Macrolides: From Toxins to Therapeutics
Abstract
1. Introduction
2. Macrolide Classes
2.1. Toxins
2.1.1. Structures
2.1.2. Mechanisms of Action
Inhibition of Actin Polymerization
Increased Actin Polymerization
Modulation of Actomyosin ATPase Activity
Prevention of Tubulin Polymerization
Wiskott Aldrich Syndrome Proteins (WASPs)
Host Immunomodulation
Prevention of Protein Secretion
mTOR Downregulation
2.1.3. Human Health Applications
2.2. Antibiotics
2.2.1. Structures
2.2.2. Mechanisms of Action
Inhibition of Bacterial Protein Synthesis
Impairment of Biofilm Synthesis
2.2.3. Pharmacology
2.2.4. Side Effects and Human Targets
Gastrointestinal Motility
Electrophysiological Effects on Heart
Cholestatic Hepatitis
Immunomodulation
2.3. Antivirals and Antiparasitics
2.3.1. Structures
2.3.2. Mechanism of Action
Modulation of Parasite Ion Channels and Receptors
Inhibition of Nuclear Transport
Protein Kinase C Modulation
Reactivation of Latent HIV
2.3.3. Pharmacology
2.3.4. Side Effects and Human Targets
2.4. Antifungals
2.4.1. Structures
2.4.2. Mechanism of Action
Ergosterol Binding
Oxidative Damage
Inhibition of ATP Hydrolysis
2.4.3. Pharmacology
2.4.4. Side Effects and Human Targets
2.5. Immunosuppressants
2.5.1. Structures
2.5.2. Mechanism of Action
Inhibition of Calcineurin
Inhibition of the Mammalian Target of Rapamycin (mTOR)
2.5.3. Pharmacology
2.5.4. Side Effects and Human Targets
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhanel, G.G.; Dueck, M.; Hoban, D.J.; Vercaigne, L.M.; Embil, J.M.; Gin, A.S.; Karlowsky, J.A. Review of Macrolides and Ketolides. Drugs 2001, 61, 443–498. [Google Scholar] [CrossRef] [PubMed]
- Bryskier, A.; Bergogne-Bérézin, E. Macrolides. In Antimicrobial Agents; American Society of Microbiology: Washington, DC, USA, 2005. [Google Scholar] [CrossRef]
- Kanoh, S.; Rubin, B.K. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin. Microbiol. Rev. 2010, 23, 590–615. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zou, J.; Yan, X.; Chen, J.; Cao, X.; Wu, J.; Liu, Y.; Wang, T. Marine-Derived Macrolides 1990–2020: An Overview of Chemical and Biological Diversity. Mar. Drugs 2021, 19, 180. [Google Scholar] [CrossRef] [PubMed]
- Guenin-Macé, L.; Ruf, M.-T.; Pluschke, G.; Demangel, C. Mycolactone: More than Just a Cytotoxin. In Buruli Ulcer: Mycobacterium Ulcerans Disease; Pluschke, G., Röltgen, K., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 117–134. [Google Scholar] [CrossRef]
- Saito, S.Y. Toxins affecting actin filaments and microtubules. Prog. Mol. Subcell. Biol. 2009, 46, 187–219. [Google Scholar] [CrossRef]
- Klenchin, V.A.; Allingham, J.S.; King, R.; Tanaka, J.; Marriott, G.; Rayment, I. Trisoxazole macrolide toxins mimic the binding of actin-capping proteins to actin. Nat. Struct. Biol. 2003, 10, 1058–1063. [Google Scholar] [CrossRef]
- Vincent, E.; Saxton, J.; Baker-Glenn, C.; Moal, I.; Hirst, J.D.; Pattenden, G.; Shaw, P.E. Effects of ulapualide A and synthetic macrolide analogues on actin dynamics and gene regulation. Cell. Mol. Life Sci. 2007, 64, 487–497. [Google Scholar] [CrossRef]
- Saito, S.; Watabe, S.; Ozaki, H.; Fusetani, N.; Karaki, H. Mycalolide B, a novel actin depolymerizing agent. J. Biol. Chem. 1994, 269, 29710–29714. [Google Scholar] [CrossRef]
- Wada, S.; Matsunaga, S.; Saito, S.; Fusetani, N.; Watabe, S. Actin-binding specificity of marine macrolide toxins, mycalolide B and kabiramide D. J. Biochem. 1998, 123, 946–952. [Google Scholar] [CrossRef]
- Dominguez, R. Actin-binding proteins—A unifying hypothesis. Trends Biochem. Sci. 2004, 29, 572–578. [Google Scholar] [CrossRef]
- Tanaka, J.; Yan, Y.; Choi, J.; Bai, J.; Klenchin, V.A.; Rayment, I.; Marriott, G. Biomolecular mimicry in the actin cytoskeleton: Mechanisms underlying the cytotoxicity of kabiramide C and related macrolides. Proc. Natl. Acad. Sci. USA 2003, 100, 13851–13856. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kawazoe, K.; Okamoto, T.; Sasaki, T.; Kitagawa, I. Marine natural products. XXXI. Structure-activity correlation of a potent cytotoxic dimeric macrolide swinholide A, from the Okinawan marine sponge Theonella swinhoei, and its isomers. Chem. Pharm. Bull. 1994, 42, 19–26. [Google Scholar] [CrossRef]
