. 2014, 98, 1387–1397. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.J.; Yu, J.J.; Zhang, Y.N.; Zhang, X.; Cheng, C.J.; Wang, J.X.; Hollomon, D.W.; Fan, P.S.; Zhou, M.G. Effect of carbendazim resistance on trichothecene production and aggressiveness of Fusarium graminearum. Mol. Plant Microbe Interact. 2009, 22, 1143–1150. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.; Shi, J. Genetic relationships, carbendazim sensitivity and mycotoxin production of the Fusarium graminearum populations from maize, wheat and rice in eastern China. Toxins 2014, 6, 2291–2309. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.J.; Wang, J.X.; Luo, Q.Q.; Yuan, S.K.; Zhou, M.G. Characterization and fitness of carbendazim-resistant strains of Fusarium graminearum (wheat scab). Pest. Manag. Sci. 2007, 63, 1201–1207. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, C.; Wang, J.; Jin, L.; Zhou, M. Genetic study on JS399-19 resistance in hyphal fusion of Fusarium graminearum by using nitrate nonutilizing mutants as genetic markers. J. Genet. Genom. 2007, 34, 469–476. [Google Scholar] [CrossRef]
- Mikaberidze, A.; Paveley, N.; Bonhoeffer, S.; van den Bosch, F. Emergence of resistance to fungicides: The role of fungicide dose. Phytopathology 2017, 107, 545–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Hatmi, A.M.; Meis, J.F.; de Hoog, G.S. Fusarium: Molecular diversity and intrinsic drug resistance. PLoS Pathog. 2016, 12, e1005464. [Google Scholar] [CrossRef] [PubMed]
- Cordova-Alcantara, I.M.; Venegas-Cortes, D.L.; Martinez-Rivera, M.A.; Perez, N.O.; Rodriguez-Tovar, A.V. Biofilm characterization of Fusarium solani keratitis isolate: Increased resistance to antifungals and UV light. J. Microbiol. 2019, 57, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Muregi, F.W. Antimalarial drugs and their useful therapeutic lives: Rational drug design lessons from pleiotropic action of quinolines and artemisinins. Curr. Drug Discov. Technol. 2010, 7, 280–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deising, H.B.; Reimann, S.; Pascholati, S.F. Mechanisms and significance of fungicide resistance. Braz. J. Microbiol. 2008, 39, 286–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Bakri, A.G.; Othman, G.; Bustanji, Y. The assessment of the antibacterial and antifungal activities of aspirin, EDTA and aspirin-EDTA combination and their effectiveness as antibiofilm agents. J. Appl. Microbiol. 2009, 107, 280–286. [Google Scholar] [CrossRef]
- Taha, M.O.; Al-Bakri, A.G.; Zalloum, W.A. Discovery of potent inhibitors of pseudomonal quorum sensing via pharmacophore modeling and in silico screening. ACS Med. Chem. Lett. 2006, 16, 5902–5906. [Google Scholar] [CrossRef]
- Cikes, M.; Vranjesevic, B.; Tomic, M.; Jamnicky, O. A successful treatment of formation damage caused by high-density brine. Spe. Prod. Eng. 1990, 5, 175–179. [Google Scholar] [CrossRef]
- Crabtree, M.; Eslinger, D.; Fletcher, P.; Miller, M.; King, G. Fighting scale-removal and prevention. Oilfield Rev. 1999, 11, 30–45. [Google Scholar]
- Banin, E.; Brady, K.M.; Greenberg, E.P. Chelator-induced dispersal and killing of Pseudomonas aeruginosa cells in a biofilm. Appl. Environ. Microbiol. 2006, 72, 2064–2069. [Google Scholar] [CrossRef] [Green Version]
- Gil, M.L.; Casanova, M.; Martinez, J.P. Changes in the cell wall glycoprotein composition of Candida albicans associated to the inhibition of germ tube formation by EDTA. Arch. Microbiol. 1994, 161, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Gray, G.W.; Wilkinson, S.G. The effect of ethylenediaminetetra-acetic acid on the cell walls of some gram-negative bacteria. J. Gen. Microbiol. 1965, 39, 385–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramage, G.; Wickes, B.L.; López-Ribot, J.L. Inhibition on Candida albicans biofilm formation using divalent cation chelators (EDTA). Mycopathologia 2007, 164, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Saadat, M.; Roudbarmohammadi, S.; Yadegari, M.; Khavarinejad, R. Evaluation of antibacterial effects of catechin and EDTA on planktonic and biofilm cells of Pseudomonas aeruginosa. Malays. J. Microbiol. 2013, 9, 184–188. [Google Scholar] [CrossRef] [Green Version]
