Cannabis: A Toxin-Producing Plant with Potential Therapeutic Uses
Abstract
1. Introduction
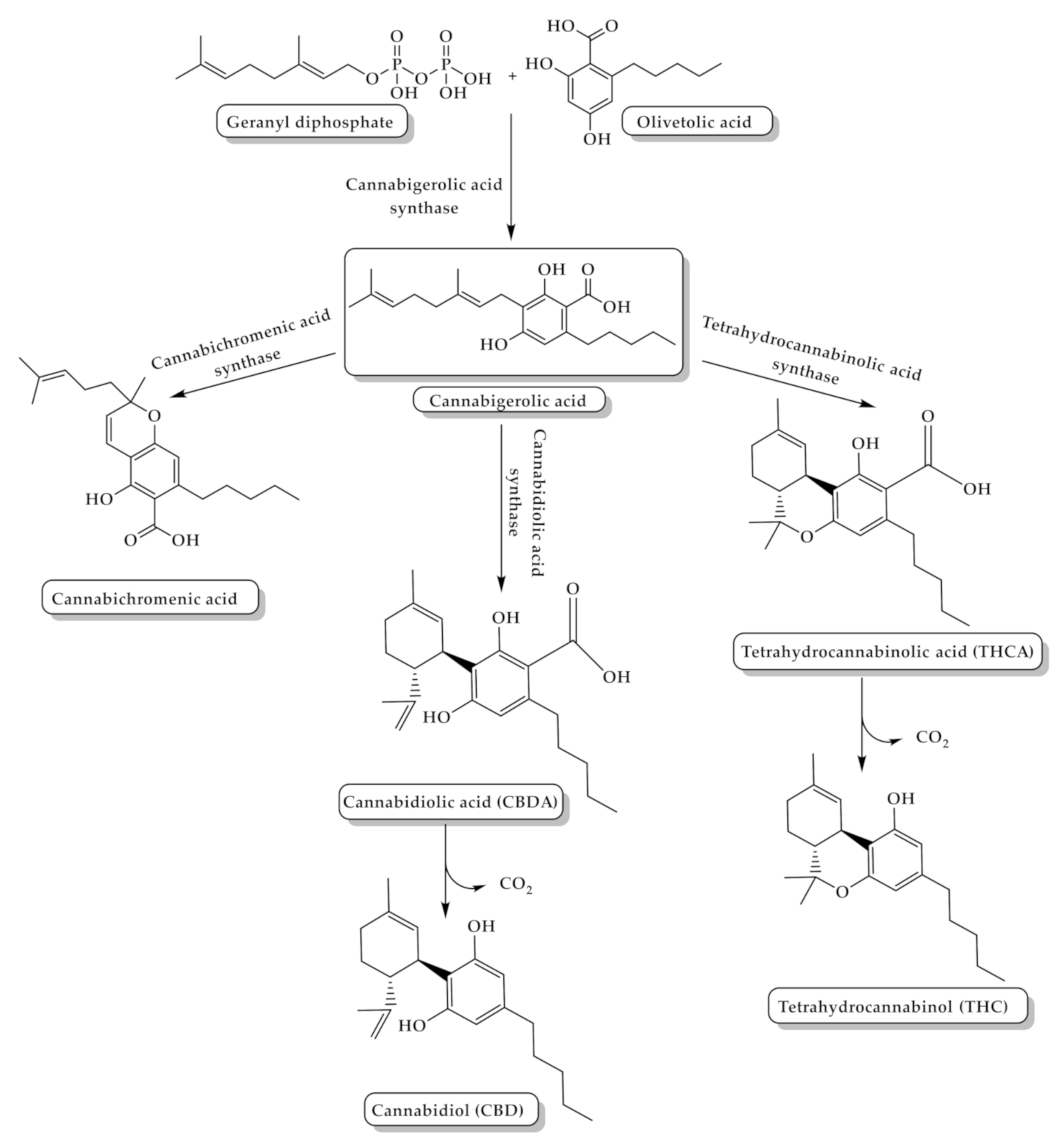
2. Cannabis Chemistry and Pharmacology
3. Cannabis and Cannabinoids: Potential Therapeutic Uses
3.1. Pain Treatment with Cannabis
| Analgesic Cannabinoids | Theraputic Actions |
|---|---|
| Dronabinol (Marinol (30), Figure 5) | A synthetic oral form of THC and a partial agonist at the CB1 receptor. Approved in the USA in 1985 for nausea associated with chemotherapy and for appetite stimulation in HIV/AIDS [69]. Used for treating the pain of patients with multiple sclerosis. [70]. |
| Nabilone (Cesamet (31), Figure 5) | A synthetic orally administrated dimethyl heptyl analog of THC [71]. Approved in 1981 by the US FDA for the treatment of nausea and vomiting induced by chemotherapy [72], and is reported to be dispensed off-label for the management of pain [73], and treatment of fibromyalgia in some studies [74,75]. |
| Ajulemic acid (AJA (32), Figure 5) | An oral synthetic non-intoxicating analog of THC. Stimulate endogenous eicosanoids that limit inflammation progress and resolve fibrosis [76,77]. AJA is still under study to evaluate its effect and toxicity [76,78]. |
| Sativex | A cannabis-based oromucosal spray containing a mixture of a 1:1 ratio of THC and CBD. Approved in several European countries. Used as an add-on therapy of multiple sclerosis (MS) related spasticity in patients non-responsive to conventional anti-spastic therapies [79]. |
| WIN-55,212-2 ((R)-(+)-[2,3-Dihydro-5-methyl-3[(4-morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl)methanone) (WIN (17), Figure 4) | A synthetic cannabinoid that binds non-selectively to CB1 and CB2 receptors Has the ability to alleviate neuropathic pain by suppressing mechanical allodynia and thermal hyperalgesia [80,81]. |
| Selective CB2 agonists (HU-308, AM1241, JWH-133 and GW405833); | HU308 (4-[4-(1,1-diemethylheptyl)-2,6-dimethoxyphenyl]-6,6-dimethyl-bicyclo[3.1.1]hept-2-ene-2-methanol (33), Figure 5) binds selectively to CB2 and its agonist activity results in peripheral antihyperalgesic and anti-inflammatory effects [82]. AM1241 (2-iodo-5-nitro-phenyl)-[1-(1-methyl-piperidin-2-ylmethyl)-1H-indol-3-yl]-methanone (34), Figure 5) acts by producing peripheral-mediated antinociception, inducing CB2-mediated antihyperalgesic effects and stimulating the release of β-endorphin from skin keratinocytes [82]. JWH-133 ((6aR,10aR)-3-(1,1-dimethylbutyl)-6a,7,10,10a-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b,d]pyran (35), Figure 5) inhibits inflammatory and neuropathic hyperalgesia [82]. Another CB2 selective agonist form called JWH015 is given intrathecal for the treatment of bone cancer pain where it has the ability to downregulate IL-1β and IL-6 [83]. GW405833 (2,3-dichloro-phenyl)-[5-methoxy-2-methyl-3-(2-morpholin-4-yl-ethyl)-indol-1-yl]-methanone) ((36), Figure 5) [82] also has an antihyperalgesic effect and reduces allodynia in inflammatory pain [84]. |
3.2. Cannabis as an Anti-Emetic and Appetite Stimulant
3.3. Cannabis and Multiple Sclerosis (MS)
3.4. Cancer Treatment by Cannabis
4. Cannabis Poisoning
4.1. Cannabis’ Effects on the Cardiovascular System
4.2. Cognitive, Psychiatric, and Psychomotor Effects
4.3. Effects on the Respiratory System
4.4. Effects on the Hormonal System and Fertility
4.5. Maternal Cannabis Exposure and Infant Outcome
4.6. Cannabis-Tolerance and Dependence
4.7. Cannabinoids Drug Interactions
5. Legalization of Cannabis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315. [Google Scholar] [CrossRef]
- Meissner, H.; Cascella, M. Cannabidiol (CBD); StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Gonçalves, J.; Rosado, T.; Soares, S.; Simão, A.Y.; Caramelo, D.; Luís, Â.; Fernández, N.; Barroso, M.; Gallardo, E.; Duarte, A.P. Cannabis and Its Secondary Metabolites: Their Use as Therapeutic Drugs, Toxicological Aspects, and Analytical Determination. Medicines 2019, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- McPartland, J.M. Cannabis Systematics at the Levels of Family, Genus, and Species. Cannabis Cannabinoid Res. 2018, 3, 203–212. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research; The National Academies Press: Washington, DC, USA, 2017; p. 486. [Google Scholar] [CrossRef]
- Sharma, P.; Murthy, P.; Bharath, M.S. Chemistry, metabolism, and toxicology of cannabis: Clinical implications. Iran. J. Psychiatry 2012, 7, 149. [Google Scholar]
- Hazekamp, A.; Grotenhermen, F. Review on clinical studies with cannabis and cannabinoids 2005–2009. Cannabinoids 2010, 5, 1–21. [Google Scholar]
- Russell, C.; Rueda, S.; Room, R.; Tyndall, M.; Fischer, B. Routes of administration for cannabis use—Basic prevalence and related health outcomes: A scoping review and synthesis. Int. J. Drug Policy 2018, 52, 87–96. [Google Scholar] [CrossRef]
- Barrus, D.G.; Capogrossi, K.L.; Cates, S.C.; Gourdet, C.K.; Peiper, N.C.; Novak, S.P.; Lefever, T.W.; Wiley, J.L. Tasty THC: Promises and Challenges of Cannabis Edibles. Methods Rep. 2016, 2016. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis sativa L. In Phytocannabinoids; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–36. [Google Scholar]
- Solymosi, K.; Köfalvi, A. Cannabis: A treasure trove or pandora’s box? Mini Rev. Med. Chem. 2017, 17, 1223–1291. [Google Scholar] [CrossRef]
- Turner, S.E.; Williams, C.M.; Iversen, L.; Whalley, B.J. Molecular pharmacology of phytocannabinoids. In Phytocannabinoids; Springer: Berlin/Heidelberg, Germany, 2017; pp. 61–101. [Google Scholar]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef]
- Champagne, A.S.; McFaull, S.R.; Thompson, W.; Bang, F. Surveillance from the high ground: Sentinel surveillance of injuries and poisonings associated with cannabis. Health Promot. Chronic Dis. Prev. Can. Res. Policy Pract. 2020, 40, 184–192. [Google Scholar] [CrossRef]
- Colizzi, M.; Ruggeri, M.; Bhattacharyya, S. Unraveling the Intoxicating and Therapeutic Effects of Cannabis Ingredients on Psychosis and Cognition. Front. Psychol. 2020, 11, 833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.W.; Ho, R.C. The Cannabis Dilemma: A Review of Its Associated Risks and Clinical Efficacy. J. Addict. 2015, 2015, 707596. [Google Scholar] [CrossRef]
- Drugs, O.O. Crime. In World Drug Report 2014; United Nations Development Programm: New York, NY, USA, 2014. [Google Scholar]
- Pratt, M.; Stevens, A.; Thuku, M.; Butler, C.; Skidmore, B.; Wieland, L.S.; Clemons, M.; Kanji, S.; Hutton, B. Benefits and harms of medical cannabis: A scoping review of systematic reviews. Syst. Rev. 2019, 8, 320. [Google Scholar] [CrossRef] [PubMed]
- Memedovich, K.A.; Dowsett, L.E.; Spackman, E.; Noseworthy, T.; Clement, F. The adverse health effects and harms related to marijuana use: An overview review. CMAJ Open 2018, 6, E339–E346. [Google Scholar] [CrossRef] [PubMed]
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis sativa L. Prog. Chem. Org. Nat. Prod. 2017, 103, 1–36. [Google Scholar] [CrossRef]
- Citti, C.; Linciano, P.; Russo, F.; Luongo, L.; Iannotta, M.; Maione, S.; Lagana, A.; Capriotti, A.L.; Forni, F.; Vandelli, M.A.; et al. A novel phytocannabinoid isolated from Cannabis sativa L. with an in vivo cannabimimetic activity higher than Delta(9)-tetrahydrocannabinol: Delta(9)-Tetrahydrocannabiphorol. Sci. Rep. 2019, 9, 20335. [Google Scholar] [CrossRef] [PubMed]