- Andrianasolo, E.H.; Gross, H.; Goeger, D.; Musafija-Girt, M.; McPhail, K.; Leal, R.M.; Mooberry, S.L.; Gerwick, W.H. Isolation of swinholide A and related glycosylated derivatives from two field collections of marine cyanobacteria. Org. Lett. 2005, 7, 1375–1378. [Google Scholar] [CrossRef]
- Spector, I.; Braet, F.; Shochet, N.R.; Bubb, M.R. New anti-actin drugs in the study of the organization and function of the actin cytoskeleton. Microsc. Res. Tech. 1999, 47, 18–37. [Google Scholar] [CrossRef]
- Kita, M.; Kigoshi, H. Marine natural products that interfere with multiple cytoskeletal protein interactions. Nat. Prod. Rep. 2015, 32, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, Y.; Yamagishi, K.; Suzuki, T.; Kawagishi, H.; Kita, M.; Kigoshi, H. Analysis of the aplyronine A-induced protein-protein interaction between actin and tubulin by surface plasmon resonance. Bioorg. Med. Chem. 2016, 24, 2809–2814. [Google Scholar] [CrossRef]
- Allingham, J.S.; Zampella, A.; D’Auria, M.V.; Rayment, I. Structures of microfilament destabilizing toxins bound to actin provide insight into toxin design and activity. Proc. Natl. Acad. Sci. USA 2005, 102, 14527–14532. [Google Scholar] [CrossRef] [PubMed]
- Mitchison, T.J.; Cramer, L.P. Actin-based cell motility and cell locomotion. Cell 1996, 84, 371–379. [Google Scholar] [CrossRef]
- Mizuno, K.; Nakahata, N.; Ito, E.; Murakami, M.; Yamaguchi, K.; Ohizumi, Y. Goniodomin A, an Antifungal Polyether Macrolide, Increases the Filamentous Actin Content of 1321N1 Human Astrocytoma Cells. J. Pharm. Pharmacol. 1998, 50, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Sakai, K.; Watanabe, S.; Maruyama, K.; Murakami, M.; Yamaguchi, K.; Ohizumi, Y. Goniodomin A induces modulation of actomyosin ATPase activity mediated through conformational change of actin. J. Biol. Chem. 1993, 268, 26026–26031. [Google Scholar] [CrossRef]
- Daranas, A.H.; Norte, M.; Fernández, J.J. Toxic marine microalgae. Toxicon 2001, 39, 1101–1132. [Google Scholar] [CrossRef]
- Yamada, K.; Ojika, M.; Ishigaki, T.; Yoshida, Y.; Ekimoto, H.; Arakawa, M. Aplyronine A, a potent antitumor substance and the congeners aplyronines B and C isolated from the sea hare Aplysia kurodai. J. Am. Chem. Soc. 1993, 115, 11020–11021. [Google Scholar] [CrossRef]
- Bai, R.; Cichacz, Z.A.; Herald, C.L.; Pettit, G.R.; Hamel, E. Spongistatin 1, a highly cytotoxic, sponge-derived, marine natural product that inhibits mitosis, microtubule assembly, and the binding of vinblastine to tubulin. Mol. Pharmacol. 1993, 44, 757–766. [Google Scholar] [PubMed]
- Xu, Q.; Huang, K.C.; Tendyke, K.; Marsh, J.; Liu, J.; Qiu, D.; Littlefield, B.A.; Nomoto, K.; Atasoylu, O.; Risatti, C.A.; et al. In vitro and in vivo anticancer activity of (+)-spongistatin 1. Anticancer Res. 2011, 31, 2773–2779. [Google Scholar]
- Kuznetsov, G.; Towle, M.J.; Cheng, H.; Kawamura, T.; TenDyke, K.; Liu, D.; Kishi, Y.; Yu, M.J.; Littlefield, B.A. Induction of Morphological and Biochemical Apoptosis following Prolonged Mitotic Blockage by Halichondrin B Macrocyclic Ketone Analog E7389. Cancer Res. 2004, 64, 5760–5766. [Google Scholar] [CrossRef]
- Jordan, M.A.; Kamath, K.; Manna, T.; Okouneva, T.; Miller, H.P.; Davis, C.; Littlefield, B.A.; Wilson, L. The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol. Cancer Ther. 2005, 4, 1086–1095. [Google Scholar] [CrossRef]
- Sarfo, F.S.; Phillips, R.; Wansbrough-Jones, M.; Simmonds, R.E. Recent advances: Role of mycolactone in the pathogenesis and monitoring of Mycobacterium ulcerans infection/Buruli ulcer disease. Cell. Microbiol. 2016, 18, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Coutanceau, E.; Decalf, J.; Martino, A.; Babon, A.; Winter, N.; Cole, S.T.; Albert, M.L.; Demangel, C. Selective suppression of dendritic cell functions by Mycobacterium ulcerans toxin mycolactone. J. Exp. Med. 2007, 204, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Pahlevan, A.A.; Wright, D.J.; Andrews, C.; George, K.M.; Small, P.L.; Foxwell, B.M. The inhibitory action of Mycobacterium ulcerans soluble factor on monocyte/T cell cytokine production and NF-kappa B function. J. Immunol. 1999, 163, 3928–3935. [Google Scholar]
