Roles of Nutrient Limitation on Western Lake Erie CyanoHAB Toxin Production
Abstract
:1. Introduction
2. Results
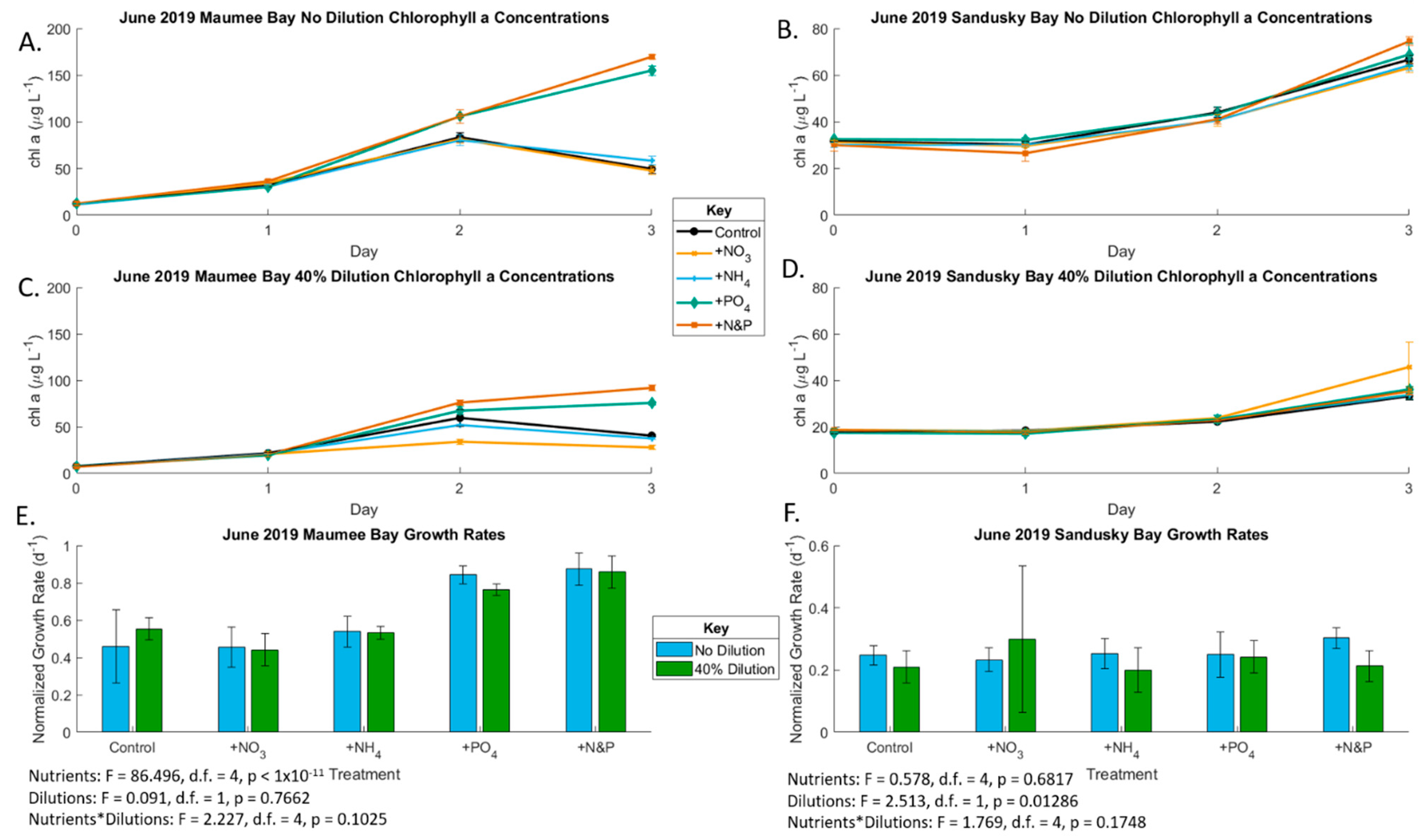
2.1. June 2019 Experiement
2.2. August 2019 Experiment
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Bioassay Methods
5.2. Phytoplankton Biomass Determination
5.3. Nutrient Concentration Determination
5.4. Anatoxin and Microcystin Determinations
5.5. Data Transformation and Analysis
5.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haas, M.; Baumann, F.; Castella, D.; Haghipour, N.; Reusch, A.; Strasser, M.; Eglinton, T.I.; Dubois, N. Roman-driven cultural eutrophication of Lake Murten, Switzerland. Earth Planet. Sci. Lett. 2019, 505, 110–117. [Google Scholar] [CrossRef]
- Mohamed, M.N.; Wellen, C.; Parsons, C.T.; Taylor, W.D.; Arhonditsis, G.; Chomicki, K.M.; Boyd, D.; Weidman, P.; Mundle, S.O.C.; Van Cappellen, P.; et al. Understanding and managing the re-eutrophication of Lake Erie: Knowledge gaps and research priorities. Freshw. Sci. 2019, 38, 675–691. [Google Scholar] [CrossRef]
- Smith, V.H.; Tilman, G.D.; Nekola, J.C. Eutrophication: Impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ. Pollut. 1999, 100, 179–196. [Google Scholar] [CrossRef]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Controlling eutrophication: Nitrogen and phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Environmental Pollution Panel President’s Science Advisory Committee in Restoring the Quality of Our Environment; White House Publishing: Cottage Grove, OR, USA, 1965.