- Fischedick, J.T. Identification of Terpenoid Chemotypes Among High (-)-trans-Delta(9)- Tetrahydrocannabinol-Producing Cannabis sativa L. Cultivars. Cannabis Cannabinoid Res. 2017, 2, 34–47. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Mehmedic, Z.; Foster, S.; Gon, C.; Chandra, S.; Church, J.C. Changes in Cannabis Potency Over the Last 2 Decades (1995–2014): Analysis of Current Data in the United States. Biol. Psychiatry 2016, 79, 613–619. [Google Scholar] [CrossRef]
- VanDolah, H.J.; Bauer, B.A.; Mauck, K.F. Clinicians’ Guide to Cannabidiol and Hemp Oils. Mayo Clin. Proc. 2019, 94, 1840–1851. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.C.; Mackie, K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef]
- Almogi-Hazan, O.; Or, R. Cannabis, the Endocannabinoid System and Immunity-the Journey from the Bedside to the Bench and Back. Int. J. Mol. Sci. 2020, 21, 4448. [Google Scholar] [CrossRef]
- Elphick, M.R. The evolution and comparative neurobiology of endocannabinoid signalling. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3201–3215. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ruiz, J.; Sagredo, O.; Pazos, M.R.; García, C.; Pertwee, R.; Mechoulam, R.; Martínez-Orgado, J. Cannabidiol for neurodegenerative disorders: Important new clinical applications for this phytocannabinoid? Br. J. Clin. Pharmacol. 2013, 75, 323–333. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Peigneur, S.; Hendrickx, L.A.; Tytgat, J. Targeting Cannabinoid Receptors: Current Status and Prospects of Natural Products. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef]
- Pertwee, R.G. The pharmacology of cannabinoid receptors and their ligands: An overview. Int. J. Obes. 2006, 30 (Suppl. 1), S13–S18. [Google Scholar] [CrossRef]
- Howlett, A.C.; Abood, M.E. CB1 and CB2 Receptor Pharmacology. Adv. Pharmacol. 2017, 80, 169–206. [Google Scholar] [CrossRef] [PubMed]
- Console-Bram, L.; Marcu, J.; Abood, M.E. Cannabinoid receptors: Nomenclature and pharmacological principles. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 38, 4–15. [Google Scholar] [CrossRef]
- Hillard, C.J.; Manna, S.; Greenberg, M.J.; DiCamelli, R.; Ross, R.A.; Stevenson, L.A.; Murphy, V.; Pertwee, R.G.; Campbell, W.B. Synthesis and characterization of potent and selective agonists of the neuronal cannabinoid receptor (CB1). J. Pharmacol. Exp. Ther. 1999, 289, 1427–1433. [Google Scholar] [PubMed]
- Price, M.R.; Baillie, G.L.; Thomas, A.; Stevenson, L.A.; Easson, M.; Goodwin, R.; McLean, A.; McIntosh, L.; Goodwin, G.; Walker, G.; et al. Allosteric modulation of the cannabinoid CB1 receptor. Mol. Pharmacol. 2005, 68, 1484–1495. [Google Scholar] [CrossRef]
- Morales, P.; Goya, P.; Jagerovic, N.; Hernandez-Folgado, L. Allosteric Modulators of the CB1 Cannabinoid Receptor: A Structural Update Review. Cannabis Cannabinoid Res. 2016, 1, 22–30. [Google Scholar] [CrossRef]
- Gado, F.; Di Cesare Mannelli, L.; Lucarini, E.; Bertini, S.; Cappelli, E.; Digiacomo, M.; Stevenson, L.A.; Macchia, M.; Tuccinardi, T.; Ghelardini, C.; et al. Identification of the First Synthetic Allosteric Modulator of the CB2 Receptors and Evidence of Its Efficacy for Neuropathic Pain Relief. J. Med. Chem. 2019, 62, 276–287. [Google Scholar] [CrossRef]
- Tham, M.; Yilmaz, O.; Alaverdashvili, M.; Kelly, M.E.M.; Denovan-Wright, E.M.; Laprairie, R.B. Allosteric and orthosteric pharmacology of cannabidiol and cannabidiol-dimethylheptyl at the type 1 and type 2 cannabinoid receptors. Br. J. Pharmacol. 2019, 176, 1455–1469. [Google Scholar] [CrossRef]
- Staton, P.C.; Hatcher, J.P.; Walker, D.J.; Morrison, A.D.; Shapland, E.M.; Hughes, J.P.; Chong, E.; Mander, P.K.; Green, P.J.; Billinton, A. The putative cannabinoid receptor GPR55 plays a role in mechanical hyperalgesia associated with inflammatory and neuropathic pain. Pain 2008, 139, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.M.; Bisogno, T.; Petros, T.J.; Chang, S.Y.; Zavitsanos, P.A.; Zipkin, R.E.; Sivakumar, R.; Coop, A.; Maeda, D.Y.; De Petrocellis, L. Identification of a new class of molecules, the arachidonyl amino acids, and characterization of one member that inhibits pain. J. Biol. Chem. 2001, 276, 42639–42644. [Google Scholar] [CrossRef] [PubMed]
- Laezza, C.; Pagano, C.; Navarra, G.; Pastorino, O.; Proto, M.C.; Fiore, D.; Piscopo, C.; Gazzerro, P.; Bifulco, M. The Endocannabinoid System: A Target for Cancer Treatment. Int. J. Mol. Sci. 2020, 21, 747. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B.; Burnett, A.; Hall, B.; Parker, K.K. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem. Res. 2005, 30, 1037–1043. [Google Scholar] [CrossRef]
- Scavone, J.; Sterling, R.; Van Bockstaele, E. Cannabinoid and opioid interactions: Implications for opiate dependence and withdrawal. Neuroscience 2013, 248, 637–654. [Google Scholar] [CrossRef] [PubMed]
- Fattore, L.; Fratta, W. Beyond THC: The New Generation of Cannabinoid Designer Drugs. Front. Behav. Neurosci. 2011, 5, 60. [Google Scholar] [CrossRef]
- Cohen, K.; Weinstein, A.M. Synthetic and Non-synthetic Cannabinoid Drugs and Their Adverse Effects-A Review From Public Health Prospective. Front. Public Health 2018, 6, 162. [Google Scholar] [CrossRef]
- Sheikh, N.K.; Dua, A. Cannabinoids; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- MacCallum, C.A.; Russo, E.B. Practical considerations in medical cannabis administration and dosing. Eur. J. Intern. Med. 2018, 49, 12–19. [Google Scholar] [CrossRef]
- Hall, W.; Stjepanović, D.; Caulkins, J.; Lynskey, M.; Leung, J.; Campbell, G.; Degenhardt, L. Public health implications of legalising the production and sale of cannabis for medicinal and recreational use. Lancet 2019, 394, 1580–1590. [Google Scholar] [CrossRef]
- Russo, E.B. Cannabinoids in the management of difficult to treat pain. Ther. Clin. Risk Manag. 2008, 4, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Elikkottil, J.; Gupta, P.; Gupta, K. The analgesic potential of cannabinoids. J. Opioid Manag. 2009, 5, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Boehnke, K.F.; Litinas, E.; Clauw, D.J. Medical Cannabis Use Is Associated With Decreased Opiate Medication Use in a Retrospective Cross-Sectional Survey of Patients With Chronic Pain. J. Pain Off. J. Am. Pain Soc. 2016, 17, 739–744. [Google Scholar] [CrossRef]
- Philpot, L.M.; Ebbert, J.O.; Hurt, R.T. A survey of the attitudes, beliefs and knowledge about medical cannabis among primary care providers. BMC Fam. Pract. 2019, 20, 17. [Google Scholar] [CrossRef] [PubMed]
- Reiman, A.; Welty, M.; Solomon, P. Cannabis as a Substitute for Opioid-Based Pain Medication: Patient Self-Report. Cannabis Cannabinoid Res. 2017, 2, 160–166. [Google Scholar] [CrossRef]
- Sexton, M.; Cuttler, C.; Finnell, J.S.; Mischley, L.K. A Cross-Sectional Survey of Medical Cannabis Users: Patterns of Use and Perceived Efficacy. Cannabis Cannabinoid Res. 2016, 1, 131–138. [Google Scholar] [CrossRef]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA 2015, 313, 2456–2473. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.; Hall, W.D.; Peacock, A.; Lintzeris, N.; Bruno, R.; Larance, B.; Nielsen, S.; Cohen, M.; Chan, G.; Mattick, R.P.; et al. Effect of cannabis use in people with chronic non-cancer pain prescribed opioids: Findings from a 4-year prospective cohort study. Lancet Public Health 2018, 3, e341–e350. [Google Scholar] [CrossRef]