- Van der Bruggen, T.; Nijenhuis, S.; Van Raaij, E.; Verhoef, J.; Sweder van Asbeck, B. Lipopolysaccharide-Induced Tumor Necrosis Factor Alpha Production by Human Monocytes Involves the Raf-1/MEK1-MEK2/ERK1-ERK2 Pathway. Infect. Immun. 1999, 67, 3824–3829. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Rapoport, T.A. Mechanisms of Sec61/SecY-mediated protein translocation across membranes. Annu. Rev. Biophys. 2012, 41, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Paatero, A.O.; Kellosalo, J.; Dunyak, B.M.; Almaliti, J.; Gestwick, J.E.; Gerwick, W.H.; Taunton, J.; Paavilainen, V.O. Apratoxin Kills Cells by Direct Blockade of the Sec61 Protein Translocation Channel. Cell Chem. Biol. 2016, 23, 561–566. [Google Scholar] [CrossRef] [PubMed]
- McKenna, M.; Simmonds, R.E.; High, S. Mechanistic insights into the inhibition of Sec61-dependent co- and post-translational translocation by mycolactone. J. Cell Sci. 2016, 129, 1404–1415. [Google Scholar] [CrossRef] [PubMed]
- Baron, L.; Paatero, A.O.; Morel, J.-D.; Impens, F.; Guenin-Macé, L.; Saint-Auret, S.; Blanchard, N.; Dillmann, R.; Niang, F.; Pellegrini, S.; et al. Mycolactone subverts immunity by selectively blocking the Sec61 translocon. J. Exp. Med. 2016, 213, 2885–2896. [Google Scholar] [CrossRef] [PubMed]
- Bieri, R.; Scherr, N.; Ruf, M.-T.; Dangy, J.-P.; Gersbach, P.; Gehringer, M.; Altmann, K.-H.; Pluschke, G. The Macrolide Toxin Mycolactone Promotes Bim-Dependent Apoptosis in Buruli Ulcer through Inhibition of mTOR. ACS Chem. Biol. 2017, 12, 1297–1307. [Google Scholar] [CrossRef]
- Bae, S.Y.; Kim, G.D.; Jeon, J.E.; Shin, J.; Lee, S.K. Anti-proliferative effect of (19Z)-halichondramide, a novel marine macrolide isolated from the sponge Chondrosia corticata, is associated with G2/M cell cycle arrest and suppression of mTOR signaling in human lung cancer cells. Toxicol. In Vitro 2013, 27, 694–999. [Google Scholar] [CrossRef]
- Konishi, H.; Kikuchi, S.; Ochiai, T.; Ikoma, H.; Kubota, T.; Ichikawa, D.; Fujiwara, H.; Okamoto, K.; Sakakura, C.; Sonoyama, T.; et al. Latrunculin A Has a Strong Anticancer Effect in a Peritoneal Dissemination Model of Human Gastric Cancer in Mice. Anticancer Res. 2009, 29, 2091–2097. [Google Scholar]
- Shin, Y.; Kim, G.D.; Jeon, J.-e.; Shin, J.; Lee, S.K. Antimetastatic Effect of Halichondramide, a Trisoxazole Macrolide from the Marine Sponge Chondrosia corticata, on Human Prostate Cancer Cells via Modulation of Epithelial-to-Mesenchymal Transition. Mar. Drugs 2013, 11, 2472–2485. [Google Scholar] [CrossRef]
- NCI. Eribulin Mesylate. Available online: https://www.cancer.gov/about-cancer/treatment/drugs/eribulinmesylate (accessed on 22 February 2021).
- Agoulnik, S.; Kuznetsov, G.; Tendyke, K.; Parent, L.A.; Marsh, J.P.; Twine, N.; Renshaw, F.G.; Silberman, S.; Littlefield, B.A. Sensitivity to halichondrin analog E7389 and hemiasterlin analog E7974 correlates with βIII tubulin isotype expression in human breast cancer cell lines. J. Clin. Oncol. 2012, 23. [Google Scholar] [CrossRef]
- Kernan, M.R.; Faulkner, D.J. Halichondramide, an antifungal macrolide from the sponge halichondria sp. Tetrahedron Lett. 1987, 28, 2809–2812. [Google Scholar] [CrossRef]
- Yarlagadda, V.; Medina, R.; Wright, G.D. Venturicidin A, A Membrane-active natural product inhibitor of Atp synthase potentiates Aminoglycoside Antibiotics. Sci. Rep. 2020, 10, 8134. [Google Scholar] [CrossRef]
- Patterson, G.M.L.; Bolis, C.M. Fungal cellwall polysaccharides elicit and antifungal secondary metabolite (phytoalexin) in the Cyanobacterium Scytonema ocelutum. J. Phycol. 2008, 33, 54–60. [Google Scholar] [CrossRef]
- Nathan, C.; Cars, O. Antibiotic resistance—Problems, progress, and prospects. N. Engl. J. Med. 2014, 371, 1761–1763. [Google Scholar] [CrossRef] [PubMed]
- Crosbie, P.A.; Woodhead, M.A. Long-term macrolide therapy in chronic inflammatory airway diseases. Eur. Respir. J. 2009, 33, 171–181. [Google Scholar] [CrossRef]
- Dinos, G.P. The macrolide antibiotic renaissance. Br. J. Pharmacol. 2017, 174, 2967–2983. [Google Scholar] [CrossRef] [PubMed]
- Brisson-Noel, A.; Trieu-Cuot, P.; Courvalin, P. Mechanism of action of spiramycin and other macrolides. J. Antimicrob. Chemother. 1988, 22, 13–23. [Google Scholar] [CrossRef]
- Araújo, L.; Demoly, P. Macrolides allergy. Curr. Pharm. Des. 2008, 14, 2840–2862. [Google Scholar] [CrossRef]