- Sharpley, A.N.; Herron, S.; Daniel, T. Overcoming the challenges of phosphorus-based management in poultry farming. J. Soil Water Conserv. 2007, 62, 375–389. [Google Scholar]
- Bullerjahn, G.S.; McKay, R.M.; Davis, T.W.; Baker, D.B.; Boyer, G.L.; D’Anglada, L.V.; Doucette, G.J.; Ho, J.C.; Irwin, E.G.; Kling, C.L.; et al. Global solutions to regional problems: Collecting global expertise to address the problem of harmful cyanobacterial blooms. A Lake Erie case study. Harmful Algae 2016, 54, 223–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harke, M.J.; Steffen, M.M.; Gobler, C.J.; Otten, T.G.; Wilhelm, S.W.; Wood, S.A.; Paerl, H.W. A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 2016, 54, 4–20. [Google Scholar] [CrossRef] [Green Version]
- Paerl, H.W.; Scott, J.T.; McCarthy, M.J.; Newell, S.E.; Gardner, W.S.; Havens, K.E.; Hoffman, D.K.; Wilhelm, S.W.; Wurtsbaugh, W.A. It takes two to tango: when and where dual nutrient (N & P) reductions are needed to protect lakes and downstream ecosystems. Environ. Sci. Technol. 2016, 50, 10805–10813. [Google Scholar] [CrossRef] [Green Version]
- Paerl, H.W.; Otten, T.G.; Kudela, R. Mitigating the expansion of harmful algal blooms across the freshwater-to-marine continuum. Environ. Sci. Technol. 2018, 52, 5519–5529. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Climate change: A catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep. 2009, 1, 27–37. [Google Scholar] [CrossRef]
- Chapra, S.C.; Boehlert, B.; Fant, C.; Bierman, V.J.; Henderson, J.; Mills, D.; Mas, D.M.L.; Rennels, L.; Jantarasami, L.; Martinich, J.; et al. Climate change impacts on harmful algal blooms in U.S. freshwaters: a screening-level assessment. Environ. Sci. Technol. 2017, 51, 8933–8943. [Google Scholar] [CrossRef] [PubMed]
- Backer, L.C.; McNeel, S.V.; Barber, T.; Kirkpatrick, B.; Williams, C.; Irvin, M.; Zhou, Y.; Johnson, T.B.; Nierenberg, K.; Aubel, M.; et al. Recreational exposure to microcystins during algal blooms in two California lakes. Toxicon 2010, 55, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Plaas, H.E.; Paerl, H.W. Toxic cyanobacteria: a growing threat to water and air quality. Environ. Sci. Technol. 2021, 55, 44–64. [Google Scholar] [CrossRef] [PubMed]
- Smith, Z.; Martin, R.; Wei, B.; Wilhelm, S.; Boyer, G. Spatial and temporal variation in paralytic shellfish toxin production by benthic Microseira (Lyngbya) wollei in a freshwater New York lake. Toxins (Basel) 2019, 11, 44. [Google Scholar] [CrossRef] [Green Version]
- Steffen, M.M.; Belisle, B.S.; Watson, S.B.; Boyer, G.L.; Wilhelm, S.W. Status, causes and controls of cyanobacterial blooms in Lake Erie. J. Great Lakes Res. 2014, 40, 215–225. [Google Scholar] [CrossRef]
- Pauer, J.J.; Anstead, A.M.; Melendez, W.; Taunt, K.W.; Kreis, R.G. Revisiting the Great Lakes Water Quality Agreement phosphorus targets and predicting the trophic status of Lake Michigan. J. Great Lakes Res. 2011, 37, 26–32. [Google Scholar] [CrossRef]
- Steffen, M.M.; Davis, T.W.; McKay, R.M.L.; Bullerjahn, G.S.; Krausfeldt, L.E.; Stough, J.M.A.; Neitzey, M.L.; Gilbert, N.E.; Boyer, G.L.; Johengen, T.H.; et al. Ecophysiological examination of the Lake Erie Microcystis bloom in 2014: linkages between biology and the water supply shutdown of Toledo, OH. Environ. Sci. Technol. 2017, 51, 6745–6755. [Google Scholar] [CrossRef]
- Chaffin, J.D.; Bridgeman, T.B.; Bade, D.L.; Mobilian, C.N. Summer phytoplankton nutrient limitation in Maumee Bay of Lake Erie during high-flow and low-flow years. J. Great Lakes Res. 2014, 40, 524–531. [Google Scholar] [CrossRef]
- Belisle, B.S.; Steffen, M.M.; Pound, H.L.; Watson, S.B.; DeBruyn, J.M.; Bourbonniere, R.A.; Boyer, G.L.; Wilhelm, S.W. Urea in Lake Erie: Organic nutrient sources as potentially important drivers of phytoplankton biomass. J. Great Lakes Res. 2016, 42, 599–607. [Google Scholar] [CrossRef] [Green Version]
- Wilhelm, S.; DeBruyn, J.; Gillor, O.; Twiss, M.; Livingston, K.; Bourbonniere, R.; Pickell, L.; Trick, C.; Dean, A.; McKay, R. Effect of phosphorus amendments on present day plankton communities in pelagic Lake Erie. Aquat. Microb. Ecol. 2003, 32, 275–285. [Google Scholar] [CrossRef]