- Hill, K.P.; Palastro, M.D.; Johnson, B.; Ditre, J.W. Cannabis and Pain: A Clinical Review. Cannabis Cannabinoid Res. 2017, 2, 96–104. [Google Scholar] [CrossRef]
- Russo, E.B.; Hohmann, A.G. Role of Cannabinoids in Pain Management. In Comprehensive Treatment of Chronic Pain by Medical, Interventional, and Integrative Approaches; Springer: New York, NY, USA, 2013; pp. 181–197. [Google Scholar] [CrossRef]
- Randall, M.D. Endocannabinoids and the haematological system. Br. J. Pharmacol. 2007, 152, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Lochte, B.C.; Beletsky, A.; Samuel, N.K.; Grant, I. The Use of Cannabis for Headache Disorders. Cannabis Cannabinoid Res. 2017, 2, 61–71. [Google Scholar] [CrossRef]
- Bloomfield, M.A.; Ashok, A.H.; Volkow, N.D.; Howes, O.D. The effects of Delta(9)-tetrahydrocannabinol on the dopamine system. Nature 2016, 539, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.F.; Oz, M.; Yang, R.; Lichtman, A.H.; Lupica, C.R. Opposing actions of chronic Delta9-tetrahydrocannabinol and cannabinoid antagonists on hippocampal long-term potentiation. Learn. Mem. 2007, 14, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid Ligands Targeting TRP Channels. Front. Mol. Neurosci. 2018, 11, 487. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef]
- Han, K.H.; Lim, S.; Ryu, J.; Lee, C.W.; Kim, Y.; Kang, J.H.; Kang, S.S.; Ahn, Y.K.; Park, C.S.; Kim, J.J. CB1 and CB2 cannabinoid receptors differentially regulate the production of reactive oxygen species by macrophages. Cardiovasc. Res. 2009, 84, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Jean-Gilles, L.; Braitch, M.; Latif, M.L.; Aram, J.; Fahey, A.J.; Edwards, L.J.; Robins, R.A.; Tanasescu, R.; Tighe, P.J.; Gran, B.; et al. Effects of pro-inflammatory cytokines on cannabinoid CB1 and CB2 receptors in immune cells. Acta Physiol. 2015, 214, 63–74. [Google Scholar] [CrossRef]
- Nichols, J.M.; Kaplan, B.L.F. Immune responses regulated by cannabidiol. Cannabis Cannabinoid Res. 2020, 5, 12–31. [Google Scholar] [CrossRef]
- O’Donnell, B.; Gupta, V. Dronabinol; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Bruni, N.; Della Pepa, C.; Oliaro-Bosso, S.; Pessione, E.; Gastaldi, D.; Dosio, F. Cannabinoid Delivery Systems for Pain and Inflammation Treatment. Molecules 2018, 23, 2478. [Google Scholar] [CrossRef]
- Burstein, S.H.; Zurier, R.B. Cannabinoids, endocannabinoids, and related analogs in inflammation. AAPS J. 2009, 11, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Receptors and channels targeted by synthetic cannabinoid receptor agonists and antagonists. Curr. Med. Chem. 2010, 17, 1360–1381. [Google Scholar] [CrossRef] [PubMed]
- Vuckovic, S.; Srebro, D.; Vujovic, K.S.; Vucetic, C.; Prostran, M. Cannabinoids and Pain: New Insights From Old Molecules. Front. Pharmacol. 2018, 9, 1259. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.C.; Giudice, M.G. Nabilone for the Management of Pain. Pharmacotherapy 2016, 36, 273–286. [Google Scholar] [CrossRef]
- McGolrick, D.; Frey, N. Nabilone for Chronic Pain Management: A Review of Clinical Effectiveness and Guidelines—An Update; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2018. [Google Scholar]
- Burstein, S.H. Ajulemic acid: Potential treatment for chronic inflammation. Pharmacol. Res. Perspect. 2018, 6, e00394. [Google Scholar] [CrossRef]
- Zurier, R.B.; Sun, Y.P.; George, K.L.; Stebulis, J.A.; Rossetti, R.G.; Skulas, A.; Judge, E.; Serhan, C.N. Ajulemic acid, a synthetic cannabinoid, increases formation of the endogenous proresolving and anti-inflammatory eicosanoid, lipoxin A4. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2009, 23, 1503–1509. [Google Scholar] [CrossRef] [PubMed]
- Burstein, S. Ajulemic acid (IP-751): Synthesis, proof of principle, toxicity studies, and clinical trials. AAPS J. 2005, 7, E143–E148. [Google Scholar] [CrossRef]
- Giacoppo, S.; Bramanti, P.; Mazzon, E. Sativex in the management of multiple sclerosis-related spasticity: An overview of the last decade of clinical evaluation. Mult. Scler. Relat. Disord. 2017, 17, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; Colleoni, M.; Conti, S.; Trovato, A.E.; Bianchi, M.; Sotgiu, M.L.; Giagnoni, G. Repeated treatment with the synthetic cannabinoid WIN 55,212-2 reduces both hyperalgesia and production of pronociceptive mediators in a rat model of neuropathic pain. Br. J. Pharmacol. 2004, 141, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Hama, A.; Sagen, J. Sustained antinociceptive effect of cannabinoid receptor agonist WIN 55,212-2 over time in rat model of neuropathic spinal cord injury pain. J. Rehabil. Res. Dev. 2009, 46, 135–143. [Google Scholar] [CrossRef]
- Guindon, J.; Hohmann, A.G. Cannabinoid CB2 receptors: A therapeutic target for the treatment of inflammatory and neuropathic pain. Br. J. Pharmacol. 2008, 153, 319–334. [Google Scholar] [CrossRef]
- Mao, Y.; Huang, Y.; Zhang, Y.; Wang, C.; Wu, H.; Tian, X.; Liu, Y.; Hou, B.; Liang, Y.; Rong, H.; et al. Cannabinoid receptor 2selective agonist JWH015 attenuates bone cancer pain through the amelioration of impaired autophagy flux induced by inflammatory mediators in the spinal cord. Mol. Med. Rep. 2019, 20, 5100–5110. [Google Scholar] [CrossRef] [PubMed]
- Li, A.L.; Carey, L.M.; Mackie, K.; Hohmann, A.G. Cannabinoid CB2 Agonist GW405833 Suppresses Inflammatory and Neuropathic Pain through a CB1 Mechanism that is Independent of CB2 Receptors in Mice. J. Pharmacol. Exp. Ther. 2017, 362, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, K.A.; Darmani, N.A.; Parker, L.A. Regulation of nausea and vomiting by cannabinoids and the endocannabinoid system. Eur. J. Pharmacol. 2014, 722, 134–146. [Google Scholar] [CrossRef]
- Torii, Y.; Saito, H.; Matsuki, N. Selective blockade of cytotoxic drug-induced emesis by 5-HT3 receptor antagonists in Suncus murinus. Jpn. J. Pharmacol. 1991, 55, 107–113. [Google Scholar] [CrossRef]
- Parker, L.A.; Rock, E.M.; Limebeer, C.L. Regulation of nausea and vomiting by cannabinoids. Br. J. Pharmacol. 2011, 163, 1411–1422. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.N.; Sauls, R.S. Cannaboinoid Antiemetic Therapy; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Fraguas-Sanchez, A.I.; Torres-Suarez, A.I. Medical Use of Cannabinoids. Drugs 2018, 78, 1665–1703. [Google Scholar] [CrossRef]
- Brierley, D.I.; Samuels, J.; Duncan, M.; Whalley, B.J.; Williams, C.M. Cannabigerol is a novel, well-tolerated appetite stimulant in pre-satiated rats. Psychopharmacology 2016, 233, 3603–3613. [Google Scholar] [CrossRef]
- Kirkham, T.C. Endocannabinoids in the regulation of appetite and body weight. Behav. Pharmacol. 2005, 16, 297–313. [Google Scholar] [CrossRef] [PubMed]
- Beal, J.E.; Olson, R.; Laubenstein, L.; Morales, J.O.; Bellman, P.; Yangco, B.; Lefkowitz, L.; Plasse, T.F.; Shepard, K.V. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J. Pain Symptom Manag. 1995, 10, 89–97. [Google Scholar] [CrossRef]
- Struwe, M.; Kaempfer, S.H.; Geiger, C.J.; Pavia, A.T.; Plasse, T.F.; Shepard, K.V.; Ries, K.; Evans, T.G. Effect of dronabinol on nutritional status in HIV infection. Ann. Pharmacother. 1993, 27, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Abrams, D.I. Integrating cannabis into clinical cancer care. Curr. Oncol. 2016, 23, S8–S14. [Google Scholar] [CrossRef]