- Jorro, G.; Morales, C.; Brasó, J.V.; Peláez, A. Anaphylaxis to erythromycin. Ann. Allergy Asthma Immunol. 1996, 77, 456–458. [Google Scholar] [CrossRef]
- Lee, C.E.; Zembower, T.R.; Fotis, M.A.; Postelnick, M.J.; Greenberger, P.A.; Peterson, L.R.; Noskin, G.A. The incidence of antimicrobial allergies in hospitalized patients: Implications regarding prescribing patterns and emerging bacterial resistance. Arch. Intern. Med. 2000, 160, 2819–2822. [Google Scholar] [CrossRef]
- Pascual, C.; Crespo, J.F.; Quiralte, J.; Lopez, C.; Wheeler, G.; Martin-Esteban, M. In vitro detection of specific IgE antibodies to erythromycin. J. Allergy Clin. Immunol. 1995, 95, 668–671. [Google Scholar] [CrossRef]
- Mankin, A.S. Macrolide myths. Curr. Opin. Microbiol. 2008, 11, 414–421. [Google Scholar] [CrossRef]
- Kannan, K.; Mankin, A.S. Macrolide antibiotics in the ribosome exit tunnel: Species-specific binding and action. Ann. N. Y. Acad. Sci. 2011, 1241, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, W.; Mutak, S. Macrolide Antibiotics; Birkhäuser Verlag: Basel, Switzerland, 2002; pp. 73–95. [Google Scholar]
- Glanzer, S.; Pulido, S.A.; Tutz, S.; Wagner, G.E.; Kriechbaum, M.; Gubensäk, N.; Trifunovic, J.; Dorn, M.; Fabian, W.M.; Novak, P.; et al. Structural and functional implications of the interaction between macrolide antibiotics and bile acids. Chemistry 2015, 21, 4350–4358. [Google Scholar] [CrossRef]
- Mao, J.C.H.; Robinshaw, E.E. Erythromycin, a Peptidyltransferase Effector. Biochemistry 1972, 11, 4864–4872. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Otaka, T.; Kaji, A. Further Studies on the Mechanism of Erythromycin Action. Biochim. Biophys. Acta Nucleic Acids Protein Synth. 1973, 331, 128–140. [Google Scholar] [CrossRef]
- Vázquez-Laslop, N.; Mankin, A.S. How Macrolide Antibiotics Work. Trends Biochem. Sci. 2018, 43, 668–684. [Google Scholar] [CrossRef]
- Tenson, T.; Lovmar, M.; Ehrenberg, M. The Mechanism of Action of Macrolides, Lincosamides and Streptogramin B Reveals the Nascent Peptide Exit Path in the Ribosome. J. Mol. Biol. 2003, 330, 1005–1014. [Google Scholar] [CrossRef]
- Almutairi, M.M.; Svetlov, M.S.; Hansen, D.A.; Khabibullina, N.F.; Klepacki, D.; Kang, H.-Y.; Sherman, D.H.; Vázquez-Laslop, N.; Polikanov, Y.S.; Mankin, A.S. Co-produced natural ketolides methymycin and pikromycin inhibit bacterial growth by preventing synthesis of a limited number of proteins. Nucleic Acids Res. 2017, 45, 9573–9582. [Google Scholar] [CrossRef] [PubMed]
- Starosta, A.L.; Karpenko, V.V.; Shishkina, A.V.; Mikolajka, A.; Sumbatyan, N.V.; Schluenzen, F.; Korshunova, G.A.; Bogdanov, A.A.; Wilson, D.N. Interplay between the Ribosomal Tunnel, Nascent Chain, and Macrolides Influences Drug Inhibition. Chem. Biol. 2010, 17, 504–514. [Google Scholar] [CrossRef]
- Tamura, A.; Ara, T.; Imamura, Y.; Fujii, T.; Wang, P.-L. The Effects of Antibiotics on in vitro Biofilm Model of Periodontal Disease. Eur. J. Med. Res. 2008, 13, 439–445. [Google Scholar]
- Kobayashi, H. Airway Biofilm Disease: Clinical Manifestations and Therapeutic Possibilities Using Macrolides. JIC 1995, 1, 1–15. [Google Scholar] [CrossRef]
- Altenburg, J.; de Graaff, C.S.; van der Werf, T.S.; Boersma, W.G. Immunomodulatory effects of macrolide antibiotics—Part 1: Biological mechanisms. Respiration 2011, 81, 67–74. [Google Scholar] [CrossRef]
- Yanagihara, K.; Tomono, K.; Imamura, Y.; Kaneko, Y.; Kuroki, M.; Sawai, T.; Miyazaki, Y.; Hirakata, Y.; Mukae, H.; Kadota, J.-I.; et al. Effect of clarithromycin on chronic respiratory infection caused by Pseudomonas aeruginosa with biofilm formation in an experimental murine model. J. Antimicrob. Chemother. 2002, 49, 867–870. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Silva Filho, L.V.; Pinto, L.A.; Stein, R.T. Use of macrolides in lung diseases: Recent literature controversies. J. Pediatr. 2015, 91, S52–S60. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bambeke, F.V.; Tulkens, P.M. Macrolides: Pharmacokinetics and pharmacodynamics. Int. J. Antimicrob. Agents 2001, 18, S17–S23. [Google Scholar] [CrossRef]
