Design of a Quantitative LC-MS Method for Residual Toxins Adenylate Cyclase Toxin (ACT), Dermonecrotic Toxin (DNT) and Tracheal Cytotoxin (TCT) in Bordetella pertussis Vaccines
Abstract
:1. Introduction
2. Results
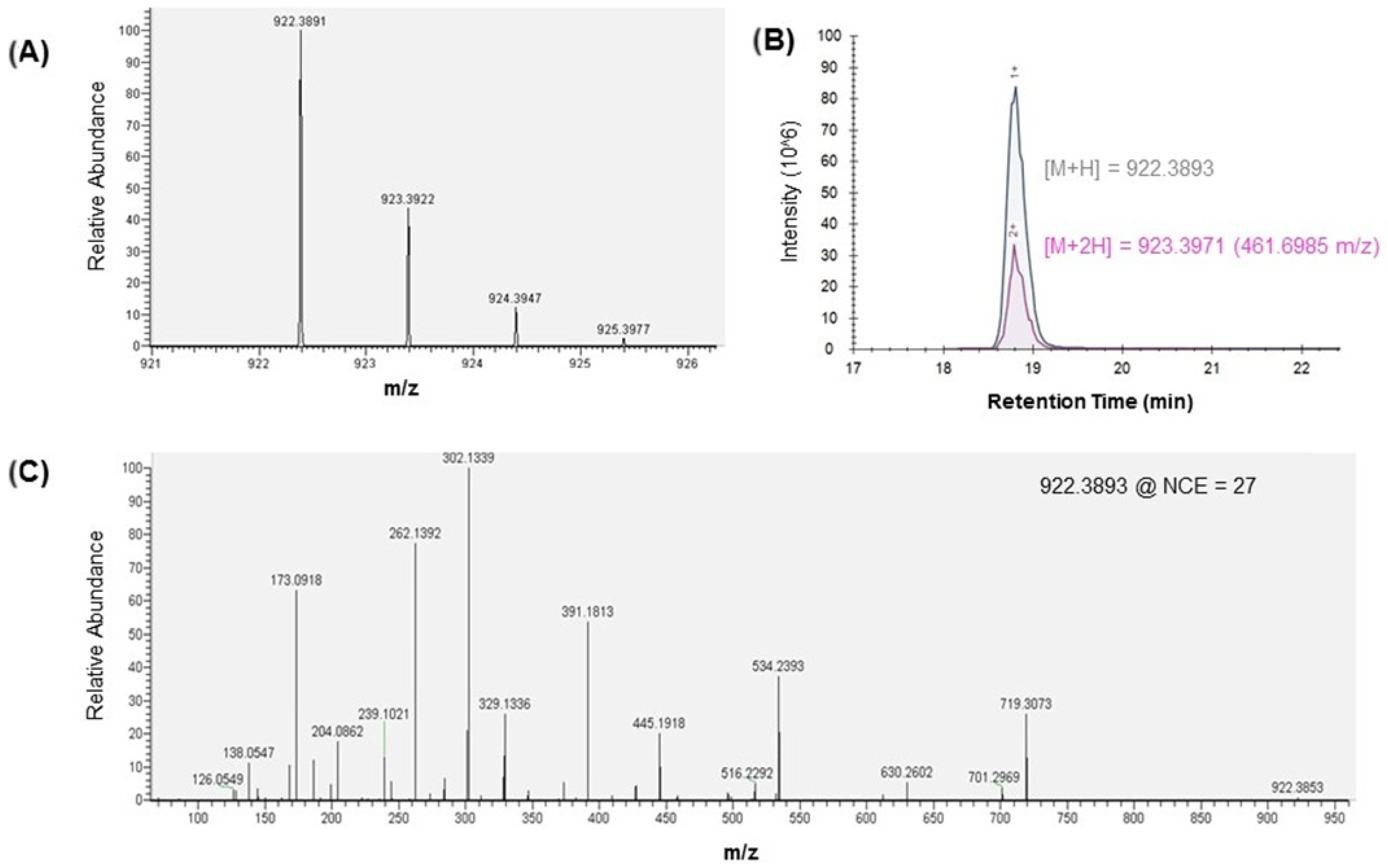
2.1. TCT Detection in B. pertussis Supernatant Harvest
2.2. MS/MS Analysis of TCT
2.3. Multiple Reaction Monitoring (MRM) Analysis
2.4. Quantitation by Multiple Reaction Monitoring (MRM)
2.5. Method Qualification
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Standard Preparation
5.2. Nano LC-MS/MS Analysis of TCT in B. pertussis Supernatant Harvest
5.3. Sample Preparation for LC-MRM
5.4. LC-MRM Experimental Design
5.5. LC-MRM Data Acquisition
5.6. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Snyder, A. Leila Denmark. Lancet 2012, 379, 2336. [Google Scholar] [CrossRef]
- Shapiro-Shapin, C.G. Pearl Kendrick, Grace Eldering, and the Pertussis Vaccine. Emerg. Infect. Dis. 2010, 16, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Greco, D.; Salmaso, S.; Mastrantonio, P.; Giuliano, M.; Tozzi, A.E.; Anemona, A.; Ciofi degli Atti, M.L.; Giammanco, A.; Panei, P.; Blackwelder, W.C.; et al. A Controlled Trial of Two Acellular Vaccines and One Whole-Cell Vaccine against Pertussis. N. Engl. J. Med. 1996, 334, 341–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherry, J.D. Pertussis: Challenges Today and for the Future. PLoS Pathog. 2013, 9, e1003418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melvin, J.A.; Scheller, E.V.; Miller, J.F.; Cotter, P.A. Bordetella Pertussis Pathogenesis: Current and Future Challenges. Nat. Rev. Microbiol. 2014, 12, 274–288. [Google Scholar] [CrossRef] [Green Version]
- Yeung, K.H.T.; Duclos, P.; Nelson, E.A.S.; Hutubessy, R.C.W. An Update of the Global Burden of Pertussis in Children Younger than 5 Years: A Modelling Study. Lancet Infect. Dis. 2017, 17, 974–980. [Google Scholar] [CrossRef]
- Clark, T.A. Changing Pertussis Epidemiology: Everything Old Is New Again. J. Infect. Dis. 2014, 209, 978–981. [Google Scholar] [CrossRef] [PubMed]