- Ingram, G.; Pearson, O.R. Cannabis and multiple sclerosis. Pract. Neurol. 2019, 19, 310–315. [Google Scholar] [CrossRef]
- Rudroff, T.; Honce, J.M. Cannabis and Multiple Sclerosis-The Way Forward. Front. Neurol. 2017, 8, 299. [Google Scholar] [CrossRef] [PubMed]
- Daris, B.; Tancer Verboten, M.; Knez, Z.; Ferk, P. Cannabinoids in cancer treatment: Therapeutic potential and legislation. Bosn. J. Basic Med Sci. 2019, 19, 14–23. [Google Scholar] [CrossRef]
- Velasco, G.; Sanchez, C.; Guzman, M. Anticancer mechanisms of cannabinoids. Curr. Oncol. 2016, 23, S23–S32. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, H.; Ning, W.; Backlund, M.G.; Dey, S.K.; DuBois, R.N. Loss of cannabinoid receptor 1 accelerates intestinal tumor growth. Cancer Res. 2008, 68, 6468–6476. [Google Scholar] [CrossRef]
- Pertwee, R.G.; Howlett, A.C.; Abood, M.E.; Alexander, S.P.; Di Marzo, V.; Elphick, M.R.; Greasley, P.J.; Hansen, H.S.; Kunos, G.; Mackie, K.; et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: Beyond CB(1) and CB(2). Pharmacol. Rev. 2010, 62, 588–631. [Google Scholar] [CrossRef]
- Borrelli, F.; Pagano, E.; Romano, B.; Panzera, S.; Maiello, F.; Coppola, D.; De Petrocellis, L.; Buono, L.; Orlando, P.; Izzo, A.A. Colon carcinogenesis is inhibited by the TRPM8 antagonist cannabigerol, a Cannabis-derived non-psychotropic cannabinoid. Carcinogenesis 2014, 35, 2787–2797. [Google Scholar] [CrossRef]
- Yuan, M.; Kiertscher, S.M.; Cheng, Q.; Zoumalan, R.; Tashkin, D.P.; Roth, M.D. Delta 9-Tetrahydrocannabinol regulates Th1/Th2 cytokine balance in activated human T cells. J. Neuroimmunol. 2002, 133, 124–131. [Google Scholar] [CrossRef]
- Blazquez, C.; Gonzalez-Feria, L.; Alvarez, L.; Haro, A.; Casanova, M.L.; Guzman, M. Cannabinoids inhibit the vascular endothelial growth factor pathway in gliomas. Cancer Res. 2004, 64, 5617–5623. [Google Scholar] [CrossRef]
- Sledzinski, P.; Zeyland, J.; Slomski, R.; Nowak, A. The current state and future perspectives of cannabinoids in cancer biology. Cancer Med. 2018, 7, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Boros, C.; Parsons, D.; Zoanetti, G.; Ketteridge, D.; Kennedy, D. Cannabis cookies: A cause of coma. J. Paediatr. Child Health 1996, 32, 194–195. [Google Scholar] [CrossRef]
- Macnab, A.; Anderson, E.; Susak, L. Ingestion of cannabis: A cause of coma in children. Pediatric Emerg. Care 1989, 5, 238–239. [Google Scholar] [CrossRef]
- Richards, J.R.; Smith, N.E.; Moulin, A.K. Unintentional cannabis ingestion in children: A systematic review. J. Pediatrics 2017, 190, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Malik, K.; Kommana, S.; Paul, J.; Krakauer, M. Synthetic cannabinoid induced ocular self-injury. Orbit 2020, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Blazquez, C.; Ruiz-Calvo, A.; Bajo-Graneras, R.; Baufreton, J.M.; Resel, E.; Varilh, M.; Pagano Zottola, A.C.; Mariani, Y.; Cannich, A.; Rodriguez-Navarro, J.A.; et al. Inhibition of striatonigral autophagy as a link between cannabinoid intoxication and impairment of motor coordination. eLife 2020, 9, e56811. [Google Scholar] [CrossRef] [PubMed]
- Gebremedhin, D.; Lange, A.R.; Campbell, W.B.; Hillard, C.J.; Harder, D.R. Cannabinoid CB1 receptor of cat cerebral arterial muscle functions to inhibit L-type Ca2+ channel current. Am. J. Physiol. Heart Circ. Physiol. 1999, 276, H2085–H2093. [Google Scholar] [CrossRef]
- Grigg, J.; Manning, V.; Arunogiri, S.; Lubman, D.I. Synthetic cannabinoid use disorder: An update for general psychiatrists. Australas. Psychiatry Bull. R. Aust. N. Z. Coll. Psychiatr. 2019, 27, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.; Degenhardt, L.; Lynskey, M. The Health and Psychological Effects of Cannabis Use, 2nd ed.; Monograph Series No. 44; Commonwealth of Australia: Canberra, Australia, 2001. [Google Scholar]
- van Amsterdam, J.; Brunt, T.; van den Brink, W. The adverse health effects of synthetic cannabinoids with emphasis on psychosis-like effects. J. Psychopharmacol. 2015, 29, 254–263. [Google Scholar] [CrossRef]
- Ware, M.A.; Adams, H.; Guy, G.W. The medicinal use of cannabis in the UK: Results of a nationwide survey. Int. J. Clin. Pract. 2005, 59, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.; Austriaco, N. A virtue analysis of recreational marijuana use. Linacre Q. 2016, 83, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Bostwick, J.M. Blurred boundaries: The therapeutics and politics of medical marijuana. Mayo Clin. Proc. 2012, 87, 172–186. [Google Scholar] [CrossRef]
- Turner, A.R.; Spurling, B.C.; Agrawal, S. Marijuana Toxicity; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Rosenkrantz, H.; Heyman, I.A.; Braude, M.C. Inhalation, parenteral and oral LD50 values of Δ9-tetrahydrocannabinol in Fischer rats. Toxicol. Appl. Pharmacol. 1974, 28, 18–27. [Google Scholar] [CrossRef]
- Ruiz, C.M.; Torrens, A.; Castillo, E.; Perrone, C.R.; Cevallos, J.; Inshishian, V.C.; Harder, E.V.; Justeson, D.N.; Huestis, M.A.; Swarup, V.; et al. Pharmacokinetic, behavioral, and brain activity effects of Delta(9)-tetrahydrocannabinol in adolescent male and female rats. Neuropsychopharmacology 2020. [Google Scholar] [CrossRef]
- Cosker, E.; Schwitzer, T.; Ramoz, N.; Ligier, F.; Lalanne, L.; Gorwood, P.; Schwan, R.; Laprevote, V. The effect of interactions between genetics and cannabis use on neurocognition. A review. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 82, 95–106. [Google Scholar] [CrossRef]
- Cooper, Z.D.; Haney, M. Investigation of sex-dependent effects of cannabis in daily cannabis smokers. Drug Alcohol Depend. 2014, 136, 85–91. [Google Scholar] [CrossRef]
- Weinberg, D.; Lande, A.; Hilton, N.; Kerns, D.L. Intoxication from accidental marijuana ingestion. Pediatrics 1983, 71, 848–850. [Google Scholar]
- Boadu, O.; Gombolay, G.Y.; Caviness, V.S.; El Saleeby, C.M. Intoxication From Accidental Marijuana Ingestion in Pediatric Patients: What May Lie Ahead. Pediatric Emerg. Care 2020, 36, e349–e354. [Google Scholar] [CrossRef]
- Chinello, M.; Scommegna, S.; Shardlow, A.; Mazzoli, F.; De Giovanni, N.; Fucci, N.; Borgiani, P.; Ciccacci, C.; Locasciulli, A.; Calvani, M. Cannabinoid poisoning by hemp seed oil in a child. Pediatric Emerg. Care 2017, 33, 344–345. [Google Scholar] [CrossRef]
- Carstairs, S.D.; Fujinaka, M.K.; Keeney, G.E.; Ly, B.T. Prolonged coma in a child due to hashish ingestion with quantitation of THC metabolites in urine. J. Emerg. Med. 2011, 41, e69–e71. [Google Scholar] [CrossRef]
- Ware, M.A.; Wang, T.; Shapiro, S.; Collet, J.-P.; Boulanger, A.; Esdaile, J.M.; Gordon, A.; Lynch, M.; Moulin, D.E.; O’Connell, C. Cannabis for the management of pain: Assessment of safety study (COMPASS). J. Pain 2015, 16, 1233–1242. [Google Scholar] [CrossRef]
- Wang, T.; Collet, J.-P.; Shapiro, S.; Ware, M.A. Adverse effects of medical cannabinoids: A systematic review. CMAJ 2008, 178, 1669–1678. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Etges, T.; Stott, C.; Wright, S.; Mohammed, A.; Robson, P. Sativex safety profile is improving over time. In Proceedings of the 21st Annual Symposium on the Cannabinoids, St. Charles, IL, USA, 5–10 July 2011; International Cannabinoid Research Society: Winston-Salem, NC, USA, 2011. [Google Scholar]
- Hartung, B.; Kauferstein, S.; Ritz-Timme, S.; Daldrup, T. Sudden unexpected death under acute influence of cannabis. Forensic Sci. Int. 2014, 237, e11–e13. [Google Scholar] [CrossRef]