- Fohner, A.; Sparreboom, A.; Altman, R.; Klein, T. PharmGKB summary: Macrolide antibiotic pathway, pharmacokinetics/pharmacodynamics. Pharmacogenet. Genom. 2017, 24, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Tsai, D.; Jamal, J.A.; Davis, J.S.; Lipman, J.; Roberts, J.A. Interethnic differences in pharmacokinetics of antibacterials. Clin. Pharmacokinet. 2015, 54, 243–260. [Google Scholar] [CrossRef]
- Sugie, M.; Asakura, E.; Zhao, Y.L.; Torita, S.; Nadai, M.; Baba, K.; Kitaichi, K.; Takagi, K.; Takagi, K.; Hasegawa, T. Possible involvement of the drug transporters P glycoprotein and multidrug resistance-associated protein Mrp2 in disposition of azithromycin. Antimicrob. Agents Chemother. 2004, 48, 809–814. [Google Scholar] [CrossRef]
- Garver, E.; Hugger, E.D.; Shearn, S.P.; Rao, A.; Dawson, P.A.; Davis, C.B.; Han, C. Involvement of intestinal uptake transporters in the absorption of azithromycin and clarithromycin in the rat. Drug Metab. Dispos. 2008, 36, 2492–2498. [Google Scholar] [CrossRef]
- Kurnik, D.; Wood, A.J.; Wilkinson, G.R. The erythromycin breath test reflects P-glycoprotein function independently of cytochrome P450 3A activity. Clin. Pharmacol. Ther. 2006, 80, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Chelvan, P.; Hamilton-Miller, J.M.; Brumfitt, W. Biliary excretion of erythromycin after parenteral administration. Br. J. Clin. Pharmacol. 1979, 8, 233–235. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hansen, M.; Scott, A.; McCullough, A.; Thorning, S.; Aronson, J.; Beller, E.; Glasziou, P.; Hoffmann, T.; Clark, J.; Del Mar, C. Adverse events in people taking macrolide antibiotics versus placebo for any indication. Cochrane Database Syst. Rev. 2019, 1. [Google Scholar] [CrossRef]
- Pilot, M.A.; Qin, X.Y. Macrolides and gastrointestinal motility. J. Antimicrob. Chemother. 1988, 22, 201–206. [Google Scholar] [CrossRef]
- Satoh, M.; Sakai, T.; Sano, I.; Fujikura, K.; Koyama, H.; Ohshima, K.; Itoh, Z.; Omura, S. EM574, an erythromycin derivative, is a potent motilin receptor agonist in human gastric antrum. J. Pharmacol. Exp. Ther. 1994, 271, 574–579. [Google Scholar] [PubMed]
- Peeters, T.; Matthijs, G.; Depoortere, I.; Cachet, T.; Hoogmartens, J.; Vantrappen, G. Erythromycin is a motilin receptor agonist. Am. J. Physiol. Gastrointest. Liver Physiol. 1989, 257, G470–G474. [Google Scholar] [CrossRef]
- Potter, T.G.; Snider, K.R. Azithromycin for the treatment of gastroparesis. Ann. Pharmacother. 2013, 47, 411–415. [Google Scholar] [CrossRef]
- Itoh, Z.; Tomoaki, S.; Nakaya, M.; Inoue, M.; Mitsuhashi, S. Gastrointestinal Motor-Stimulating Activity of Macrolide Antibiotics and Analysis of Their Side Effects on the Canine Gut. Antimicrob. Agents Chemother. 1984, 863–869. [Google Scholar] [CrossRef]
- Russo, V.; Puzio, G.; Siniscalchi, N. Azithromycin-induced QT prolongation in elderly patient. Acta. Biomed. 2006, 77, 30–32. [Google Scholar]
- Milberg, P.; Eckardt, L.; Bruns, H.-J.; Biertz, J.; Ramtin, S.; Reinsch, N.; Fleischer, D.; Kirchhof, P.; Fabritz, L.; Breithardt, G.; et al. Divergent Proarrhythmic Potential of Macrolide Antibiotics Despite Similar QT Prolongation: Fast Phase 3 Repolarization Prevents Early Afterdepolarizations and Torsade de Pointes. J. Pharmacol. Exp. Ther. 2002, 303, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Ray, W.A.; Murray, K.T.; Meredith, S.; Narasimhulu, S.S.; Hall, K.; Stein, C.M. Oral erythromycin and the risk of sudden death from cardiac causes. N. Engl. J. Med. 2004, 351, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Schoenenberger, R.A.; Haefeli, W.E.; Weiss, P.; Ritz, R.F. Association of intravenous erythromycin and potentially fatal ventricular tachycardia with Q-T prolongation (torsades de pointes). BMJ 1990, 300, 1375–1376. [Google Scholar] [CrossRef][Green Version]
- Corrao, G.; Botteri, E.; Bagnardi, V.; Zambon, A.; Carobbio, A.; Falcone, C.; Leoni, O. Generating signals of drug-adverse effects from prescription databases and application to the risk of arrhythmia associated with antibacterials. Pharmacoepidemiol. Drug Saf. 2005, 14, 31–40. [Google Scholar] [CrossRef]
- Harris, S.; Hilligoss, D.M.; Colangelo, P.M.; Eller, M.; Okerholm, R. Azithromycin and terfenadine: Lack of drug interaction. Clin. Pharmacol. Ther. 1995, 58, 310–315. [Google Scholar] [CrossRef]