- Chaffin, J.D.; Bridgeman, T.B.; Bade, D.L. Nitrogen constrains the growth of late summer cyanobacterial blooms in Lake Erie. Adv. Microbiol. 2013, 3, 16–26. [Google Scholar] [CrossRef] [Green Version]
- Davis, T.W.; Bullerjahn, G.S.; Tuttle, T.; McKay, R.M.; Watson, S.B. Effects of increasing nitrogen and phosphorus concentrations on phytoplankton community growth and toxicity during Planktothrix Blooms in Sandusky Bay, Lake Erie. Environ. Sci. Technol. 2015, 49, 7197–7207. [Google Scholar] [CrossRef] [PubMed]
- Gobler, C.J.; Burkholder, J.A.M.; Davis, T.W.; Harke, M.J.; Johengen, T.; Stow, C.A.; Van de Waal, D.B. The dual role of nitrogen supply in controlling the growth and toxicity of cyanobacterial blooms. Harmful Algae 2016, 54, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Chaffin, J.D.; Davis, T.W.; Smith, D.J.; Baer, M.M.; Dick, G.J. Interactions between nitrogen form, loading rate, and light intensity on Microcystis and Planktothrix growth and microcystin production. Harmful Algae 2018, 73, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Conroy, J.D.; Kane, D.D.; Quinlan, E.L.; Edwards, W.J.; Culver, D.A. Abiotic and biotic controls of phytoplankton biomass dynamics in a freshwater tributary, estuary, and large lake ecosystem: Sandusky Bay (lake Erie) chemostat. Inland Waters 2017, 7, 473–492. [Google Scholar] [CrossRef]
- Redfield, A.C.; Ketchum, B.H.; Richards, F.A. The influence of organisms on the composition of sea-water. In The Sea: Ideas and Observations on Progress in the Study of the Seas; Interscienc Publishers: New York, NY, USA, 1963. [Google Scholar]
- Salk, K.R.; Bullerjahn, G.S.; McKay, R.M.L.; Chaffin, J.D.; Ostrom, N.E. Nitrogen cycling in Sandusky Bay, Lake Erie: Oscillations between strong and weak export and implications for harmful algal blooms. Biogeosciences 2018, 15, 2891–2907. [Google Scholar] [CrossRef] [Green Version]
- Hampel, J.J.; McCarthy, M.J.; Neudeck, M.; Bullerjahn, G.S.; McKay, R.M.L.; Newell, S.E. Ammonium recycling supports toxic Planktothrix blooms in Sandusky Bay, Lake Erie: Evidence from stable isotope and metatranscriptome data. Harmful Algae 2019, 81, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Huang, Y.; Hu, J.; Li, P.; Zhang, C.; Li, L.; Xu, P.; Zhang, J.; Chen, X. The nitrogen reduction in eutrophic water column driven by Microcystis blooms. J. Hazard. Mater. 2020, 385, 121578. [Google Scholar] [CrossRef]
- Muenich, R.L.; Kalcic, M.; Scavia, D. Evaluating the impact of legacy p and agricultural conservation practices on nutrient loads from the Maumee River watershed. Environ. Sci. Technol. 2016, 50, 8146–8154. [Google Scholar] [CrossRef]
- Cousino, L.K.; Becker, R.H.; Zmijewski, K.A. Modeling the effects of climate change on water, sediment, and nutrient yields from the Maumee River watershed. J. Hydrol. Reg. Stud. 2015, 4, 762–775. [Google Scholar] [CrossRef]
- Michalak, A.M.; Anderson, E.J.; Beletsky, D.; Boland, S.; Bosch, N.S.; Bridgeman, T.B.; Chaffin, J.D.; Cho, K.; Confesor, R.; Daloglu, I.; et al. Record-setting algal bloom in Lake Erie caused by agricultural and meteorological trends consistent with expected future conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 6448–6452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, D.R.; Owens, P.R.; Leytem, A.B.; Warnemuende, E.A. Nutrient losses from manure and fertilizer applications as impacted by time to first runoff event. Environ. Pollut. 2007, 147, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Barnard, M.A. Mitigating the global expansion of harmful cyanobacterial blooms: Moving targets in a human- and climatically-altered world. Harmful Algae 2020, 96, 101845. [Google Scholar] [CrossRef] [PubMed]
- Krausfeldt, L.E.; Farmer, A.T.; Castro, H.F.; Boyer, G.L.; Campagna, S.R.; Wilhelm, S.W. Nitrogen flux into metabolites and microcystins changes in response to different nitrogen sources in Microcystis aeruginosa NIES-843. Environ. Microbiol. 2020, 1462–2920. [Google Scholar] [CrossRef] [PubMed]
- NOAA MODIS. Algal Bloom in Lake Erie. Available online: https://modis.gsfc.nasa.gov/gallery/individual.php?db_date=2019-08-19 (accessed on 19 August 2019).