- Bouchard, J.-F.; Lépicier, P.; Lamontagne, D. Contribution of endocannabinoids in the endothelial protection afforded by ischemic preconditioning in the isolated rat heart. Life Sci. 2003, 72, 1859–1870. [Google Scholar] [CrossRef]
- Bonz, A.; Laser, M.; Küllmer, S.; Kniesch, S.; Babin-Ebell, J.; Popp, V.; Ertl, G.; Wagner, J.A. Cannabinoids acting on CB1 receptors decrease contractile performance in human atrial muscle. J. Cardiovasc. Pharmacol. 2003, 41, 657–664. [Google Scholar] [CrossRef]
- Sidney, S. Cardiovascular consequences of marijuana use. J. Clin. Pharmacol. 2002, 42, 64S–70S. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.A.; Hu, K.; Bauersachs, J.; Karcher, J.; Wiesler, M.; Goparaju, S.K.; Kunos, G.; Ertl, G. Endogenous cannabinoids mediate hypotension after experimental myocardial infarction. J. Am. Coll. Cardiol. 2001, 38, 2048–2054. [Google Scholar] [CrossRef]
- Mukamal, K.J.; Maclure, M.; Muller, J.E.; Mittleman, M.A. An exploratory prospective study of marijuana use and mortality following acute myocardial infarction. Am. Heart J. 2008, 155, 465–470. [Google Scholar] [CrossRef]
- Bachs, L.; Mørland, H. Acute cardiovascular fatalities following cannabis use. Forensic Sci. Int. 2001, 124, 200–203. [Google Scholar] [CrossRef]
- Lee, J.; Sharma, N.; Kazi, F.; Youssef, I.; Myers, A.; Marmur, J.D.; Salifu, M.O.; McFarlane, S.I. Cannabis and Myocardial Infarction: Risk Factors and Pathogenetic Insights. SciFed J. Cardiol. 2017, 1, 1000004. [Google Scholar] [PubMed]
- Charles, R.; Holt, S.; Kirkham, N. Myocardial infarction and marijuana. Clin. Toxicol. 1979, 14, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Desai, R.; Patel, U.; Sharma, S.; Amin, P.; Bhuva, R.; Patel, M.S.; Sharma, N.; Shah, M.; Patel, S.; Savani, S.; et al. Recreational Marijuana Use and Acute Myocardial Infarction: Insights from Nationwide Inpatient Sample in the United States. Cureus 2017, 9, e1816. [Google Scholar] [CrossRef]
- Frost, L.; Mostofsky, E.; Rosenbloom, J.I.; Mukamal, K.J.; Mittleman, M.A. Marijuana use and long-term mortality among survivors of acute myocardial infarction. Am. Heart J. 2013, 165, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Nogi, M.; Fergusson, D.; Chiaco, J.M.C. Mid-ventricular variant takotsubo cardiomyopathy associated with cannabinoid hyperemesis syndrome: A case report. Hawai’i J. Med. Public Health 2014, 73, 115. [Google Scholar]
- Khalid, S.; Khalid, A.; Maroo, P. Risk factors and management of Takotsubo cardiomyopathy. Cureus 2018, 10, e2626. [Google Scholar] [CrossRef]
- Alliu, S.; Adejumo, A.; Adegbala, O.; Namana, V.; Chetana, P.; Yevgeniya, B.; Hecht, M.; Wolf, L.; Kamholz, S.; Hollander, G. Association between Cannabis use and TakoTsubo cardiomyopathy (TTC): Analysis from the NIS 2012-2014. Circ. Res. 2017, 121, A209. [Google Scholar]
- Tournebize, J.; Gibaja, V.; Puskarczyk, E.; Popovic, B.; Kahn, J.-P. Myocarditis associated with cannabis use in a 15-year-old boy: A rare case report. Int. J. Cardiol. 2016, 203, 243. [Google Scholar] [CrossRef]
- Leontiadis, E.; Morshuis, M.; Arusoglu, L.; Cobaugh, D.; Koerfer, R.; El-Banayosy, A. Thoratec left ventricular assist device removal after toxic myocarditis. Ann. Thorac. Surg. 2008, 86, 1982–1985. [Google Scholar] [CrossRef]
- Nappe, T.M.; Hoyte, C.O. Pediatric death due to myocarditis after exposure to cannabis. Clin. Pract. Cases Emerg. Med. 2017, 1, 166. [Google Scholar] [CrossRef]
- Rodríguez-Castro, C.E.; Alkhateeb, H.; Elfar, A.; Saifuddin, F.; Abbas, A.; Siddiqui, T. Recurrent myopericarditis as a complication of marijuana use. Am. J. Case Rep. 2014, 15, 60. [Google Scholar] [PubMed]
- Samaan, J.; Ferrer, G.F.; Akinyemi, B.; Junquera, P.; Oms, J.; Dumenigo, R. Synthetic cannabis overdose and withdrawal in a young adult: A case report, commentary on regulation, and review of the literature. Case Rep. Psychiatry 2016, 2016, 3640549. [Google Scholar] [CrossRef] [PubMed]
- Earleywine, M. Understanding Marijuana: A New Look at the Scientific Evidence; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Matsuda, L.A.; Bonner, T.I.; Lolait, S.J. Localization of cannabinoid receptor mRNA in rat brain. J. Comp. Neurol. 1993, 327, 535–550. [Google Scholar] [CrossRef]
- Misner, D.L.; Sullivan, J.M. Mechanism of cannabinoid effects on long-term potentiation and depression in hippocampal CA1 neurons. J. Neurosci. 1999, 19, 6795–6805. [Google Scholar] [CrossRef]
- Wise, L.E.; Thorpe, A.J.; Lichtman, A.H. Hippocampal CB 1 receptors mediate the memory impairing effects of Δ 9-tetrahydrocannabinol. Neuropsychopharmacology 2009, 34, 2072–2080. [Google Scholar] [CrossRef]
- Marsicano, G.; Wotjak, C.T.; Azad, S.C.; Bisogno, T.; Rammes, G.; Cascio, M.G.; Hermann, H.; Tang, J.; Hofmann, C.; Zieglgänsberger, W. The endogenous cannabinoid system controls extinction of aversive memories. Nature 2002, 418, 530–534. [Google Scholar] [CrossRef]
- Bowman, M.; Pihl, R.O. Cannabis: Psychological effects of chronic heavy use. Psychopharmacologia 1973, 29, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Herning, R.I.; Better, W.E.; Tate, K.; Cadet, J.L. Cerebrovascular perfusion in marijuana users during a month of monitored abstinence. Neurology 2005, 64, 488–493. [Google Scholar] [CrossRef]
- Nahas, G.G. Cannabis Physiopathology Epidemiology Detection; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar]
- Ashton, C.H. Adverse effects of cannabis and cannabinoids. Br. J. Anaesth. 1999, 83, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.M.; Stuart, G.L.; Meehan, J.C.; Rhatigan, D.; Hellmuth, J.C.; Keen, S.M. Drug abuse and aggression between intimate partners: A meta-analytic review. Clin. Psychol. Rev. 2008, 28, 247–274. [Google Scholar] [CrossRef] [PubMed]
- Eksborg, S.; Rajs, J. Causes and manners of death among users of heroin, methadone, amphetamine, and cannabis in relation to postmortem chemical tests for illegal drugs. Subst. Use Misuse 2008, 43, 1326–1339. [Google Scholar] [CrossRef] [PubMed]
- De Aquino, J.P.; Sherif, M.; Radhakrishnan, R.; Cahill, J.D.; Ranganathan, M.; D’Souza, D.C. The psychiatric consequences of cannabinoids. Clin. Ther. 2018, 40, 1448–1456. [Google Scholar] [CrossRef]
- Reece, A.S. Chronic toxicology of cannabis. Clin. Toxicol. 2009, 47, 517–524. [Google Scholar] [CrossRef]
- Wilkinson, S.T.; Radhakrishnan, R.; D’Souza, D.C. Impact of Cannabis Use on the Development of Psychotic Disorders. Curr. Addict. Rep. 2014, 1, 115–128. [Google Scholar] [CrossRef]
- Konings, M.; Henquet, C.; Maharajh, H.; Hutchinson, G.; Van Os, J. Early exposure to cannabis and risk for psychosis in young adolescents in Trinidad. Acta Psychiatr. Scand. 2008, 118, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Henquet, C.; Murray, R.; Linszen, D.; van Os, J. The environment and schizophrenia: The role of cannabis use. Schizophr. Bull. 2005, 31, 608–612. [Google Scholar] [CrossRef]
- Degenhardt, L.; Tennant, C.; Gilmour, S.; Schofield, D.; Nash, L.; Hall, W.; McKAY, D. The temporal dynamics of relationships between cannabis, psychosis and depression among young adults with psychotic disorders: Findings from a 10-month prospective study. Psychol. Med. 2007, 37, 927. [Google Scholar] [CrossRef]