- Zerbo, O.; Bartlett, J.; Goddard, K.; Fireman, B.; Lewis, E.; Klein, N.P. Acellular Pertussis Vaccine Effectiveness Over Time. Pediatrics 2019, 144. [Google Scholar] [CrossRef]
- Pertussis: A Disease Affecting All Ages—American Family Physician. Available online: https://www.aafp.org/afp/2006/0801/p420.html (accessed on 6 August 2021).
- Schwartz, K.L.; Kwong, J.C.; Deeks, S.L.; Campitelli, M.A.; Jamieson, F.B.; Marchand-Austin, A.; Stukel, T.A.; Rosella, L.; Daneman, N.; Bolotin, S.; et al. Effectiveness of Pertussis Vaccination and Duration of Immunity. CMAJ Can. Med. Assoc. J. 2016, 188, E399–E406. [Google Scholar] [CrossRef] [Green Version]
- Meade, B.D.; Plotkin, S.A.; Locht, C. Possible Options for New Pertussis Vaccines. J. Infect. Dis. 2014, 209 (Suppl. 1), S24–S27. [Google Scholar] [CrossRef] [PubMed]
- Ladant, D. Bioengineering of Bordetella Pertussis Adenylate Cyclase Toxin for Vaccine Development and Other Biotechnological Purposes. Toxins 2021, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tan, Y.; Asokanathan, C.; Zhang, S.; Xing, D.; Wang, J. Characterization of Co-Purified Acellular Pertussis Vaccines. Hum. Vaccines Immunother. 2015, 11, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Scheller, E.V.; Cotter, P.A. Bordetella Filamentous Hemagglutinin and Fimbriae: Critical Adhesins with Unrealized Vaccine Potential. Pathog. Dis. 2015, 73, ftv079. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhang, Y.; Wang, Q.; Li, Z.; Liu, Y.; Zhang, S.; Zhang, G.; Ma, G.; Luo, J.; Su, Z. Purification Design and Practice for Pertactin, the Third Component of Acellular Pertussis Vaccine, from Bordetella Pertussis. Vaccine 2016, 34, 4032–4039. [Google Scholar] [CrossRef]
- Carbonetti, N.H. Pertussis Toxin and Adenylate Cyclase Toxin: Key Virulence Factors of Bordetella Pertussis and Cell Biology Tools. Future Microbiol. 2010, 5, 455–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guiso, N. Bordetella Adenylate Cyclase-Hemolysin Toxins. Toxins 2017, 9, 277. [Google Scholar] [CrossRef]
