Enterotoxin Gene Distribution and Genotypes of Bacillus cereus sensu lato Isolated from Cassava Starch
Abstract
:1. Introduction
2. Results
2.1. Detection of B. cereus s.l. on Cassava Starch
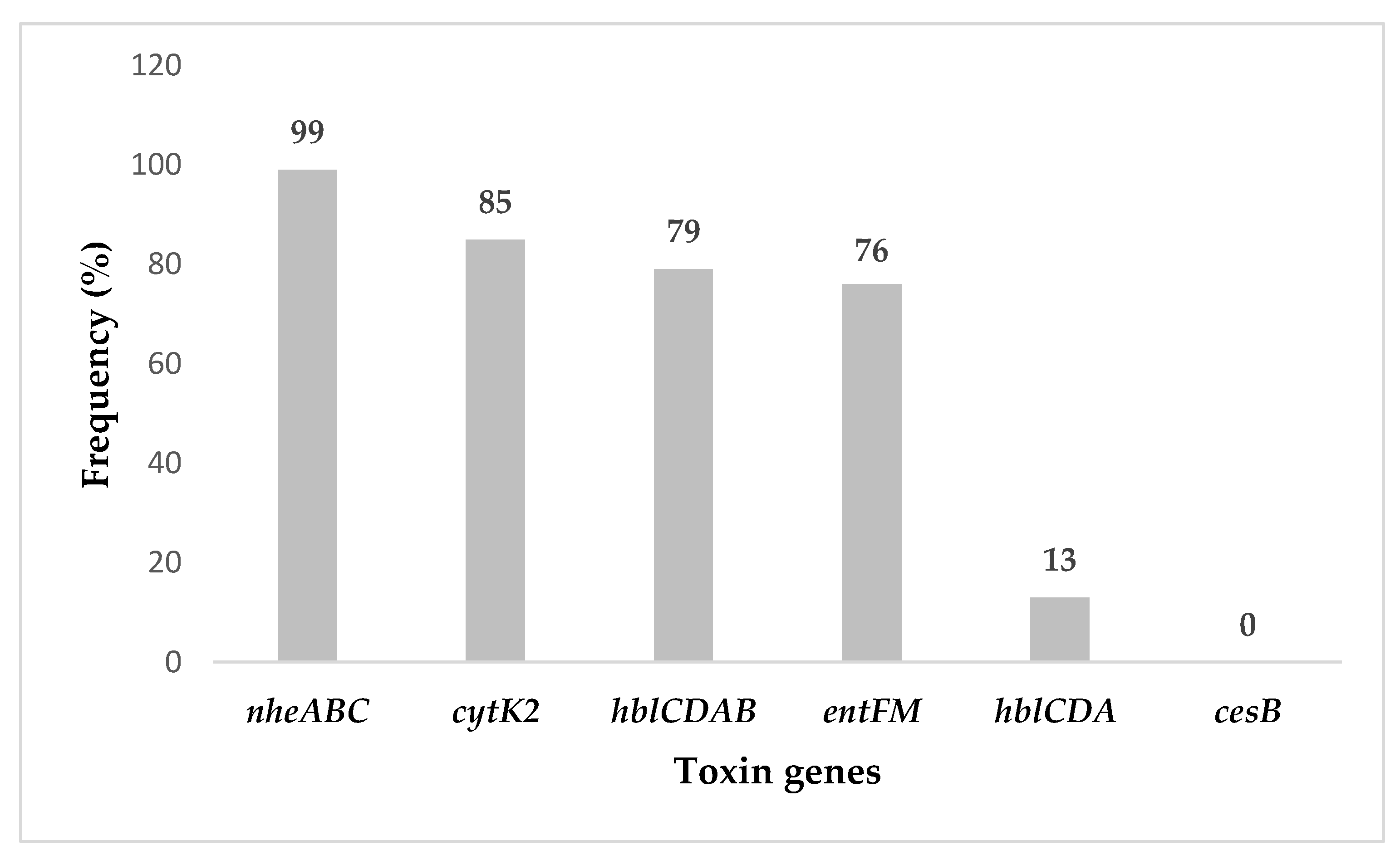
2.2. Bacillus cereus s.l. Toxigenic Profiles
2.3. Genotyping of B. cereus s.l.
3. Discussion
4. Materials and Methods
4.1. Cassava Starch Samples and B. cereus s.l. Isolation
4.2. B. cereus s.l. Toxin Genes Detection
4.3. Bacillus cereus s.l. (GTG)5-PCR Fingerprinting
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ceuppens, S.; Boon, N.; Uyttendaele, M. Diversity of Bacillus cereus group strains is reflected in their broad range of pathogenicity and diverse ecological lifestyles. FEMS Microbiol. Ecol. 2013, 84, 433–450. [Google Scholar] [CrossRef] [Green Version]
- Ehling-Schulz, M.; Koehler, T.M. The Bacillus cereus Group: Bacillus species with Pathogenic Potential. Microbiol. Spec. 2019, 7. [Google Scholar] [CrossRef]
- Miller, R.A.; Beno, S.M.; Kent, D.J.; Carroll, L.M.; Martin, N.H.; Boor, K.J.; Kovac, J. Bacillus wiedmannii sp. nov., a psychrotolerant and cytotoxic Bacillus cereus group species isolated from dairy foods and dairy environments. Int. J. Syst. Evol. Microbiol. 2016, 66, 4744–4753. [Google Scholar] [CrossRef]
- Liu, Y.; Du, J.; Lai, Q.; Zeng, R.; Ye, D.; Xu, J.; Shao, Z. Proposal of nine novel species of the Bacillus cereus group. Int. J. Syst. Evol. Microbiol. 2017, 67, 2499–2508. [Google Scholar] [CrossRef] [PubMed]
- Logan, N.A. Bacillus and relatives in foodborne illness. J. Appl. Microbiol. 2012, 112, 417–429. [Google Scholar] [CrossRef] [PubMed]
- De Jonghe, V.; Coorevits, A.; Vandroemme, J.; Heyrman, J.; Herman, L.; De Vos, P.; Heyndrickx, M. Intraspecific genotypic diversity of Bacillus species from raw milk. Int. Dairy J. 2008, 18, 496–505. [Google Scholar] [CrossRef]