- Coulston, C.M.; Perdices, M.; Tennant, C.C. The neuropsychological correlates of cannabis use in schizophrenia: Lifetime abuse/dependence, frequency of use, and recency of use. Schizophr. Res. 2007, 96, 169–184. [Google Scholar] [CrossRef]
- Ksir, C.; Hart, C.L. Cannabis and psychosis: A critical overview of the relationship. Curr. Psychiatry Rep. 2016, 18, 12. [Google Scholar] [CrossRef]
- Hamilton, I. Cannabis, psychosis and schizophrenia: Unravelling a complex interaction. Addiction 2017, 112, 1653–1657. [Google Scholar] [CrossRef] [PubMed]
- Fergusson, D.M.; Horwood, L.; Swain-Campbell, N. Cannabis dependence and psychotic symptoms in young people. Psychol. Med. 2003, 33, 15–21. [Google Scholar] [CrossRef]
- Moore, T.H.; Zammit, S.; Lingford-Hughes, A.; Barnes, T.R.; Jones, P.B.; Burke, M.; Lewis, G. Cannabis use and risk of psychotic or affective mental health outcomes: A systematic review. Lancet 2007, 370, 319–328. [Google Scholar] [CrossRef]
- Lagerberg, T.V.; Sundet, K.; Aminoff, S.R.; Berg, A.O.; Ringen, P.A.; Andreassen, O.A.; Melle, I. Excessive cannabis use is associated with earlier age at onset in bipolar disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2011, 261, 397–405. [Google Scholar] [CrossRef]
- Lev-Ran, S.; Le Foll, B.; McKenzie, K.; George, T.P.; Rehm, J. Bipolar disorder and co-occurring cannabis use disorders: Characteristics, co-morbidities and clinical correlates. Psychiatry Res. 2013, 209, 459–465. [Google Scholar] [CrossRef] [PubMed]
- van Rossum, I.; Boomsma, M.; Tenback, D.; Reed, C.; van Os, J. Does cannabis use affect treatment outcome in bipolar disorder? A longitudinal analysis. J. Nerv. Ment. Dis. 2009, 197, 35–40. [Google Scholar] [CrossRef]
- Bovasso, G.B. Cannabis abuse as a risk factor for depressive symptoms. Am. J. Psychiatry 2001, 158, 2033–2037. [Google Scholar] [CrossRef]
- Degenhardt, L.; Hall, W.; Lynskey, M. Exploring the association between cannabis use and depression. Addiction 2003, 98, 1493–1504. [Google Scholar] [CrossRef]
- Wu, T.-C.; Tashkin, D.P.; Djahed, B.; Rose, J.E. Pulmonary hazards of smoking marijuana as compared with tobacco. N. Engl. J. Med. 1988, 318, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.K.; Smith, R.P.; Morrison, D.; Laszlo, G.; White, R.J. Large lung bullae in marijuana smokers. Thorax 2000, 55, 340–342. [Google Scholar] [CrossRef]
- Huber, G.L.; First, M.W.; Grubner, O. Marijuana and tobacco smoke gas-phase cytotoxins. Pharmacol. Biochem. Behav. 1991, 40, 629–636. [Google Scholar] [CrossRef]
- Roth, M.D.; Marques-Magallanes, J.A.; Yuan, M.; Sun, W.; Tashkin, D.P.; Hankinson, O. Induction and regulation of the carcinogen-metabolizing enzyme CYP1A1 by marijuana smoke and Δ9-tetrahydrocannabinol. Am. J. Respir. Cell Mol. Biol. 2001, 24, 339–344. [Google Scholar] [CrossRef]
- MacPhee, D. Effects of Marijuana on Cell Nuclei: A Review of the Literature Relating to the Genotoxicity of Cannabis. The Health Effects of Cannabis; Centre for Addiction and Mental Health: Toronto, ON, Canada, 1999; pp. 435–458. [Google Scholar]
- Hall, W. The respiratory risks of cannabis smoking. Addiction 1998, 93, 1461. [Google Scholar]
- Thompson, C.; White, R. Lung bullae and marijuana. Thorax 2002, 57, 563. [Google Scholar] [CrossRef][Green Version]
- Fiorelli, A.; Accardo, M.; Vicidomini, G.; Messina, G.; Laperuta, P.; Santini, M. Does cannabis smoking predispose to lung bulla formation? Asian Cardiovasc. Thorac. Ann. 2014, 22, 65–71. [Google Scholar] [CrossRef]
- Reece, A. Cannabis as a cause of giant cystic lung disease. QJM Int. J. Med. 2008, 101, 503. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.I.; Ind, P.W. Effect of cannabis smoking on lung function and respiratory symptoms: A structured literature review. NPJ Prim. Care Respir. Med. 2016, 26, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.R.; Hall, W. Respiratory health effects of cannabis: Position statement of the Thoracic Society of Australia and New Zealand. Intern. Med. J. 2003, 33, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Mehra, R.; Moore, B.A.; Crothers, K.; Tetrault, J.; Fiellin, D.A. The association between marijuana smoking and lung cancer: A systematic review. Arch. Intern. Med. 2006, 166, 1359–1367. [Google Scholar] [CrossRef]
- Berthiller, J.; Straif, K.; Boniol, M.; Voirin, N.; Benhaïm-Luzon, V.; Ayoub, W.B.; Dari, I.; Laouamri, S.; Hamdi-Cherif, M.; Bartal, M. Cannabis smoking and risk of lung cancer in men: A pooled analysis of three studies in Maghreb. J. Thorac. Oncol. 2008, 3, 1398–1403. [Google Scholar] [CrossRef]
- Callaghan, R.C.; Allebeck, P.; Sidorchuk, A. Marijuana use and risk of lung cancer: A 40-year cohort study. Cancer Causes Control 2013, 24, 1811–1820. [Google Scholar] [CrossRef] [PubMed]
- Melamede, R. Cannabis and tobacco smoke are not equally carcinogenic. Harm Reduct. J. 2005, 2, 1–4. [Google Scholar]
- Hart, S.; Fischer, O.M.; Ullrich, A. Cannabinoids induce cancer cell proliferation via tumor necrosis factor α-converting enzyme (TACE/ADAM17)-mediated transactivation of the epidermal growth factor receptor. Cancer Res. 2004, 64, 1943–1950. [Google Scholar] [CrossRef]
- Tetrault, J.M.; Crothers, K.; Moore, B.A.; Mehra, R.; Concato, J.; Fiellin, D.A. Effects of marijuana smoking on pulmonary function and respiratory complications: A systematic review. Arch. Intern. Med. 2007, 167, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Ghasemiesfe, M.; Ravi, D.; Vali, M.; Korenstein, D.; Arjomandi, M.; Frank, J.; Austin, P.C.; Keyhani, S. Marijuana use, respiratory symptoms, and pulmonary function: A systematic review and meta-analysis. Ann. Intern. Med. 2018, 169, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Winhusen, T.; Theobald, J.; Kaelber, D.C.; Lewis, D. Regular cannabis use, with and without tobacco co-use, is associated with respiratory disease. Drug Alcohol Depend. 2019, 204, 107557. [Google Scholar] [CrossRef] [PubMed]
- Maykut, M.O. Health consequences of acute and chronic marihuana use. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 1985, 9, 209–238. [Google Scholar] [CrossRef]
- Baldwin, G.C.; Tashkin, D.P.; Buckley, D.M.; Park, A.N.; Dubinett, S.M.; Roth, M.D. Marijuana and cocaine impair alveolar macrophage function and cytokine production. Am. J. Respir. Crit. Care Med. 1997, 156, 1606–1613. [Google Scholar] [CrossRef]
- Russo, E.B. Cannabis and Cannabinoids: Pharmacology, Toxicology, and Therapeutic Potential; Routledge: Abingdon, UK, 2013. [Google Scholar]
- Dalterio, S.; Bartke, A.; Mayfield, D. Effects of Δ9-tetrahydrocannabinol on testosterone production in vitro: Influence of Ca++, Mg++ or glucose. Life Sci. 1985, 37, 1425–1433. [Google Scholar] [CrossRef]
- Rettori, V.; Wenger, T.; Snyder, G.; Dalterio, S.; McCann, S.M. Hypothalamic action of delta-9-tetrahydrocannabinol to inhibit the release of prolactin and growth hormone in the rat. Neuroendocrinology 1988, 47, 498–503. [Google Scholar] [CrossRef]
- Payne, K.S.; Mazur, D.J.; Hotaling, J.M.; Pastuszak, A.W. Cannabis and male fertility: A systematic review. J. Urol. 2019, 202, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Agirregoitia, E.; Carracedo, A.; Subirán, N.; Valdivia, A.; Agirregoitia, N.; Peralta, L.; Velasco, G.; Irazusta, J. The CB2 cannabinoid receptor regulates human sperm cell motility. Fertil. Steril. 2010, 93, 1378–1387. [Google Scholar] [CrossRef]