- Teruya, S.; Hiramatsu, Y.; Nakamura, K.; Fukui-Miyazaki, A.; Tsukamoto, K.; Shinoda, N.; Motooka, D.; Nakamura, S.; Ishigaki, K.; Shinzawa, N.; et al. Bordetella Dermonecrotic Toxin Is a Neurotropic Virulence Factor That Uses CaV3.1 as the Cell Surface Receptor. mBio 2020, 11, e03146-19. [Google Scholar] [CrossRef] [Green Version]
- Fukui, A.; Horiguchi, Y. Bordetella Dermonecrotic Toxin Exerting Toxicity through Activation of the Small GTPase Rho. J. Biochem. 2004, 136, 415–419. [Google Scholar] [CrossRef]
- Goldman, W.E.; Klapper, D.G.; Baseman, J.B. Detection, Isolation, and Analysis of a Released Bordetella Pertussis Product Toxic to Cultured Tracheal Cells. Infect. Immun. 1982, 36, 782–794. [Google Scholar] [CrossRef] [Green Version]
- Luker, K.E.; Collier, J.L.; Kolodziej, E.W.; Marshall, G.R.; Goldman, W.E. Bordetella Pertussis Tracheal Cytotoxin and Other Muramyl Peptides: Distinct Structure-Activity Relationships for Respiratory Epithelial Cytopathology. Proc. Natl. Acad. Sci. USA 1993, 90, 2365–2369. [Google Scholar] [CrossRef] [Green Version]
- Locht, C. Molecular Aspects of Bordetella Pertussis Pathogenesis. Int. Microbiol. Off. J. Span. Soc. Microbiol. 1999, 2, 137–144. [Google Scholar]
- Recommendations to Assure the Quality, Safety and Efficacy of Acellular Pertussis Vaccines; WHO Technical Report Series No. 979; World Health Organization: Geneva, Switzerland, 2013.
- Eby, J.C.; Gray, M.C.; Warfel, J.M.; Paddock, C.D.; Jones, T.F.; Day, S.R.; Bowden, J.; Poulter, M.D.; Donato, G.M.; Merkel, T.J.; et al. Quantification of the Adenylate Cyclase Toxin of Bordetella Pertussis In Vitro and during Respiratory Infection. Infect. Immun. 2013, 81, 1390–1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Publication Office of the European Union. Replacement, Reduction and Refinement of Animal Testing in the Quality Control of Human Vaccines: Brief Description of Ongoing Projects. Available online: http://op.europa.eu/en/publication-detail/-/publication/42c3a466-bf43-11e5-9e54-01aa75ed71a1/language-en (accessed on 24 April 2020).