- Yakandawala, N.; Gawande, P.V.; LoVetri, K.; Madhyastha, S. Effect of ovotransferrin, protamine sulfate and EDTA combination on biofilm formation by catheter-associated bacteria. J. Appl. Microbiol. 2010, 102, 722–727. [Google Scholar] [CrossRef]
- Chauhan, A.A. Full and broad-spectrum in vivo eradication of catheter-associated biofilms using gentamicin-EDTA antibiotic lock therapy. Antimicrob. Agents Chemother. 2012, 56, 6310–6318. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, A.I.; Musaddiq, M. Antibiotic-EDTA combination induced dispersal of Pseudomonas aeruginosa biofilm. J. Pure Appl. Microbiol. 2012, 6, 363–367. [Google Scholar]
- Bartnicki-Garcia, S. Cell wall chemistry, morphogenesis, and taxonomy of fungi. Annu. Rev. Microbiol. 1968, 22, 87–108. [Google Scholar] [CrossRef]
- Brent, K.J. Fungicide Resistance in Crop Pathogens: How Can It Be Managed? 2nd ed.; Fungicide Resistance Action Committee: Basel, Switzerland, 1995; Available online: http://www.frac.info (accessed on 27 December 2020).
- Dekker, J. Prospects for the use of systemic fungicides in view of the resistance problem. Lab. Phytopathol. 1976, 3, 60–66. [Google Scholar]
- Grimmer, M.K.; van den Bosch, F.; Powers, S.J.; Paveley, N.D. Evaluation of a matrix to calculate fungicide resistance risk. Pest Manag. Sci. 2014, 70, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- van den Bosch, F.; Gilligan, C.A. Models of fungicide resistance dynamics. Annu. Rev. Phytopathol. 2008, 46, 123–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ates, M.; Akdeniz, B.G.; Sen, B.H. The effect of calcium chelating or binding agents on Candida albicans. Oral Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2005, 100, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Alborzi, S.; Bastarrachea, L.J.; Tikekar, R.V. Novel sanitization approach based on synergistic action of UV-A light and benzoic acid: Inactivation mechanism and a potential application in washing fresh produce. Food Microbiol. 2018, 72, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-W.; Campbell, L.T.; Wilkins, M.R.; Pang, C.N.I.; Chen, S.; Carter, D.A. Synergy and antagonism between iron chelators and antifungal drugs in Cryptococcus. Int. J. Antimicrob. Agents 2016, 48, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Mesterhazy, A.; Toth, B.; Varga, M.; Bartok, T.; Szabo-Hever, A.; Farady, L.; Lehoczki-Krsjak, S. Role of fungicides, application of nozzle types, and the resistance level of wheat varieties in the control of Fusarium head blight and deoxynivalenol. Toxins 2011, 3, 1453–1483. [Google Scholar] [CrossRef] [Green Version]
- Latge, J.P. The cell wall: A carbohydrate armour for the fungal cell. Mol. Microbiol. 2007, 66, 279–290. [Google Scholar] [CrossRef]
- Holmes, A.R.; Cannon, R.D.; Shepherd, M.G. Effect of calcium ion uptake on Candida albicans morphology. FEMS Microbiol. Lett. 2010, 77, 187–194. [Google Scholar] [CrossRef]
- Robertson, E.J.; Wolf, J.M.; Casadevall, A. EDTA inhibits biofilm formation, extracellular vesicular secretion, and shedding of the capsular polysaccharide glucuronoxylomannan by Cryptococcus neoformans. Appl. Environ. Microbiol. 2012, 78, 7977–7984. [Google Scholar] [CrossRef] [Green Version]
- Choi, W.J.; Cabib, E. The use of divalent cations and pH for the determination of specific yeast chitin synthetases. Anal. Biochem. 1994, 219, 368. [Google Scholar] [CrossRef]