- Wacklin, P.; Hoffmann, L.; Komarek, J. Nomenclatural validation of the genetically revised cyanobacterial genus Dolichospermum (RALFS ex BORNET et FLAHAULT) comb. nova. Fottea 2009, 9, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Davis, T.W.; Berry, D.L.; Boyer, G.L.; Gobler, C.J. The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae 2009, 8, 715–725. [Google Scholar] [CrossRef]
- Rinta-Kanto, J.M.; Konopko, E.A.; DeBruyn, J.M.; Bourbonniere, R.A.; Boyer, G.L.; Wilhelm, S.W. Lake Erie Microcystis: Relationship between microcystin production, dynamics of genotypes and environmental parameters in a large lake. Harmful Algae 2009, 8, 665–673. [Google Scholar] [CrossRef]
- Newell, S.E.; Davis, T.W.; Johengen, T.H.; Gossiaux, D.; Burtner, A.; Palladino, D.; McCarthy, M.J. Reduced forms of nitrogen are a driver of non-nitrogen-fixing harmful cyanobacterial blooms and toxicity in Lake Erie. Harmful Algae 2019, 81, 86–93. [Google Scholar] [CrossRef]
- Osswald, J.; Rellán, S.; Gago, A.; Vasconcelos, V. Toxicology and detection methods of the alkaloid neurotoxin produced by cyanobacteria, anatoxin-a. Environ. Int. 2007, 33, 1070–1089. [Google Scholar] [CrossRef]
- U.S. Action Plan for Lake Erie; United States Environmental Protection Agency: Washington, DC, USA, 2018.
- Phosphorus Loading Targets For Lake Erie; Annex 4 Objectives and Targets Task Team Final Report to the Nutrients Annex Subcommittee; United States Environmental Protection Agency: Washington, DC, USA, 2015.
- Environment and Climate Change Canada. Canada-Ontario Lake Erie Action Plan Partnering on Achieving Phosphorus Loading Reductions to Lake Erie from Canadian Sources; Ontario Ministry of the Environment and Climate Change: Toronto, ON, Canada, 2018.
- Maccoux, M.J.; Dove, A.; Backus, S.M.; Dolan, D.M. Total and soluble reactive phosphorus loadings to Lake Erie: A detailed accounting by year, basin, country, and tributary. J. Great Lakes Res. 2016, 42, 1151–1165. [Google Scholar] [CrossRef] [Green Version]
- Scavia, D.; DePinto, J.V.; Bertani, I. A multi-model approach to evaluating target phosphorus loads for Lake Erie. J. Great Lakes Res. 2016, 42, 1139–1150. [Google Scholar] [CrossRef]
- Yan, P.; Guo, J.S.; Zhang, P.; Xiao, Y.; Li, Z.; Zhang, S.Q.; Zhang, Y.X.; He, S.X. The role of morphological changes in algae adaptation to nutrient stress at the single-cell level. Sci. Total Environ. 2021, 754, 142076. [Google Scholar] [CrossRef] [PubMed]
- Spivak, A.C.; Vanni, M.J.; Mette, E.M. Moving on up: Can results from simple aquatic mesocosm experiments be applied across broad spatial scales? Freshw. Biol. 2011, 56, 279–291. [Google Scholar] [CrossRef]
- Del Giorgio, P.; Williams, P. Respiration in Aquatic Ecosystems; Oxford University Press: Oxford, UK, 2005; ISBN 9780191713347. [Google Scholar]
- Nogueira, P.; Domingues, R.B.; Barbosa, A.B. Are microcosm volume and sample pre-filtration relevant to evaluate phytoplankton growth? J. Exp. Mar. Bio. Ecol. 2014, 461, 323–330. [Google Scholar] [CrossRef]
- Chaffin, J.D.; Bridgeman, T.B. Organic and inorganic nitrogen utilization by nitrogen-stressed cyanobacteria during bloom conditions. J. Appl. Phycol. 2014, 26, 299–309. [Google Scholar] [CrossRef]
- Chaffin, J.D.; Bridgeman, T.B.; Heckathorn, S.A.; Mishra, S. Assessment of Microcystis growth rate potential and nutrient status across a trophic gradient in western Lake Erie. J. Great Lakes Res. 2011, 37, 92–100. [Google Scholar] [CrossRef]
- North, R.L.; Guildford, S.J.; Smith, R.E.H.; Havens, S.M.; Twiss, M.R. Evidence for phosphorus, nitrogen, and iron colimitation of phytoplankton communities in Lake Erie. Limnol. Oceanogr. 2007, 52, 315–328. [Google Scholar] [CrossRef] [Green Version]
- Moon, J.; Carrick, H. Seasonal variation of phytoplankton nutrient limitation in Lake Erie. Aquat. Microb. Ecol. 2007, 48, 61–71. [Google Scholar] [CrossRef]
- Likens, G.E. Nutrients and Eutrophication: The Limiting-Nutrient Controversy: Proceedings; American Society of Limnology and Oceanography: Waco, TX, USA, 1972. [Google Scholar]
- Motew, M.; Chen, X.; Booth, E.G.; Carpenter, S.R.; Pinkas, P.; Zipper, S.C.; Loheide, S.P.; Donner, S.D.; Tsuruta, K.; Vadas, P.A.; et al. The Influence of Legacy P on Lake Water Quality in a Midwestern Agricultural Watershed. Ecosystems 2017, 20, 1468–1482. [Google Scholar] [CrossRef]
- Schindler, D.W. The dilemma of controlling cultural eutrophication of lakes. Proc. R. Soc. B Biol. Sci. 2012, 279, 4322–4333. [Google Scholar] [CrossRef] [Green Version]