- Uhlrich, S.; Coppens, E.; Moysan, F.; Nelson, S.; Nougarede, N. 3Rs in Quality Control of Human Vaccines: Opportunities and Barriers. In Alternatives to Animal Testing; Kojima, H., Seidle, T., Spielmann, H., Eds.; Springer: Singapore, 2019; pp. 76–82. [Google Scholar] [CrossRef] [Green Version]
- Szymkowicz, L.; Wilson, D.J.; James, D.A. Development of a Targeted NanoLC-MS/MS Method for Quantitation of Residual Toxins from Bordetella Pertussis. J. Pharm. Biomed. Anal. 2020, 188, 113395. [Google Scholar] [CrossRef]
- Kawasaki, A.; Karasudani, Y.; Otsuka, Y.; Hasegawa, M.; Inohara, N.; Fujimoto, Y.; Fukase, K. Synthesis of Diaminopimelic Acid Containing Peptidoglycan Fragments and Tracheal Cytotoxin (TCT) and Investigation of Their Biological Functions. Chem. Weinh. Bergstr. Ger. 2008, 14, 10318–10330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cookson, B.T.; Tyler, A.N.; Goldman, W.E. Primary Structure of the Peptidoglycan-Derived Tracheal Cytotoxin of Bordetella Pertussis. Biochemistry 1989, 28, 1744–1749. [Google Scholar] [CrossRef]
- Goldman, W.E.; Cookson, B.T. Structure and Functions of the Bordetella Tracheal Cytotoxin. Tokai J. Exp. Clin. Med. 1988, 13, 187–191. [Google Scholar]
- Zhao, P.; Viner, R.; Teo, C.F.; Boons, G.-J.; Horn, D.; Wells, L. Combining High-Energy C-Trap Dissociation and Electron Transfer Dissociation for Protein O-GlcNAc Modification Site Assignment. J. Proteome Res. 2011, 10, 4088–4104. [Google Scholar] [CrossRef] [Green Version]
- Martin, S.A.; Rosenthal, R.S.; Biemann, K. Fast Atom Bombardment Mass Spectrometry and Tandem Mass Spectrometry of Biologically Active Peptidoglycan Monomers from Neisseria Gonorrhoeae. J. Biol. Chem. 1987, 262, 7514–7522. [Google Scholar] [CrossRef]
- European Pharmacopoeia. Current Edition European Monograph for Adsorbed Pertussis Vaccines (Acellular Component); Council of Europe: Strasbourg, France, 2013; p. 1356. [Google Scholar]
- Gu, H.; Zhao, Y.; DeMichele, M.; Zheng, N.; Zhang, Y.J.; Pillutla, R.; Zeng, J. In-Sample Calibration Curve Using Multiple Isotopologue Reaction Monitoring of a Stable Isotopically Labeled Analyte for Instant LC-MS/MS Bioanalysis and Quantitative Proteomics. Anal. Chem. 2019, 91, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Liu, G.; Wang, J.; Aubry, A.-F.; Arnold, M.E. Selecting the Correct Weighting Factors for Linear and Quadratic Calibration Curves with Least-Squares Regression Algorithm in Bioanalytical LC-MS/MS Assays and Impacts of Using Incorrect Weighting Factors on Curve Stability, Data Quality, and Assay Performance. Anal. Chem. 2014, 86, 8959–8966. [Google Scholar] [CrossRef] [PubMed]