- Hembree, W.C.; Nahas, G.G.; Zeidenberg, P.; Huang, H.F. Changes in human spermatozoa associated with high-dose marihuana smoking. In Marihuana and medicine; Springer: Berlin/Heidelberg, Germany, 1999; pp. 367–378. [Google Scholar]
- du Plessis, S.S.; Agarwal, A.; Syriac, A. Marijuana, phytocannabinoids, the endocannabinoid system, and male fertility. J. Assist. Reprod. Genet. 2015, 32, 1575–1588. [Google Scholar] [CrossRef]
- Harclerode, J. Endocrine effects of marijuana in the male: Preclinical studies. NIDA Res. Monogr. 1984, 44, 46–64. [Google Scholar] [PubMed]
- Hollister, L.E. Health aspects of cannabis. Pharmacol. Rev. 1986, 38, 1–20. [Google Scholar] [CrossRef]
- Ghosh, S. Cannabis Effect on Female Reproductive Health. In Natural Products-From Bioactive Molecules to Human Health; IntechOpen Limited: London, UK; Rijeka, Croatia, 2020. [Google Scholar] [CrossRef]
- Mendelson, J.H.; Mello, N.; Ellingboe, J. Acute effects of marihuana smoking on prolactin levels in human females. J. Pharmacol. Exp. Ther. 1985, 232, 220–222. [Google Scholar]
- Block, R.I.; Farinpour, R.; Schlechte, J.A. Effects of chronic marijuana use on testosterone, luteinizing hormone, follicle stimulating hormone, prolactin and cortisol in men and women. Drug Alcohol Depend. 1991, 28, 121–128. [Google Scholar] [CrossRef]
- Corsi, D.J.; Murphy, M.; Cook, J. The Effects of Cannabis on Female Reproductive Health Across the Life Course. Cannabis Cannabinoid Res. 2020. [Google Scholar] [CrossRef]
- Gold, M. Marijuana. In Drugs of Abuse: A Comprehensive Series for Clinicians; Plenum Press: London, UK, 1989; Volume 1. [Google Scholar]
- Sims, E.D.; Anvari, S.; Lee, Y.; Samaan, Z.; Banfield, L.; Thabane, L.; Samaan, M.C. The effect of cannabis exposure on pubertal outcomes: A systematic review. Adolesc. Health Med. Ther. 2018, 9, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Copeland, K.C.; Underwood, L.E.; Van Wyk, J.J. Marihuana smoking and pubertal arrest. J. Pediatr. 1980, 96, 1079–1080. [Google Scholar] [CrossRef]
- Volkow, N.D.; Han, B.; Compton, W.M.; McCance-Katz, E.F. Self-reported medical and nonmedical cannabis use among pregnant women in the United States. JAMA 2019, 322, 167–169. [Google Scholar] [CrossRef]
- Roncero, C.; Valriberas-Herrero, I.; Mezzatesta-Gava, M.; Villegas, J.L.; Aguilar, L.; Grau-López, L. Cannabis use during pregnancy and its relationship with fetal developmental outcomes and psychiatric disorders. A systematic review. Reprod. Health 2020, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lozano, J.; García-Algar, O.; Marchei, E.; Vall, O.; Monleon, T.; Giovannandrea, R.D.; Pichini, S. Prevalence of gestational exposure to cannabis in a Mediterranean city by meconium analysis. Acta Paediatr. 2007, 96, 1734–1737. [Google Scholar] [CrossRef] [PubMed]
- El Marroun, H.; Tiemeier, H.; Jaddoe, V.W.; Hofman, A.; Mackenbach, J.P.; Steegers, E.A.; Verhulst, F.C.; van den Brink, W.; Huizink, A.C. Demographic, emotional and social determinants of cannabis use in early pregnancy: The Generation R study. Drug Alcohol Depend. 2008, 98, 218–226. [Google Scholar] [CrossRef]
- Jaques, S.C.; Kingsbury, A.; Henshcke, P.; Chomchai, C.; Clews, S.; Falconer, J.; Abdel-Latif, M.E.; Feller, J.M.; Oei, J.L. Cannabis, the pregnant woman and her child: Weeding out the myths. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2014, 34, 417–424. [Google Scholar] [CrossRef]
- Bailey, J.; Cunny, H.; Paule, M.; Slikker Jr, W. Fetal disposition of Δ9-tetrahydrocannabinol (THC) during late pregnancy in the rhesus monkey. Toxicol. Appl. Pharmacol. 1987, 90, 315–321. [Google Scholar] [CrossRef]
- Gómez, M.; Hernández, M.; Johansson, B.; de Miguel, R.; Ramos, J.A.; Fernández-Ruiz, J. Prenatal cannabinoid exposure and gene expression for neural adhesion molecule L1 in the fetal rat brain. Dev. Brain Res. 2003, 147, 201–207. [Google Scholar] [CrossRef]
- Smith, A.M.; Mioduszewski, O.; Hatchard, T.; Byron-Alhassan, A.; Fall, C.; Fried, P.A. Prenatal marijuana exposure impacts executive functioning into young adulthood: An fMRI study. Neurotoxicol. Teratol. 2016, 58, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Fried, P.A.; Hogan, M.J.; Cameron, I. Effects of prenatal marijuana on visuospatial working memory: An fMRI study in young adults. Neurotoxicol. Teratol. 2006, 28, 286–295. [Google Scholar] [CrossRef]
- Fried, P.A.; Watkinson, B.; Gray, R. Differential effects on cognitive functioning in 13-to 16-year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicol. Teratol. 2003, 25, 427–436. [Google Scholar] [CrossRef]
- Smith, A.M.; Longo, C.A.; Fried, P.A.; Hogan, M.J.; Cameron, I. Effects of marijuana on visuospatial working memory: An fMRI study in young adults. Psychopharmacology 2010, 210, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, L.; Richardson, G.A.; Willford, J.; Day, N.L. Prenatal marijuana exposure and intelligence test performance at age 6. J. Am. Acad. Child Adolesc. Psychiatry 2008, 47, 254–263. [Google Scholar] [CrossRef]
- Sharapova, S.R.; Phillips, E.; Sirocco, K.; Kaminski, J.W.; Leeb, R.T.; Rolle, I. Effects of prenatal marijuana exposure on neuropsychological outcomes in children aged 1–11 years: A systematic review. Paediatr. Perinat. Epidemiol. 2018, 32, 512–532. [Google Scholar] [CrossRef] [PubMed]
- de Moraes Barros, M.C.; Guinsburg, R.; de Araújo Peres, C.; Mitsuhiro, S.; Chalem, E.; Laranjeira, R.R. Exposure to marijuana during pregnancy alters neurobehavior in the early neonatal period. J. Pediatrics 2006, 149, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Fried, P.A.; Makin, J. Neonatal behavioural correlates of prenatal exposure to marihuana, cigarettes and alcohol in a low risk population. Neurotoxicol. Teratol. 1987, 9, 1–7. [Google Scholar] [CrossRef]
- Lester, B.M.; Dreher, M. Effects of marijuana use during pregnancy on newborn cry. Child Dev. 1989, 60, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Forrester, M.B.; Merz, R.D. Risk of selected birth defects with prenatal illicit drug use, Hawaii, 1986–2002. J. Toxicol. Environ. Health Part A 2006, 70, 7–18. [Google Scholar] [CrossRef]
- Robison, L.L.; Buckley, J.D.; Daigle, A.E.; Wells, R.; Benjamin, D.; Arthur, D.C.; Hammond, G.D. Maternal drug use and risk of childhood nonlymphoblastic leukemia among offspring. An epidemiologic investigation implicating marijuana (a report from the Childrens Cancer Study Group). Cancer 1989, 63, 1904–1911. [Google Scholar] [CrossRef]
- Huizink, A. Prenatal cannabis exposure and infant outcomes: Overview of studies. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 52, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; De Fonseca, F.R.; Hernandez, M.; Ramos, J.; Fernandez-Ruiz, J. Motor behavior and nigrostriatal dopaminergic activity in adult rats perinatally exposed to cannabinoids. Pharmacol. Biochem. Behav. 1994, 47, 47–58. [Google Scholar] [CrossRef]
- Antonelli, T.; Tomasini, M.C.; Tattoli, M.; Cassano, T.; Tanganelli, S.; Finetti, S.; Mazzoni, E.; Trabace, L.; Steardo, L.; Cuomo, V. Prenatal exposure to the CB1 receptor agonist WIN 55,212-2 causes learning disruption associated with impaired cortical NMDA receptor function and emotional reactivity changes in rat offspring. Cereb. Cortex 2005, 15, 2013–2020. [Google Scholar] [CrossRef]