- Owens, R.C., Jr. QT prolongation with antimicrobial agents: Understanding the significance. Drugs 2004, 64, 1091–1124. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, F. Cholestatic Hepatitis Caused by Esters of Erythromycin and Oleandomycin. JAMA 1962, 1048–1050. [Google Scholar] [CrossRef][Green Version]
- Derby, L.E.; Jick, H.; Henry, D.A.; Dean, A.D. Erythromycin-associated cholestatic hepatitis. Med. J. Aust. 1993, 158, 600–602. [Google Scholar] [CrossRef] [PubMed]
- Leitner, J.M.; Graninger, W.; Thalhammer, F. Hepatotoxicity of antibacterials: Pathomechanisms and clinical. Infection 2010, 38, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.J.; Speelman, P.; Hack, C.E.; Buurman, W.A.; van Deventer, S.J.; van Der Poll, T. Intravenous infusion of erythromycin inhibits CXC chemokine production, but augments neutrophil degranulation in whole blood stimulated with Streptococcus pneumoniae. J. Antimicrob. Chemother. 2000, 46, 235–240. [Google Scholar] [CrossRef]
- Kadota, J.; Sakito, O.; Kohno, S.; Sawa, H.; Mukae, H.; Oda, H.; Kawakami, K.; Fukushima, K.; Hiratani, K.; Hara, K. A mechanism of erythromycin treatment in patients with diffuse panbronchiolitis. Am. Rev. Respir. Dis. 1993, 147, 153–159. [Google Scholar] [CrossRef]
- Oda, H.; Kadota, J.; Kohno, S.; Hara, K. Erythromycin inhibits neutrophil chemotaxis in bronchoalveoli of diffuse panbronchiolitis. Chest 1994, 106, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Ianaro, A.; Ialenti, A.; Maffia, P.; Sautebin, L.; Rombolà, L.; Carnuccio, R.; Iuvone, T.; D’Acquisto, F.; Di Rosa, M. Anti-inflammatory activity of macrolide antibiotics. J. Pharmacol. Exp. Ther. 2000, 292, 156–163. [Google Scholar]
- Tamaoki, J. The effects of macrolides on inflammatory cells. Chest 2004, 125, 41S–50S. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Wang, C.H.; Liu, C.Y.; Yu, C.T.; Kuo, H.P. Erythromycin inhibits beta2-integrins (CD11b/CD18) expression, interleukin-8 release and intracellular oxidative metabolism in neutrophils. Respir. Med. 2000, 94, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Kusano, S.; Kadota, J.; Kohno, S.; Iida, K.; Kawakami, K.; Morikawa, T.; Hara, K. Effect of roxithromycin on peripheral neutrophil adhesion molecules in patients with chronic lower respiratory tract disease. Respiration 1995, 62, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.; Nelson, D.K.; Koyama, S.; Hoyt, J.C.; Robbins, R.A. Erythromycin modulates eosinophil chemotactic cytokine production by human lung fibroblasts in vitro. Antimicrob. Agents Chemother. 2001, 45, 401–406. [Google Scholar] [CrossRef][Green Version]
- Burg, R.W.; Miller, B.M.; Baker, E.E.; Birnbaum, J.; Currie, S.A.; Hartman, R.; Kong, Y.-L.; Monaghan, R.L.; Olson, G.; Putter, I.; et al. Avermectins, New Family of Potent Anthelmintic Agents: Producing Organism and Fermentation. Antimicrob. Agents Chemother. 1979, 361–367. [Google Scholar] [CrossRef]
- Crump, A. Ivermectin: Enigmatic multifaceted ‘wonder’ drug continues to surprise and exceed expectations. J. Antibiot. 2017, 70, 495–505. [Google Scholar] [CrossRef]
- Crump, A.; Omura, S. Ivermectin, ‘Wonder drug’ from Japan: The human use perspective. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2011, 87, 13–28. [Google Scholar] [CrossRef]
- González Canga, A.; Sahagún Prieto, A.M.; Diez Liébana, M.J.; Fernández Martínez, N.; Sierra Vega, M.; García Vieitez, J.J. The pharmacokinetics and interactions of ivermectin in humans—A mini-review. AAPS J. 2008, 10, 42–46. [Google Scholar] [CrossRef]
- Shushni, M.A.; Singh, R.; Mentel, R.; Lindequist, U. Balticolid: A new 12-membered macrolide with antiviral activity from an ascomycetous fungus of marine origin. Mar. Drugs 2011, 9, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.A. Marine natural products. Annu. Rep. Prog. Chem. Sect. B Org. Chem. 2012, 108, 131–146. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Nikzad, S.; Kadir, H.A.; Abubakar, S.; Zandi, K. Potential Antiviral Agents from Marine Fungi: An Overview. Mar. Drugs 2015, 13, 4520–4538. [Google Scholar] [CrossRef] [PubMed]
- Berkow, R.L.; Kraft, A.S. Bryostatin, a non-phorbol macrocyclic lactone, activates intact human polymorphonuclear leukocytes and binds to the phorbol ester receptor. Biochem. Biophys. Res. Commun. 1985, 131, 1109–1116. [Google Scholar] [CrossRef]