- Choi, W.J.; Sburlati, A.; Cabib, E. Chitin synthase 3 from yeast has zymogenic properties that depend on both the CAL1 and the CAL3 genes. Proc. Natl. Acad. Sci. USA 1994, 91, 4727–4730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, W.; Song, X.S.; Li, H.P.; Cao, L.H.; Sun, K.; Qiu, X.L.; Xu, Y.B.; Yang, P.; Huang, T.; Zhang, J.B.; et al. Host-induced gene silencing of an essential chitin synthase gene confers durable resistance to Fusarium head blight and seedling blight in wheat. Plant Biotechnol. J. 2015, 13, 1335–1345. [Google Scholar] [CrossRef] [PubMed]
- Orlean, P. Two chitin synthases in Saccharomyces cerevisiae. J. Biol. Chem. 1987, 262, 5732–5739. [Google Scholar] [PubMed]
- Kim, S.W.; Park, J.K.; Lee, C.H.; Hahn, B.S.; Koo, J.C. Comparison of the antimicrobial properties of chitosan oligosaccharides (COS) and EDTA against Fusarium fujikuroi causing rice bakanae disease. Curr. Microbiol. 2016, 72, 496–502. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, M.G. Characterization of Fusarium graminearum isolates resistant to both carbendazim and a new fungicide JS399-19. Phytopathology 2009, 99, 441–446. [Google Scholar] [CrossRef] [Green Version]
- Duvick, J.P.; Rood, T.; Rao, A.G.; Marshak, D.R. Purification and characterization of a novel antimicrobial peptide from maize (Zea mays L.) kernels. J. Biol. Chem. 1992, 267, 18814–18820. [Google Scholar]
- Song, X.S.; Li, H.P.; Zhang, J.B.; Song, B.; Huang, T.; Du, X.M.; Gong, A.D.; Liu, Y.K.; Feng, Y.N.; Agboola, R.S.; et al. Trehalose 6-phosphate phosphatase is required for development, virulence and mycotoxin biosynthesis apart from trehalose biosynthesis in Fusarium graminearum. Fungal. Genet. Biol. 2014, 63, 24–41. [Google Scholar] [CrossRef]
- Audenaert, K.; Callewaert, E.; Hofte, M.; De Saeger, S.; Haesaert, G. Hydrogen peroxide induced by the fungicide prothioconazole triggers deoxynivalenol (DON) production by Fusarium graminearum. BMC Microbiol. 2010, 10, 112. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Duan, Y.; Zhou, M. Carbendazim-resistance associated β2-tubulin substitutions increase deoxynivalenol biosynthesis by reducing the interaction between β2-tubulin and IDH3 in Fusarium graminearum. Environ. Microbiol. 2020, 22, 598–614. [Google Scholar] [CrossRef]
- Xu, C.; Hou, Y.; Wang, J.; Yang, G.; Liang, X.; Zhou, M. Activity of a novel strobilurin fungicide benzothiostrobin against Sclerotinia sclerotiorum. Pestic Biochem. Physiol. 2014, 115, 32–38. [Google Scholar] [CrossRef]
- Valentin, J.L.; Watling, R.J. Provenance establishment of coffee using solution ICP-MS and ICP-AES. Food Chem. 2013, 141, 98–104. [Google Scholar] [CrossRef] [PubMed]




| Year | Experimental Date | Rainy Period | Temperature a (°C) | ||
|---|---|---|---|---|---|
| Tav | Tmax | Tmin | |||
| 2018 | 20 Apr.–4 May. | 21 Apr.–23 Apr., 29 Apr.–1 May | 21 | 30 | 11 |
| 2019 | 20 Apr.–11 May. | 21 Apr., 27 Apr. and 28 Apr. | 18 | 30 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.-S.; Gu, K.-X.; Gao, J.; Wang, J.-X.; Ding, S.-C.; Zhou, M. Ethylenediaminetetraacetic Acid Disodium Salt Acts as an Antifungal Candidate Molecule against Fusarium graminearum by Inhibiting DON Biosynthesis and Chitin Synthase Activity. Toxins 2021, 13, 17. https://doi.org/10.3390/toxins13010017
Song X-S, Gu K-X, Gao J, Wang J-X, Ding S-C, Zhou M. Ethylenediaminetetraacetic Acid Disodium Salt Acts as an Antifungal Candidate Molecule against Fusarium graminearum by Inhibiting DON Biosynthesis and Chitin Synthase Activity. Toxins. 2021; 13(1):17. https://doi.org/10.3390/toxins13010017
Chicago/Turabian StyleSong, Xiu-Shi, Kai-Xin Gu, Jing Gao, Jian-Xin Wang, Shao-Chen Ding, and Mingguo Zhou. 2021. "Ethylenediaminetetraacetic Acid Disodium Salt Acts as an Antifungal Candidate Molecule against Fusarium graminearum by Inhibiting DON Biosynthesis and Chitin Synthase Activity" Toxins 13, no. 1: 17. https://doi.org/10.3390/toxins13010017