- Picotti, P.; Aebersold, R. Selected Reaction Monitoring–Based Proteomics: Workflows, Potential, Pitfalls and Future Directions. Nat. Methods 2012, 9, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Kellermann, J.; Lottspeich, F. A Novel Strategy for Quantitative Proteomics Using Isotope-Coded Protein Labels. Proteomics 2005, 5, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; Wei, C.; Guo, Z.; Ma, X.; Li, Y.; Yao, J.; Ji, F.; Li, C.; Huang, T. Determination of tracheal cytotoxin in pertussis and diphtheria tetanus acellular pertussis vaccines using liquid chromatography-tandem mass spectrometry. Se Pu Chin. J. Chromatogr. 2019, 37, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Shuford, C.M.; Walters, J.J.; Holland, P.M.; Sreenivasan, U.; Askari, N.; Ray, K.; Grant, R.P. Absolute Protein Quantification by Mass Spectrometry: Not as Simple as Advertised. Anal. Chem. 2017, 89, 7406–7415. [Google Scholar] [CrossRef]
- Kuipers, B.J.H.; Gruppen, H. Prediction of Molar Extinction Coefficients of Proteins and Peptides Using UV Absorption of the Constituent Amino Acids at 214 Nm To Enable Quantitative Reverse Phase High-Performance Liquid Chromatography−Mass Spectrometry Analysis. J. Agric. Food Chem. 2007, 55, 5445–5451. [Google Scholar] [CrossRef]
- Li, C.; Bhavaraju, S.; Thibeault, M.-P.; Melanson, J.; Blomgren, A.; Rundlöf, T.; Kilpatrick, E.; Swann, C.J.; Rudd, T.; Aubin, Y.; et al. Survey of Peptide Quantification Methods and Comparison of Their Reproducibility: A Case Study Using Oxytocin. J. Pharm. Biomed. Anal. 2019, 166, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Stanek, O.; Linhartova, I.; Holubova, J.; Bumba, L.; Gardian, Z.; Malandra, A.; Bockova, B.; Teruya, S.; Horiguchi, Y.; Osicka, R.; et al. Production of Highly Active Recombinant Dermonecrotic Toxin of Bordetella Pertussis. Toxins 2020, 12, 596. [Google Scholar] [CrossRef]
- Pratt, J.M.; Simpson, D.M.; Doherty, M.K.; Rivers, J.; Gaskell, S.J.; Beynon, R.J. Multiplexed Absolute Quantification for Proteomics Using Concatenated Signature Peptides Encoded by QconCAT Genes. Nat. Protoc. 2006, 1, 1029–1043. [Google Scholar] [CrossRef]
- Cheung, C.S.F.; Anderson, K.W.; Wang, M.; Turko, I.V. Natural Flanking Sequences for Peptides Included in a Quantification Concatamer Internal Standard. Anal. Chem. 2015, 87, 1097–1102. [Google Scholar] [CrossRef]