- Wu, R.; Chen, H.; Chang, N.; Xu, Y.; Jiao, J.; Zhang, H. Unlocking the Drug Potential of the Bryostatin Family: Recent Advances in Product Synthesis and Biomedical Applications. Chem. Eur. J. 2020, 26, 1166–1195. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.H.; Ogbourne, S. Eco-toxicological effects of the avermectin family with a focus on abamectin and ivermectin. Chemosphere 2016, 154, 204–214. [Google Scholar] [CrossRef]
- Chen, I.-S.; Kubo, Y. Ivermectin and its target molecules: Shared and unique modulation mechanisms of ion channels and receptors by ivermectin. J. Physiol. 2018, 1833–1845. [Google Scholar] [CrossRef]
- Bowie, D. Shared and unique aspects of ligand- and voltage-gated ion-channel gating. J. Physiol. 2018, 596, 1829–1832. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Messerli, S.M.; Sudo, T.; Maruta, H. Ivermectin inactivates the kinase PAK1 and blocks the PAK1-dependent growth of human ovarian cancer and NF2 tumor cell lines. Drug Discov. Ther. 2009, 3, 243–246. [Google Scholar] [PubMed]
- Althoff, T.; Hibbs, R.; Banerjee, S.; Gouaux, E. X-ray structures of GluCl in apo states reveal gating mechanism of Cys-loop receptors. Nature 2014, 512, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, E. Ivermectin, antiviral properties and COVID-19: A possible new mechanism of action. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 1153–1156. [Google Scholar] [CrossRef]
- Wagstaff, K.M.; Rawlinson, S.M.; Hearps, A.C.; Jans, D.A. An AlphaScreen®-based assay for high-throughput screening for specific inhibitors of nuclear import. J. Biomol. Screen. 2011, 16, 192–200. [Google Scholar] [CrossRef]
- Wagstaff, K.M.; Sivakumaran, H.; Heaton, S.M.; Harrich, D.; Jans, D.A. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem. J. 2012, 443, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Tay, M.Y.F.; Fraser, J.E.; Chan, W.K.K.; Moreland, N.J.; Rathore, A.P.; Wang, C.; Vasudevan, S.G.; Jans, D.A. Nuclear localization of dengue virus (DENV) 1-4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin. Antivir. Res. 2013, 99, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.N.Y.; Atkinson, S.C.; Wang, C.; Lee, A.; Bogoyevitch, M.A.; Borg, N.A.; Jansa, D.A. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antivir. Res. 2020, 177, 104760. [Google Scholar] [CrossRef]
- Lundberg, L.; Pinkham, C.; Baer, A.; Amaya, M.; Narayanan, A.; Wagstaff, K.M.; Jans, D.A.; Kehn-Hall, K. Nuclear import and export inhibitors alter capsid protein distribution in mammalian cells and reduce Venezuelan Equine Encephalitis Virus replication. Antivir. Res. 2013, 100, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Götz, V.; Magar, L.; Dornfeld, D.; Giese, S.; Pohlmann, A.; Höper, D.; Kong, B.-W.; Jans, D.A.; Beer, M.; Haller, O.; et al. Influenza A viruses escape from MxA restriction at the expense of efficient nuclear vRNP import. Sci. Rep. 2016, 6, 23138. [Google Scholar] [CrossRef] [PubMed]
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020, 178, 104787. [Google Scholar] [CrossRef]
- Pott-Junior, H.; Paoliello, M.M.B.; Miguel, A.Q.C.; da Cunha, A.F.; Freire, C.C.; Neves, F.F.; de Avó, L.R.; Roscani, M.G.; dos Santos, S.D.S.; Chachá, S.G.F. Use of ivermectin in the treatment of Covid-19: A pilot trial. Toxicol. Rep. 2021, 8, 505–510. [Google Scholar] [CrossRef]
- Pettit, G.R.; Kamano, Y.; Herald, C.L. Antineoplastic agents, 118. Isolation and structure of bryostatin 9. J. Nat. Prod. 1986, 49, 661–664. [Google Scholar] [CrossRef]
- Staveness, D.; Abdelnabi, R.; Schrier, A.J.; Loy, B.A.; Verma, V.A.; DeChristopher, B.A.; Near, K.E.; Neyts, J.; Delang, L.; Leyssen, P.; et al. Simplified Bryostatin Analogues Protect Cells from Chikungunya Virus-Induced Cell Death. J. Nat. Prod. 2016, 79, 675–679. [Google Scholar] [CrossRef]
- Alexaki, A.; Liu, Y.; Wigdahl, B. Cellular reservoirs of HIV-1 and their role in viral persistence. Curr. HIV Res. 2008, 6, 388–400. [Google Scholar] [CrossRef]
- Díaz, L.; Martínez-Bonet, M.; Sánchez, J.; Fernández-Pineda, A.; Jiménez, J.L.; Muñoz, E.; Moreno, S.; Álvarez, S.; Muñoz-Fernández, M.Á. Bryostatin activates HIV-1 latent expression in human astrocytes through a PKC and NF-ĸB-dependent mechanism. Sci. Rep. 2015, 5, 12442. [Google Scholar] [CrossRef]