- Schindler, D.W.; Hecky, R.E.; Findlay, D.L.; Stainton, M.P.; Parker, B.R.; Paterson, M.J.; Beaty, K.G.; Lyng, M.; Kasian, S.E.M. Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37-year whole-ecosystem experiment. Proc. Natl. Acad. Sci. USA 2008, 105, 11254–11258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, J.T.; McCarthy, M.J.; Paerl, H.W. Nitrogen transformations differentially affect nutrient-limited primary production in lakes of varying trophic state. Limnol. Oceanogr. Lett. 2019. [Google Scholar] [CrossRef] [Green Version]
- Davis, T.; Harke, M.; Marcoval, M.; Goleski, J.; Orano-Dawson, C.; Berry, D.; Gobler, C. Effects of nitrogenous compounds and phosphorus on the growth of toxic and non-toxic strains of Microcystis during cyanobacterial blooms. Aquat. Microb. Ecol. 2010, 61, 149–162. [Google Scholar] [CrossRef] [Green Version]
- Jankowiak, J.; Hattenrath-Lehmann, T.; Kramer, B.J.; Ladds, M.; Gobler, C.J. Deciphering the effects of nitrogen, phosphorus, and temperature on cyanobacterial bloom intensification, diversity, and toxicity in western Lake Erie. Limnol. Oceanogr. 2019, 64, 1347–1370. [Google Scholar] [CrossRef] [Green Version]
- Stucken, K.; John, U.; Cembella, A.; Murillo, A.A.; Soto-Liebe, K.; Fuentes-Valdés, J.J.; Friedel, M.; Plominsky, A.M.; Vásquez, M.; Glöckner, G. the smallest known genomes of multicellular and toxic cyanobacteria: comparison, minimal gene sets for linked traits and the evolutionary implications. PLoS ONE 2010, 5, e9235. [Google Scholar] [CrossRef] [Green Version]
- McKindles, K.M.; Manes, M.A.; DeMarco, J.R.; McClure, A.; McKay, R.M.; Davis, T.W.; Bullerjahn, G.S. Dissolved microcystin release coincident with lysis of a Microcystis-dominated bloom in western Lake Erie attributed to a novel cyanophage. Appl. Environ. Microbiol. 2020. [Google Scholar] [CrossRef]
- Thees, A.; Atari, E.; Birbeck, J.; Westrick, J.A.; Huntley, J.F. Isolation and characterization of Lake Erie bacteria that degrade the cyanobacterial microcystin toxin MC-LR. J. Great Lakes Res. 2019, 45, 138–149. [Google Scholar] [CrossRef]
- Dolman, A.M.; Rücker, J.; Pick, F.R.; Fastner, J.; Rohrlack, T.; Mischke, U.; Wiedner, C. Cyanobacteria and cyanotoxins: the influence of nitrogen versus phosphorus. PLoS ONE 2012, 7, e38757. [Google Scholar] [CrossRef]
- Beversdorf, L.J.; Miller, T.R.; McMahon, K.D. Long-term monitoring reveals carbon-nitrogen metabolism key to microcystin production in eutrophic lakes. Front. Microbiol. 2015, 6, 456. [Google Scholar] [CrossRef] [Green Version]
- Harris, T.D.; Smith, V.H.; Graham, J.L.; de Waa, D.B.V.; Tedesco, L.P.; Clercin, N. Combined effects of nitrogen to phosphorus and nitrate to ammonia ratios on cyanobacterial metabolite concentrations in eutrophic Midwestern USA reservoirs. Inl. Waters 2016, 6, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Jover, L.F.; Effler, T.C.; Buchan, A.; Wilhelm, S.W.; Weitz, J.S. The elemental composition of virus particles: Implications for marine biogeochemical cycles. Nat. Rev. Microbiol. 2014, 12, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.M.; Lee, S.J.; Jang, M.H.; Yoon, B.D. Microcystin production by Microcystis aeruginosa in a phosphorus-limited chemostat. Appl. Environ. Microbiol. 2000, 66, 176–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viaggiu, E.; Melchiorre, S.; Volpi, F.; Di Corcia, A.; Mancini, R.; Garibaldi, L.; Crichigno, G.; Bruno, M. Anatoxin-a toxin in the cyanobacterium Planktothrix rubescens from a fishing pond in northern Italy. Environ. Toxicol. 2004, 19, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, D.; Moreno, E.; Paniagua, T.; de Hoyos, C.; Wormer, L.; Sanchis, D.; Cirés, S.; Martín-del-Pozo, D.; Codd, G.A.; Quesada, A. Anatoxin-a occurrence and potential cyanobacterial anatoxin-a producers in Spanish reservoirs. J. Phycol. 2007, 43, 1120–1125. [Google Scholar] [CrossRef]
- Toporowska, M.; Pawlik-Skowrońska, B.; Kalinowska, R. Mass development of diazotrophic cyanobacteria (Nostocales) and production of neurotoxic anatoxin-a in a Planktothrix (Oscillatoriales) dominated temperate lake. Water Air Soil Pollut. 2016, 227, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Smith, Z.J.; Conroe, D.E.; Schulz, K.L.; Boyer, G.L. Limnological differences in a two-basin lake help to explain the occurrence of anatoxin-a, paralytic shellfish poisoning toxins, and microcystins. Toxins (Basel) 2020, 12, 559. [Google Scholar] [CrossRef]