- Stenfors Arnesen, L.P.; Fagerlund, A.; Granum, P.E. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 2008, 32, 579–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajkovic, A.; Uyttendaele, M.; Vermeulen, A.; Andjelkovic, M.; Fitz-James, I.; In’t Veld, P.; Denon, Q.; Vérhe, R.; Debevere, J. Heat resistance of Bacillus cereus emetic toxin, cereulide. Lett. Appl. Microbiol. 2008, 46, 536–541. [Google Scholar] [CrossRef]
- Bhunia, A.K. Bacillus cereus and Bacillus anthracis. In Foodborne Microbial Pathogens; Springer: New York, NY, USA, 2007; pp. 135–148. [Google Scholar]
- Fagerlund, A.; Lindbäck, T.; Storset, A.K.; Granum, P.E.; Hardy, S.P. Bacillus cereus Nhe is a pore-forming toxin with structural and functional properties similar to the ClyA (HlyE, SheA) family of haemolysins, able to induce osmotic lysis in epithelia. Microbiology 2008, 154, 693–704. [Google Scholar] [CrossRef] [Green Version]
- Samapundo, S.; Heyndrickx, M.; Xhaferi, R.; Devlieghere, F. Incidence, diversity and toxin gene characteristics of Bacillus cereus group strains isolated from food products marketed in Belgium. Int. J. Food Microbiol. 2011, 150, 34–41. [Google Scholar] [CrossRef]
- Sánchez-Chica, J.; Correa, M.M.; Aceves-Diez, A.E.; Rasschaert, G.; Heyndrickx, M.; Castañeda-Sandoval, L.M. Genomic and Toxigenic Heterogeneity of Bacillus cereus sensu lato Isolated from Ready-to-Eat Foods and Powdered Milk in Day Care Centers in Colombia. Foodborne Pathog. Dis. 2020, 17, 340–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Chica, J.; Correa, M.M.; Aceves-Diez, A.E.; Castañeda-Sandoval, L.M. Genetic and toxigenic diversity of Bacillus cereus group isolated from powdered foods. J. Food Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Gao, T.; Ding, Y.; Wu, Q.; Wang, J.; Zhang, J.; Yu, S.; Yu, P.; Liu, C.; Kong, L.; Feng, Z.; et al. Prevalence, Virulence Genes, Antimicrobial Susceptibility, and Genetic Diversity of Bacillus cereus Isolated From Pasteurized Milk in China. Front. Microbiol. 