- Scott, K.B.; Turko, I.V.; Phinney, K.W. Chapter Eleven—QconCAT: Internal Standard for Protein Quantification. In Methods in Enzymology; Kelman, Z., Ed.; Isotope Labeling of Biomolecules—Applications; Academic Press: Cambridge, MA, USA, 2016; Volume 566, pp. 289–303. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of Protein Using Bicinchoninic Acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Saveliev, S.; Bratz, M.; Zubarev, R.; Szapacs, M.; Budamgunta, H.; Urh, M. Trypsin/Lys-C Protease Mix for Enhanced Protein Mass Spectrometry Analysis. Nat. Methods 2013, 10, 1134. [Google Scholar] [CrossRef]
- Yu, Y.-Q.; Gilar, M.; Lee, P.J.; Bouvier, E.S.P.; Gebler, J.C. Enzyme-Friendly, Mass Spectrometry-Compatible Surfactant for in-Solution Enzymatic Digestion of Proteins. Anal. Chem. 2003, 75, 6023–6028. [Google Scholar] [CrossRef] [PubMed]
- MacLean, B.; Tomazela, D.M.; Shulman, N.; Chambers, M.; Finney, G.L.; Frewen, B.; Kern, R.; Tabb, D.L.; Liebler, D.C.; MacCoss, M.J. Skyline: An Open Source Document Editor for Creating and Analyzing Targeted Proteomics Experiments. Bioinformatics 2010, 26, 966–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
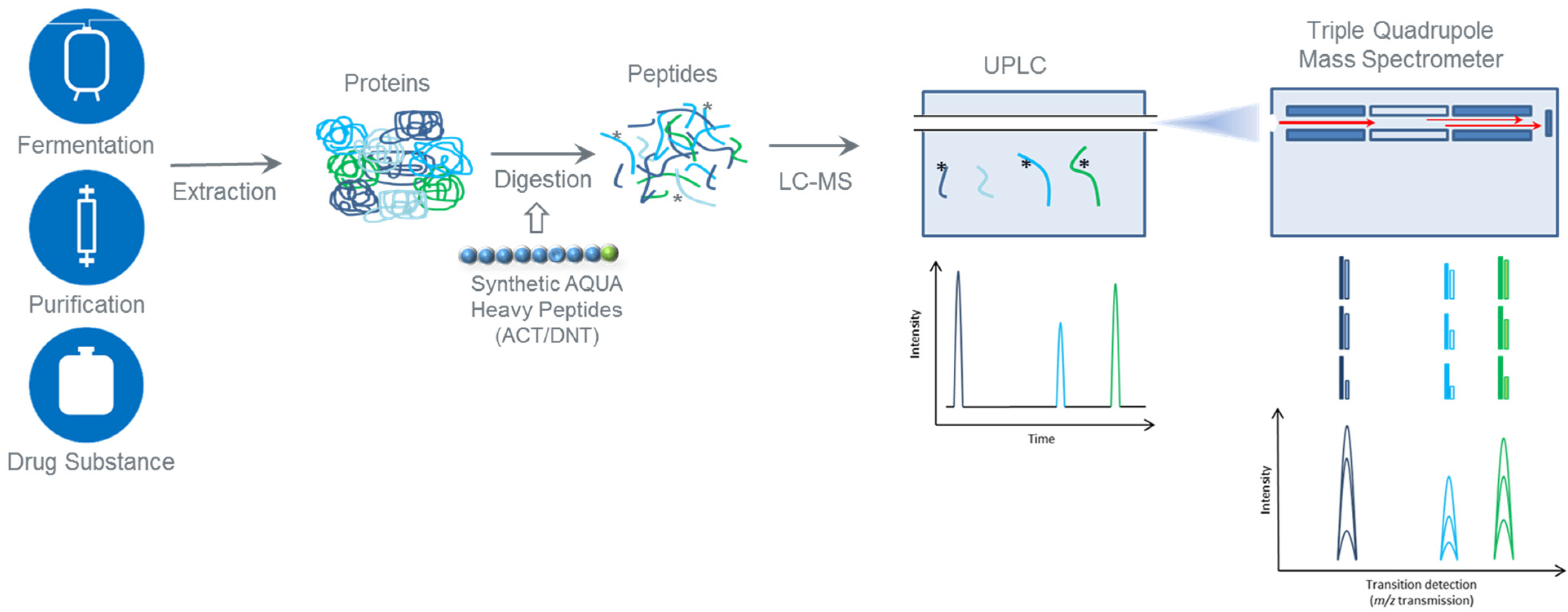

| Toxin | Theoretical Intact Molecular Weight (g/mol) | Peptide | Fragment Type | Description | Precursor (m/z) | Fragment (m/z) | Cone (V) | Collision Energy (V) |
|---|---|---|---|---|---|---|---|---|
| ACT | 177 414 | NIENAVGSAR A | y8 * | Native | 515.7674 | 803.4006 | 35 | 19 |
| Heavy AQUA | 520.7716 | 813.4089 | 35 | 19 | ||||
| y7 | Native | 515.7674 | 674.3580 | 35 | 22 | |||
| Heavy AQUA | 520.7716 | 684.3663 | 35 | 22 | ||||
| ITGDAQANVLR B | y9 | Native | 579.3173 | 943.4956 | 35 | 22 | ||
| Heavy AQUA | 584.3214 | 953.5038 | 35 | 22 | ||||
| y5 | Native | 579.3173 | 572.3515 | 35 | 22 | |||
| Heavy AQUA | 584.3214 | 582.3597 | 35 | 22 | ||||
| DNT | 160 644 | ELPALIGASGLR A | y6 * | Native | 598.8535 | 560.3151 | 35 | 21 |
| Heavy AQUA | 603.8576 | 570.3234 | 35 | 21 | ||||
| y7 | Native | 598.8535 | 673.3991 | 35 | 24 | |||
| Heavy AQUA | 603.8576 | 683.4074 | 35 | 24 | ||||
| NDDLVSIAATYDR B | y8 | Native | 726.8519 | 896.4472 | 35 | 24 | ||
| Heavy AQUA | 731.8560 | 906.4555 | 35 | 24 | ||||
| b3 | Native | 726.8519 | 345.1041 | 35 | 29 | |||
| Heavy AQUA | 731.8560 | 345.1041 | 35 | 29 | ||||
| TCT | 921 | C37H59O20N7 | Peptidoglycan | [M + H] | 922.3888 | 719.3099 | 35 | 31 |
| [M + 2H] | 461.6985 | 719.3099 | 35 | 11 |
| Toxin | Standard Type | Peptide Standard | LLOQ 1 | LOD 2 | ||
|---|---|---|---|---|---|---|
| On-Column | per 25 µg Digest | On-Column | per 25 µg Digest | |||