- Mahmood, N.A.; Carmichael, W.W. The pharmacology of anatoxin-a(s), a neurotoxin produced by the freshwater cyanobacterium Anabaena flos-aquae NRC 525-17. Toxicon 1986, 24, 425–434. [Google Scholar] [CrossRef]
- Gagnon, A.; Pick, F.R. Effect of nitrogen on cellular production and release of the neurotoxin anatoxin-a in a nitrogen-fixing cyanobacterium. Front. Microbiol. 2012, 3, 211. [Google Scholar] [CrossRef] [Green Version]
- Boopathi, T.; Ki, J.-S. Impact of environmental factors on the regulation of cyanotoxin production. Toxins (Basel) 2014, 6, 1951–1978. [Google Scholar] [CrossRef] [Green Version]
- Osswald, J.; Rellán, S.; Gago-Martinez, A.; Vasconcelos, V. Production of anatoxin-a by cyanobacterial strains isolated from Portuguese fresh water systems. Ecotoxicology 2009, 18, 1110–1115. [Google Scholar] [CrossRef]
- Neilan, B.A.; Pearson, L.A.; Muenchhoff, J.; Moffitt, M.C.; Dittmann, E. Environmental conditions that influence toxin biosynthesis in cyanobacteria. Environ. Microbiol. 2013, 15, 1239–1253. [Google Scholar] [CrossRef] [PubMed]
- Heath, M.; Wood, S.A.; Young, R.G.; Ryan, K.G. The role of nitrogen and phosphorus in regulating Phormidium sp. (cyanobacteria) growth and anatoxin production. FEMS Microbiol. Ecol. 2016, 92, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rapala, J.; Sivonen, K.; Luukkainen, R.; Niemelä, S.I. Anatoxin-a concentration in Anabaena and Aphanizomenon under different environmental conditions and comparison of growth by toxic and non-toxic Anabaena-strains—A laboratory study. J. Appl. Phycol. 1993, 5, 581–591. [Google Scholar] [CrossRef]
- Stolerman, I.P.; Albuquerque, E.X.; Garcha, H.S. Behavioural effects of anatoxin, a potent nicotinic agonist, in rats. Neuropharmacology 1992, 31, 311–314. [Google Scholar] [CrossRef]
- Rogers, E.H.; Hunter, E.S.; Moser, V.C.; Phillips, P.M.; Herkovits, J.; Muñoz, L.; Hall, L.L.; Chernoff, N. Potential developmental toxicity of anatoxin-a, a cyanobacterial toxin. J. Appl. Toxicol. 2005, 25, 527–534. [Google Scholar] [CrossRef]
- Anderson, B.; Voorhees, J.; Phillips, B.; Fadness, R.; Stancheva, R.; Nichols, J.; Orr, D.; Wood, S.A. Extracts from benthic anatoxin-producing Phormidium are toxic to 3 macroinvertebrate taxa at environmentally relevant concentrations. Environ. Toxicol. Chem. 2018, 37, 2851–2859. [Google Scholar] [CrossRef]
- Lovin, L.M.; Brooks, B.W. Global scanning of anatoxins in aquatic systems: Environment and health hazards, and research needs. Mar. Freshw. Res. 2020, 71, 689. [Google Scholar] [CrossRef]
- Richards, R.P.; Baker, D.B.; Crumrine, J.P.; Stearns, A.M. Unusually large loads in 2007 from the Maumee and Sandusky Rivers, tributaries to Lake Erie. J. Soil Water Conserv. 2010, 65, 450–462. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.G.; Chang, E.K.M.; Wong, S.; Zhang, R.; Zhang, M.; Del Genio, A. Impacts of storm track variations on wintertime extreme precipitation and moisture budgets over the Ohio Valley and northwestern United States. J. Clim. 2020, 33, 5371–5391. [Google Scholar] [CrossRef] [Green Version]
- Paerl, H.W.; Hall, N.S.; Hounshell, A.G.; Rossignol, K.L.; Barnard, M.A.; Luettich, R.A.; Rudolph, J.C.; Osburn, C.L.; Bales, J.; Harding, L.W. Recent increases of rainfall and flooding from tropical cyclones (TCs) in North Carolina (USA): Implications for organic matter and nutrient cycling in coastal watersheds. Biogeochemistry 2020, 4. [Google Scholar] [CrossRef]
- Paerl, H.W.; Havens, K.E.; Hall, N.S.; Otten, T.G.; Zhu, M.; Xu, H.; Zhu, G.; Qin, B. Mitigating a global expansion of toxic cyanobacterial blooms: Confounding effects and challenges posed by climate change. Mar. Freshw. Res. 2019. [Google Scholar] [CrossRef] [Green Version]
- Trenberth, K.E. The impact of climate change and variability on heavy precipitation, floods, and droughts. In Encyclopedia of Hydrological Sciences; American Cancer Society: Atlanta, GA, USA, 2008; ISBN 978-0-470-84894-4. [Google Scholar]
- Boedecker, A.R.; Niewinski, D.N.; Newell, S.E.; Chaffin, J.D.; McCarthy, M.J. Evaluating sediments as an ecosystem service in western Lake Erie via quantification of nutrient cycling pathways and selected gene abundances. J. Great Lakes Res. 2020. [Google Scholar] [CrossRef]
- Preventing Eutrophication: Scientific Support for Dual Nutrient Criteria Fact sheet; US EPA Office of Water: Washington, DC, USA, 2015.