2018, 9, 533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, N.; Sun, J.M.; Kwon, K.Y.; Kim, H.J.; Koo, M.; Chun, H.S. Genetic diversity, antimicrobial resistance, and toxigenic profiles of Bacillus cereus strains isolated from sunsik. J. Food Prot. 2012, 75, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Chaves, J.Q.; Pires, E.S.; Vivoni, A.M. Genetic diversity, antimicrobial resistance and toxigenic profiles of Bacillus cereus isolated from food in Brazil over three decades. Int. J. Food Microbiol. 2011, 147, 12–16. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, K.P.; Jang, S.S.; Shin, E.M.; Kim, M.J.; Oh, S.; Ryu, S. Prevalence and Toxigenic profiles of Bacillus cereus isolated from dried red peppers, rice, and sunsik in Korea. J. Food Prot. 2009, 72, 578–582. [Google Scholar] [CrossRef]
- Ngamwongsatit, P.; Buasri, W.; Pianariyanon, P.; Pulsrikarn, C.; Ohba, M.; Assavanig, A.; Panbangred, W. Broad distribution of enterotoxin genes (hblCDA, nheABC, cytK, and entFM) among Bacillus thuringiensis and Bacillus cereus as shown by novel primers. Int. J. Food Microbiol. 2008, 121, 352–356. [Google Scholar] [CrossRef]
- Sánchez-Chica, J.; Correa, M.M.; Aceves-Diez, A.E.; Castañeda-Sandoval, L.M. Direct detection of toxigenic Bacillus cereus in dietary complement for children and cassava starch. Rev. Colomb. Quim. 2014, 43, 5–9. [Google Scholar] [CrossRef]
- Augustyn, A. Cassava. Available online: https://www.britannica.com/plant/cassava (accessed on 23 November 2020).
- Arenas, F. 127 Usos Tiene la Yuca, Según Estudio de Corpoica en Espinal (Tolima)-Archivo Digital de Noticias de Colombia y el Mundo Desde 1.990-Eltiempo.com. Available online: https://www.eltiempo.com/archivo/documento/CMS-3254363 (accessed on 10 August 2020).