| ACT | Internal—AQUA peptide standards (20 fmol/µL) | NIENAVGSAR | 93.6 amol | 2.6 ng (14.6 fmol) | 21.4 amol | 0.59 ng (3.39 fmol) |
| ITGDAQANVLR | 116 amol | 3.2 ng (18.1 fmol) | 63.6 amol | 1.76 ng (9.92 fmol) | ||
| DNT | Internal—AQUA peptide standards (20 fmol/µL) | NDDLVSIAATYDR | 1.96 fmol | 49.1 ng (306 fmol) | 375 amol | 9.4 ng (58.5 fmol) |
| ELPALIGASGLR | 3.97 fmol | 99.5 ng (619 fmol) | 655 amol | 16.4 ng (102 fmol) | ||
| TCT | External—purified TCT standard | Purified TCT | 2.3 pg | 0.388 pmol (0.36 ng) | 0.423 pg | 0.069 pmol (63.6 pg) |
| Toxin | Ph. Eu. 1356 Limit | % EU Limit | Accuracy (% Recovery) | Precision (% CV) | Linearity | Qualified Range (per Dose) | LLOQ (per Antigen Dose) | |
|---|---|---|---|---|---|---|---|---|
| Repeatability | Intermediate Precision | |||||||
| ACT | 500 ng per dose | 10.0% | 87 | 2.3 | 5.3 | R2 = 0.9824 | 50.0 to 600.0 ng | 14.1 ng |
| 50.0% | 89 | 2.6 | 7.7 | |||||
| 120.0% | 81 | 4.1 | 7.8 | |||||
| TCT | 2 pmol per dose | 20.0% | 128 | 5.7 | 12.0 | R2 = 0.9931 | 0.4 to 12.8 pmol | 0.41 pmol |
| 53.1% | 118 | 5.0 | 9.5 | |||||
| 120.0% | 114 | 2.6 | 4.0 | |||||
| 265.7% | 122 | 1.1 | 5.2 | |||||
| 637.7% | 120 | 0.9 | 6.4 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymkowicz, L.; Gerard, J.; Messham, B.; Tam, W.W.V.; James, D.A. Design of a Quantitative LC-MS Method for Residual Toxins Adenylate Cyclase Toxin (ACT), Dermonecrotic Toxin (DNT) and Tracheal Cytotoxin (TCT) in Bordetella pertussis Vaccines. Toxins 2021, 13, 763. https://doi.org/10.3390/toxins13110763
Szymkowicz L, Gerard J, Messham B, Tam WWV, James DA. Design of a Quantitative LC-MS Method for Residual Toxins Adenylate Cyclase Toxin (ACT), Dermonecrotic Toxin (DNT) and Tracheal Cytotoxin (TCT) in Bordetella pertussis Vaccines. Toxins. 2021; 13(11):763. https://doi.org/10.3390/toxins13110763
Chicago/Turabian StyleSzymkowicz, Lisa, Jeffery Gerard, Benjamin Messham, Wai Wan Vivian Tam, and D. Andrew James. 2021. "Design of a Quantitative LC-MS Method for Residual Toxins Adenylate Cyclase Toxin (ACT), Dermonecrotic Toxin (DNT) and Tracheal Cytotoxin (TCT) in Bordetella pertussis Vaccines" Toxins 13, no. 11: 763. https://doi.org/10.3390/toxins13110763
APA StyleSzymkowicz, L., Gerard, J., Messham, B., Tam, W. W. V., & James, D. A. (2021). Design of a Quantitative LC-MS Method for Residual Toxins Adenylate Cyclase Toxin (ACT), Dermonecrotic Toxin (DNT) and Tracheal Cytotoxin (TCT) in Bordetella pertussis Vaccines. Toxins, 13(11), 763. https://doi.org/10.3390/toxins13110763