- Campolongo, P.; Trezza, V.; Ratano, P.; Palmery, M.; Cuomo, V. Developmental consequences of perinatal cannabis exposure: Behavioral and neuroendocrine effects in adult rodents. Psychopharmacology 2011, 214, 5–15. [Google Scholar] [CrossRef] [PubMed]
- DiNieri, J.A.; Wang, X.; Szutorisz, H.; Spano, S.M.; Kaur, J.; Casaccia, P.; Dow-Edwards, D.; Hurd, Y.L. Maternal cannabis use alters ventral striatal dopamine D2 gene regulation in the offspring. Biol. Psychiatry 2011, 70, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Scheyer, A.F.; Borsoi, M.; Wager-Miller, J.; Pelissier-Alicot, A.-L.; Murphy, M.N.; Mackie, K.; Manzoni, O.J. Cannabinoid exposure via lactation in rats disrupts perinatal programming of the gamma-aminobutyric acid trajectory and select early-life behaviors. Biol. Psychiatry 2020, 87, 666–677. [Google Scholar] [CrossRef]
- Bonnin, A.; De Miguel, R.; Hernandez, M.; Ramos, J.; Fernandez-Ruiz, J. The prenatal exposure to Δ9-tetrahydrocannabinol affects the gene expression and the activity of tyrosine hydroxylase during early brain development. Life Sci. 1995, 56, 2177–2184. [Google Scholar] [CrossRef]
- Bonnin, A.; de Miguel, R.; Castro, J.G.; Ramos, J.A.; Fernandez-Ruiz, J.J. Effects of perinatal exposure to Δ 9-tetrahydrocannabinol on the fetal and early postnatal development of tyrosine hydroxylase-containing neurons in rat brain. J. Mol. Neurosci. 1996, 7, 291–308. [Google Scholar] [CrossRef]
- Morales, P.; Hurst, D.P.; Reggio, P.H. Molecular targets of the phytocannabinoids: A complex picture. In Phytocannabinoids; Springer: Berlin/Heidelberg, Germany, 2017; pp. 103–131. [Google Scholar]
- Weimar, H.V.; Wright, H.R.; Warrick, C.R.; Brown, A.M.; Lugo, J.M.; Freels, T.G.; McLaughlin, R.J. Maternal cannabis vapor exposure causes long-term alterations in emotional reactivity, social behavior, and behavioral flexibility in offspring. bioRxiv 2020. [CrossRef]
- Panlilio, L.V.; Goldberg, S.R.; Justinova, Z. Cannabinoid abuse and addiction: Clinical and preclinical findings. Clin. Pharmacol. Ther. 2015, 97, 616–627. [Google Scholar] [CrossRef]
- Patel, J.; Marwaha, R. Cannabis Use Disorder; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Budney, A.J.; Roffman, R.; Stephens, R.S.; Walker, D. Marijuana dependence and its treatment. Addict. Sci. Clin. Pract. 2007, 4, 4–16. [Google Scholar] [CrossRef]
- Ramesh, D.; Schlosburg, J.E.; Wiebelhaus, J.M.; Lichtman, A.H. Marijuana dependence: Not just smoke and mirrors. ILAR J. 2011, 52, 295–308. [Google Scholar] [CrossRef]
- Bonnet, U.; Preuss, U.W. The cannabis withdrawal syndrome: Current insights. Subst. Abus. Rehabil. 2017, 8, 9–37. [Google Scholar] [CrossRef] [PubMed]
- Freeman, T.P.; Hindocha, C.; Baio, G.; Shaban, N.D.C.; Thomas, E.M.; Astbury, D.; Freeman, A.M.; Lees, R.; Craft, S.; Morrison, P.D.; et al. Cannabidiol for the treatment of cannabis use disorder: A phase 2a, double-blind, placebo-controlled, randomised, adaptive Bayesian trial. Lancet Psychiatry 2020, 7, 865–874. [Google Scholar] [CrossRef]
- Gilbert, D.G.; Rabinovich, N.E.; McDaniel, J.T. Nicotine patch for cannabis withdrawal symptom relief: A randomized controlled trial. Psychopharmacology 2020, 237, 1507–1519. [Google Scholar] [CrossRef]
- Sherman, B.J.; Caruso, M.A.; McRae-Clark, A.L. Exogenous progesterone for cannabis withdrawal in women: Feasibility trial of a novel multimodal methodology. Pharmacol. Biochem. Behav. 2019, 179, 22–26. [Google Scholar] [CrossRef]
- Allsop, D.J.; Copeland, J.; Lintzeris, N.; Dunlop, A.J.; Montebello, M.; Sadler, C.; Rivas, G.R.; Holland, R.M.; Muhleisen, P.; Norberg, M.M.; et al. Nabiximols as an agonist replacement therapy during cannabis withdrawal: A randomized clinical trial. JAMA Psychiatry 2014, 71, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.; Hart, C.L.; Vosburg, S.K.; Comer, S.D.; Reed, S.C.; Foltin, R.W. Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacology 2008, 197, 157–168. [Google Scholar] [CrossRef]
- Walther, L.; Gantner, A.; Heinz, A.; Majic, T. Evidence-based Treatment Options in Cannabis Dependency. Dtsch. Arztebl. Int. 2016, 113, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, T.; Bodkin, J.; Ho, J.M. Drug interactions with cannabinoids. CMAJ 2020, 192, E206. [Google Scholar] [CrossRef]
- Brown, J.D.; Winterstein, A.G. Potential Adverse Drug Events and Drug-Drug Interactions with Medical and Consumer Cannabidiol (CBD) Use. J. Clin. Med. 2019, 8, 989. [Google Scholar] [CrossRef]
- Stott, C.; White, L.; Wright, S.; Wilbraham, D.; Guy, G. A Phase I, open-label, randomized, crossover study in three parallel groups to evaluate the effect of Rifampicin, Ketoconazole, and Omeprazole on the pharmacokinetics of THC/CBD oromucosal spray in healthy volunteers. SpringerPlus 2013, 2, 236. [Google Scholar] [CrossRef] [PubMed]
- Cox, E.J.; Maharao, N.; Patilea-Vrana, G.; Unadkat, J.D.; Rettie, A.E.; McCune, J.S.; Paine, M.F. A marijuana-drug interaction primer: Precipitants, pharmacology, and pharmacokinetics. Pharmacol. Ther. 2019, 201, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Geffrey, A.L.; Pollack, S.F.; Bruno, P.L.; Thiele, E.A. Drug-drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia 2015, 56, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Zvonarev, V.; Fatuki, T.A.; Tregubenko, P. The Public Health Concerns of Marijuana Legalization: An Overview of Current Trends. Cureus 2019, 11, e5806. [Google Scholar] [CrossRef]
- Turner, A.R.; Agrawal, S. Marijuana. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Mead, A. Legal and Regulatory Issues Governing Cannabis and Cannabis-Derived Products in the United States. Front. Plant Sci. 2019, 10, 697. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Additions. Medical Use of Cannabis and Cannabinoids: Questions and Answers for Policymaking; EMCDDA: Lisbon, Portugal, 2018; p. 44. [Google Scholar]
- Bahji, A.; Stephenson, C. International Perspectives on the Implications of Cannabis Legalization: A Systematic Review & Thematic Analysis. Int. J. Environ. Res. Public Health 2019, 16, 3095. [Google Scholar] [CrossRef]
- Hall, W.; Lynskey, M. Assessing the public health impacts of legalizing recreational cannabis use: The US experience. World Psychiatry Offi. J. World Psychiatr. Assoc. 2020, 19, 179–186. [Google Scholar] [CrossRef]
- Leyton, M. Cannabis legalization: Did we make a mistake? Update 2019. J. Psychiatry Neurosci. JPN 2019, 44, 291–293. [Google Scholar] [CrossRef]
- Di Forti, M. To legalize or not to legalize cannabis, that is the question! World Psychiatry Offi. J. World Psychiatr. Assoc. 2020, 19, 188–189. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breijyeh, Z.; Jubeh, B.; Bufo, S.A.; Karaman, R.; Scrano, L. Cannabis: A Toxin-Producing Plant with Potential Therapeutic Uses. Toxins 2021, 13, 117. https://doi.org/10.3390/toxins13020117
Breijyeh Z, Jubeh B, Bufo SA, Karaman R, Scrano L. Cannabis: A Toxin-Producing Plant with Potential Therapeutic Uses. Toxins. 2021; 13(2):117. https://doi.org/10.3390/toxins13020117
Chicago/Turabian StyleBreijyeh, Zeinab, Buthaina Jubeh, Sabino A. Bufo, Rafik Karaman, and Laura Scrano. 2021. "Cannabis: A Toxin-Producing Plant with Potential Therapeutic Uses" Toxins 13, no. 2: 117. https://doi.org/10.3390/toxins13020117
APA StyleBreijyeh, Z., Jubeh, B., Bufo, S. A., Karaman, R., & Scrano, L. (2021). Cannabis: A Toxin-Producing Plant with Potential Therapeutic Uses. Toxins, 13(2), 117. https://doi.org/10.3390/toxins13020117