- Baker, D.B.; Johnson, L.T.; Confesor, R.B.; Crumrine, J.P.; Guo, T.; Manning, N.F. Needed: Early-term adjustments for Lake Erie phosphorus target loads to address western basin cyanobacterial blooms. J. Great Lakes Res. 2019, 45, 203–211. [Google Scholar] [CrossRef]
- Levy, S. Microcystis Rising: Why Phosphorus Reduction Isn’t Enough to Stop CyanoHABs. Environ. Health Perspect. 2017, 125, A34–A39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Paerl, H.W.; Qin, B.; Zhu, G.; Hall, N.S.; Wu, Y. Determining critical nutrient thresholds needed to control harmful cyanobacterial blooms in eutrophic Lake Taihu, China. Environ. Sci. Technol. 2015, 49, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Mitrovic, S.M. Phytoplankton co-limitation by nitrogen and phosphorus in a shallow reservoir: Progressing from the phosphorus limitation paradigm. Hydrobiologia 2015, 744, 255–269. [Google Scholar] [CrossRef]
- Zohary, T.; Herut, B.; Krom, M.D.; Fauzi, C.; Mantoura, R.; Pitta, P.; Psarra, S.; Rassoulzadegan, F.; Stambler, N.; Tanaka, T.; et al. P-limited bacteria but N and P co-limited phytoplankton in the Eastern Mediterranean—A microcosm experiment. Deep Sea Res. Part II Top. Stud. Oceanogr. 2005, 52, 3011–3023. [Google Scholar] [CrossRef]
- Wilhelm, S.W.; Bullerjahn, G.S.; McKay, R.M.L. The complicated and confusing ecology of Microcystis blooms. mBio 2020, 11, e00529-20. [Google Scholar] [CrossRef]
- Stow, C.A.; Glassner-Shwayder, K.; Lee, D.; Wang, L.; Arhonditsis, G.; DePinto, J.V.; Twiss, M.R. Lake Erie phosphorus targets: An imperative for active adaptive management. J. Great Lakes Res. 2020, 46, 672–676. [Google Scholar] [CrossRef]
- Testai, E.; Scardala, S.; Vichi, S.; Buratti, F.M.; Funari, E. Risk to human health associated with the environmental occurrence of cyanobacterial neurotoxic alkaloids anatoxins and saxitoxins. Crit. Rev. Toxicol. 2016, 46, 385–419. [Google Scholar] [CrossRef]
- Paerl, H.W.; Hall, N.S.; Calandrino, E.S. Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change. Sci. Total Environ. 2011, 409, 1739–1745. [Google Scholar] [CrossRef]
- Xu, H.; Paerl, H.W.; Qin, B.; Zhu, G.; Gaoa, G. Nitrogen and phosphorus inputs control phytoplankton growth in eutrophic Lake Taihu, China. Limnol. Oceanogr. 2010, 55, 420–432. [Google Scholar] [CrossRef] [Green Version]
- Hanson, P.C.; Carpenter, S.R.; Armstrong, D.E.; Stanley, E.H.; Kratz, T.K. Lake dissolved inorganic carbon and dissolved oxygen: Changing drivers from days to decades. Ecol. Monogr. 2006, 76, 343–363. [Google Scholar] [CrossRef]
- Rockwell, D.C.; Warren, G.J.; Bertram, P.E.; Salisbury, D.K.; Burns, N.M. The U.S. EPA Lake Erie indicators monitoring program 1983-2002: Trends in phosphorus, silica, and chlorophyll a in the central basin. J. Great Lakes Res. 2005, 31, 23–34. [Google Scholar] [CrossRef]
- Chapra, S.C.; Dove, A.; Warren, G.J. Long-term trends of Great Lakes major ion chemistry. J. Great Lakes Res. 2012, 38, 550–560. [Google Scholar] [CrossRef]
- Shorthouse, D.P. SimpleMappr. Available online: https://www.simplemappr.net (accessed on 14 September 2020).
- Arar, E.J.; Collins, G.B. Method 445.0 In Vitro Determination of Chlorophyll a and Pheophytin in Marine and Freshwater Algae by Fluorescence; United States Environmental Protection Agency: Washington, DC, USA, 1997.
- Arar, E.J.; Collins, G.B. Using the Turner Designs Model 10 Analog, The 10AU Field, or the TD-700 Laboratory Fluorometer with EPA Method 445.0; United States Environmental Protection Agency: Washington, DC, USA, 2001.
- Welschmeyer, N.A. Fluorometric analysis of chlorophyll a in the presence of chlorophyll b and pheopigments. Limnol. Oceanogr. 1994, 39, 1985–1992. [Google Scholar] [CrossRef]
- Chaffin, J.D.; Mishra, S.; Kane, D.D.; Bade, D.L.; Stanislawczyk, K.; Slodysko, K.N.; Jones, K.W.; Parker, E.M.; Fox, E.L. Cyanobacterial blooms in the central basin of Lake Erie: Potentials for cyanotoxins and environmental drivers. J. Great Lakes Res. 2019, 45, 277–289. [Google Scholar] [CrossRef]
- Goeyens, L.; Kindermans, N.; Abu Yusuf, M.; Elskens, M. A room temperature procedure for the manual determination of urea in seawater. Estuar. Coast. Shelf Sci. 1998, 47, 415–418. [Google Scholar] [CrossRef] [Green Version]
- Mulvenna, P.F.; Savidge, G. A modified manual method for the determination of urea in seawater using diacetylmonoxime reagent. Estuar. Coast. Shelf Sci. 1992, 34, 429–438. [Google Scholar] [CrossRef]
- Boyer, G.L. The occurrence of cyanobacterial toxins in New York lakes: Lessons from the MERHAB-Lower Great Lakes program. In Lake and Reservoir Management; Taylor & Francis Group: Abingdon, UK, 2007; Volume 23, pp. 153–160. [Google Scholar]
- Peng, G.; Martin, R.M.; Dearth, S.P.; Sun, X.; Boyer, G.L.; Campagna, S.R.; Lin, S.; Wilhelm, S.W. Seasonally relevant cool temperatures interact with N chemistry to increase microcystins produced in lab cultures of Microcystis aeruginosa NIES-843. Environ. Sci. Technol. 2018, 52, 4127–4136. [Google Scholar] [CrossRef]
- Boyer, G.L. LCMS-SOP Determination of microcystins in water samples by high performance liquid chromatography (HPLC) with single quadrupole mass spectrometry (MS). Protocols 2020. [Google Scholar] [CrossRef]
- Yang, X. Occurrence of the Cyanobacterial Neurotoxin, Anatoxin-A, in New York State Waters; State University of New York: Syracuse, NY, USA, 2007. [Google Scholar]
- Barnard, M.A. GitHub: malcolmbarnard/WLE_Barnard_et_al_Toxins. Zenodo 2021. [Google Scholar] [CrossRef]
- Mathworks MATLAB, version 9.5.0.1049112 (R2018b); The MathWorks: Natick, MA, USA, 2018.