- Aristizábal, J.; Sánchez, T. Guía Técnica Para Producción y Análisis de Almidón de Yuca; Organización de las Naciones Unidas para la agricultura y la alimentación: Roma, Italia, 2007. [Google Scholar]
- Jang, J.H.; Lee, N.A.; Woo, G.J.; Park, J. Prevalence of Bacillus cereus group in rice and distribution of enterotoxin genes. Food Sci. Biotechnol. 2006, 15, 232–237. [Google Scholar]
- Rahimi, E.; Abdos, F.; Momtaz, H.; Torki Baghbadorani, Z.; Jalali, M. Bacillus cereus in infant foods: Prevalence study and distribution of enterotoxigenic virulence factors in Isfahan Province, Iran. Sci. World J. 2013, 2013, 292571. [Google Scholar] [CrossRef] [Green Version]
- King, N.J.; Whyte, R.; Hudson, J.A. Presence and significance of Bacillus cereus in dehydrated potato products. J. Food Prot. 2007, 70, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Altayar, M.; Sutherland, A.D. Bacillus cereus is common in the environment but emetic toxin producing isolates are rare. J. Appl. Microbiol. 2006, 100, 7–14. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization ISO 7932:2004. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Presumptive Bacillus Cereus—Colony-Count Technique at 30 Degrees C; ISO: Geneva, Switzerland, 2004; p. 13. [Google Scholar]
- Kim, B.; Bang, J.; Kim, H.; Kim, Y.; Kim, B.S.; Beuchat, L.R.; Ryu, J.H. Bacillus cereus and Bacillus thuringiensis spores in Korean rice: Prevalence and toxin production as affected by production area and degree of milling. Food Microbiol. 2014, 42, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Böhm, M.E.; Huptas, C.; Krey, V.M.; Scherer, S. Massive horizontal gene transfer, strictly vertical inheritance and ancient duplications differentially shape the evolution of Bacillus cereus enterotoxin operons hbl, cytK and nhe. BMC Evol. Biol. 2015, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guinebretière, M.H.; Broussolle, V.; Nguyen-The, C. Enterotoxigenic profiles of food-poisoning and food-borne Bacillus cereus strains. J. Clin. Microbiol. 2002, 40, 3053–3056. [Google Scholar] [CrossRef] [Green Version]
- Wehrle, E.; Moravek, M.; Dietrich, R.; Bürk, C.; Didier, A.; Märtlbauer, E. Comparison of multiplex PCR, enzyme immunoassay and cell culture methods for the detection of enterotoxinogenic Bacillus cereus. J. Microbiol. Methods 2009, 78, 265–270. [Google Scholar] [CrossRef]
- Hansen, B.M.; Hendriksen, N.B. Detection of enterotoxic Bacillus cereus and Bacillus thuringiensis: Strains by PCR analysis. Appl. Environ. Microbiol. 2001, 67, 185–189. [Google Scholar] [CrossRef] [Green Version]
- Carter, L.; Chase, H.R.; Gieseker, C.M.; Hasbrouck, N.R.; Stine, C.B.; Khan, A.; Ewing-Peeples, L.J.; Tall, B.D.; Gopinath, G.R. Analysis of enterotoxigenic Bacillus cereus strains from dried foods using whole genome sequencing, multi-locus sequence analysis and toxin gene prevalence and distribution using endpoint PCR analysis. Int. J. Food Microbiol. 2018, 284, 31–39. [Google Scholar] [CrossRef]
- Osman, K.M.; Kappell, A.D.; Orabi, A.; Al-Maary, K.S.; Mubarak, A.S.; Dawoud, T.M.; Hemeg, H.A.; Moussa, I.M.I.; Hessain, A.M.; Yousef, H.M.Y.; et al. Poultry and beef meat as potential seedbeds for antimicrobial resistant enterotoxigenic Bacillus species: A materializing epidemiological and potential severe health hazard. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Økstad, O.; Kolstø, A. Genomics of Bacillus species. In Genomics of Foodborne Bacterial Pathogens; Wiedmann, M., Zhang, W., Eds.; Springer: New York, NY, USA, 2011; pp. 29–53. [Google Scholar]