| Nutrient Parameter | Maumee Bay | Sandusky Bay | ||
|---|---|---|---|---|
| No Dilution | 40% Dilution | No Dilution | 40% Dilution | |
| NO3 + NO2 (µmol L−1) | 223.67 ± 25.43 | 137.64 ± 35.00 | 101.45 ± 5.95 | 58.46 ± 8.46 |
| NH4 (µmol L−1) | 1.34 ± 1.01 | 3.67 ± 0.60 | 24.28 ± 0.66 | 17.14 ± 0.85 |
| DRP (µmol L−1) | 2.24 ± 0.23 | 1.50 ± 0.07 | 1.20 ± 0.14 | 0.85 ± 0.05 |
| Silicate (µmol L−1) | 139.14 ± 12.23 | 100.46 ± 2.53 | 130.42 ± 19.50 | 78.88 ± 16.20 |
| Nutrient Parameter | Maumee Bay | Sandusky Bay | ||
|---|---|---|---|---|
| No Dilution | 40% Dilution | No Dilution | 40% Dilution | |
| NO3 + NO2 (µmol L−1) | 127.12 ± 10.82 | 60.10 ± 12.94 | 6.59 ± 0.29 | 6.61 ± 0.05 |
| NH4 (µmol L−1) | 0.70 ± 0.42 | 1.74 ± 1.58 | 1.05 ± 0.69 | 1.04 ± 0.06 |
| Urea (µmol L−1) | 3.45 ± 0.61 | 1.59 ± 1.15 | 2.91 ± 1.46 | 3.99 ± 1.09 |
| DRP (µmol L−1) | 0.20 ± 0.20 | 0.05 ± 0.01 | 0.03 ± 0.01 | 0.10 ± 0.07 |
| Silicate (µmol L−1) | 124.33 ± 7.13 | 95.44 ± 6.24 | 51.12 ± 12.23 | 64.91 ± 18.52 |
| Ion 1 | Average Ambient Concentration (mg/L) [105] | MIS 1 Concentration (mg/L) | MIS 1 Concentration (µM) | Percent Difference between Chapra et al. [105] and MIS Concentrations |
|---|---|---|---|---|
| Ca 2+ | 32.11 | 32 | 800 | − 0.34% |
| Mg 2+ | 8.89 | 8.88 | 370 | − 0.11% |
| Na + | 8.58 | 4.6 | 200 | − 46.39% 2 |
| K + | 1.431 | 1.56 | 40 | 9.01% 3 |
| Cl - | 14.58 | 16.33 | 460 | 12.00% 3 |
| SO4 2- | 22.81 | 43.2 | 450 | 89.39% 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barnard, M.A.; Chaffin, J.D.; Plaas, H.E.; Boyer, G.L.; Wei, B.; Wilhelm, S.W.; Rossignol, K.L.; Braddy, J.S.; Bullerjahn, G.S.; Bridgeman, T.B.; et al. Roles of Nutrient Limitation on Western Lake Erie CyanoHAB Toxin Production. Toxins 2021, 13, 47. https://doi.org/10.3390/toxins13010047
Barnard MA, Chaffin JD, Plaas HE, Boyer GL, Wei B, Wilhelm SW, Rossignol KL, Braddy JS, Bullerjahn GS, Bridgeman TB, et al. Roles of Nutrient Limitation on Western Lake Erie CyanoHAB Toxin Production. Toxins. 2021; 13(1):47. https://doi.org/10.3390/toxins13010047
Chicago/Turabian StyleBarnard, Malcolm A., Justin D. Chaffin, Haley E. Plaas, Gregory L. Boyer, Bofan Wei, Steven W. Wilhelm, Karen L. Rossignol, Jeremy S. Braddy, George S. Bullerjahn, Thomas B. Bridgeman, and et al. 2021. "Roles of Nutrient Limitation on Western Lake Erie CyanoHAB Toxin Production" Toxins 13, no. 1: 47. https://doi.org/10.3390/toxins13010047
APA StyleBarnard, M. A., Chaffin, J. D., Plaas, H. E., Boyer, G. L., Wei, B., Wilhelm, S. W., Rossignol, K. L., Braddy, J. S., Bullerjahn, G. S., Bridgeman, T. B., Davis, T. W., Wei, J., Bu, M., & Paerl, H. W. (2021). Roles of Nutrient Limitation on Western Lake Erie CyanoHAB Toxin Production. Toxins, 13(1), 47. https://doi.org/10.3390/toxins13010047