- Carroll, L.M.; Wiedmann, M.; Mukherjee, M.; Nicholas, D.C.; Mingle, L.A.; Dumas, N.B.; Cole, J.A.; Kovac, J. Characterization of Emetic and Diarrheal Bacillus cereus Strains From a 2016 Foodborne Outbreak Using Whole-Genome Sequencing: Addressing the Microbiological, Epidemiological, and Bioinformatic Challenges. Front. Microbiol. 2019, 10, 144. [Google Scholar] [CrossRef] [Green Version]
- Ehling-Schulz, M.; Svensson, B.; Guinebretiere, M.H.; Lindbäck, T.; Andersson, M.; Schulz, A.; Fricker, M.; Christiansson, A.; Granum, P.E.; Märtlbauer, E.; et al. Emetic toxin formation of Bacillus cereus is restricted to a single evolutionary lineage of closely related strains. Microbiology 2005, 151, 183–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.; Yu, P.; Wang, J.; Li, C.; Guo, H.; Liu, C.; Kong, L.; Yu, L.; Wu, S.; Lei, T.; et al. A Study on Prevalence and Characterization of Bacillus cereus in Ready-to-Eat Foods in China. Front. Microbiol. 2020, 10, 3043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Alessandro, B.; Antúnez, K.; Piccini, C.; Zunino, P. DNA extraction and PCR detection of Paenibacillus larvae spores from naturally contaminated honey and bees using spore-decoating and freeze-thawing techniques. World J. Microbiol. Biotechnol. 2007. [Google Scholar] [CrossRef]


| Place | Type | Collected Samples (n) | Positive Samples (n) | B. cereus s.l. (n) |
|---|---|---|---|---|
| A | Bakery | 3 | 1 | 1 |
| B | Bakery | 4 | 3 | 9 |
| C | Bakery | 1 | 1 | 4 |
| D | Bakery | 1 | 1 | 1 |
| E | Bakery | 14 | 9 | 14 |
| F | Bakery | 9 | 2 | 7 |
| G | Bakery | 1 | 1 | 1 |
| H | Powdered foods company | 14 | 13 | 34 |
| I | Bakery | 10 | 5 | 13 |
| J | Bakery | 11 | 3 | 6 |
| K | Bakery | 5 | 4 | 8 |
| L | Bakery | 1 | 0 | 0 |
| M | Bakery | 1 | 0 | 0 |
| TOTAL | 75 | 43 | 98 |
| Profile | Toxin Genes | Number of B. cereus s.l. (%) |
|---|---|---|
| I | nheABC, hblCDAB, cytK2, entFM | 49 (50) |
| II | nheABC, hblCDA, cytK2, entFM | 8 (8) |
| III | nheABC, hblCDAB, cytK2 | 19 (20) |
| IV | nheABC, hblCDA, cytK2 | 1 (1) |
| V | nheABC, hblCDAB, entFM | 8 (8) |
| VI | nheABC, hblCDA, entFM | 2 (2) |
| VII | nheABC, cytK2, entFM | 5 (5) |
| VIII | nheABC, cytK2 | 1 (1) |
| IX | nheABC, hblCDA | 2 (2) |
| X | nheABC, entFM | 1 (1) |
| XI | hblCDAB, entFM | 1 (1) |
| XII | nheABC | 1 (1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Chica, J.; Correa, M.M.; Aceves-Diez, A.E.; Castañeda-Sandoval, L.M. Enterotoxin Gene Distribution and Genotypes of Bacillus cereus sensu lato Isolated from Cassava Starch. Toxins 2021, 13, 131. https://doi.org/10.3390/toxins13020131
Sánchez-Chica J, Correa MM, Aceves-Diez AE, Castañeda-Sandoval LM. Enterotoxin Gene Distribution and Genotypes of Bacillus cereus sensu lato Isolated from Cassava Starch. Toxins. 2021; 13(2):131. https://doi.org/10.3390/toxins13020131
Chicago/Turabian StyleSánchez-Chica, Jennifer, Margarita M. Correa, Angel E. Aceves-Diez, and Laura M. Castañeda-Sandoval. 2021. "Enterotoxin Gene Distribution and Genotypes of Bacillus cereus sensu lato Isolated from Cassava Starch" Toxins 13, no. 2: 131. https://doi.org/10.3390/toxins13020131
APA StyleSánchez-Chica, J., Correa, M. M., Aceves-Diez, A. E., & Castañeda-Sandoval, L. M. (2021). Enterotoxin Gene Distribution and Genotypes of Bacillus cereus sensu lato Isolated from Cassava Starch. Toxins, 13(2), 131. https://doi.org/10.3390/toxins13020131