- Mehla, R.; Bivalkar-Mehla, S.; Zhang, R.; Handy, I.; Albrecht, H.; Giri, S.; Nagarkatti, P.; Nagarkatti, M.; Chauhan, A. Bryostatin Modulates Latent HIV-1 Infection via PKC and AMPK Signaling but Inhibits Acute Infection in a Receptor Independent Manner. PLoS ONE 2010, 5, e11160. [Google Scholar] [CrossRef]
- Gutiérrez, C.; Serrano-Villar, S.; Madrid-Elena, N.; Pérez-Elías, M.J.; Martín, M.E.; Barbas, C.; Ruipérez, J.; Muñoz, E.; Muñoz-Fernández, M.A.; Castor, T.; et al. Bryostatin-1 for latent virus reactivation in HIV-infected patients on antiretroviral therapy. AIDS 2016, 30, 1385–1392. [Google Scholar] [CrossRef]
- Fisher, M.H.; Mrozik, H. The Chemistry and Pharmacology of Avermectins. Annu. Rev. Pharmacol. Toxicol. 1992, 32, 537–553. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.L.; Williams, J.F.; Dave, V. Pharmacology of Ivermectin. Parasitol. Today 1988, 4, 226–228. [Google Scholar] [CrossRef]
- Ottesen, E.A.; Campbell, W.C. Ivermectin in human medicine. J. Antimicrob. Chemother. 1994, 34, 195–203. [Google Scholar] [CrossRef]
- Sun, M.K.; Alkon, D.L. Bryostatin-1: Pharmacology and Therapeutic Potential as a CNS Drug. CNS Drug Rev. 2006, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, R.; Zhao, H.; Cai, H.; Gush, K.; Kerr, R.; Pettit, G.R.; Kraft, A.S. Preclinical Pharmacology of the Natural Product Anticancer Agent Bryostatin 1, an Activator of Protein Kinase C. Cancer Res. 1996, 56, 802–808. [Google Scholar]
- Rothova, A.; Stilma, J.S.; Van der Lelij, A.; Wilson, W.R.; Barbe, R.F. Side-effects of Ivermectin in Treatment of Onchocerciasis. Lancet 1989, 1439–1441. [Google Scholar] [CrossRef]
- Dominguez-Gomez, G.; Chavez-Blanco, A.; Medina-Franco, J.L.; Saldivar-Gonzalez, F.; Flores-Torrontegui, Y.; Juarez, M.; Díaz-Chávez, J.; Gonzalez-Fierro, A.; Dueñas-González, A. Ivermectin as an inhibitor of cancer stem-like cells. Mol. Med. Rep. 2018, 17, 3397–3403. [Google Scholar] [CrossRef]
- Nörenberg, W.; Sobottka, H.; Hempel, C.; Plötz, T.; Fischer, W.; Schmalzing, G.; Schaefer, M. Positive allosteric modulation by ivermectin of human but not murine P2X7 receptors. Br. J. Pharmacol. 2012, 167, 48–66. [Google Scholar] [CrossRef] [PubMed]
- Dagur, R.S.; Hambarde, S.; Chandna, S. Bryostatin-1 causes radiosensitization of BMG-1 malignant glioma cells through differential activation of protein kinase-Cδ not evident in the non-malignant AA8 fibroblasts. Mol. Cell. Biochem. 2015, 401, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.K.; Alkon, D.L. Induced depressive behavior impairs learning and memory in rats. Neuroscience 2003, 129, 129–139. [Google Scholar] [CrossRef]
- Nelson, T.J.; Sun, M.K.; Lim, C.; Sen, A.; Khan, T.; Chirila, F.V.; Alkon, D.L. Bryostatin Effects on Cognitive Function and PKCε in Alzheimer’s Disease Phase IIa and Expanded Access Trials. J. Alzheimers Dis. 2017, 58, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Lucke-Wold, B.P.; Logsdon, A.F.; Smith, K.E.; Turner, R.C.; Alkon, D.L.; Tan, Z.; Naser, Z.J.; Knotts, C.M.; Huber, J.D.; Rosen, C.L. Bryostatin-1 Restores Blood Brain Barrier Integrity following Blast-Induced Traumatic Brain Injury. Mol. Neurobiol. 2015, 52, 1119–1134. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ng, A.; Wasan, K.M.; Lopez-Berestein, G. Development of liposomal polyene antibiotics: An historical perspective. J. Pharm. Pharm. Sci. 2003, 6, 67–83. [Google Scholar] [PubMed]
- Espinel-Ingroff, A. Medical Mycology and Training in the United States: A Historical Analysis (1894–1996); Springer Science & Business Media: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Cooper, C. Nystatin and the Women Who Founded the Antifungal Era. In Proceedings of the 20th Congress of the International Society for Human and Animal Mycology, Amsterdam, The Netherlands, 30 June–4 July 2018. [Google Scholar]
- Lemke, A.; Kiderlen, A.; Kayser, O. Amphotericin B. Appl. Microbiol. Biotechnol. 2005, 68, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Branen, A.L.; Davidson, P.M.; Salminen, S.; Thorngate, J. Food Additives, 2nd ed.; Marcel Dekker Inc.: New York, NY, USA, 2003. [Google Scholar]
- Whitfield, G.B.; Brock, T.D.; Ammann, A.; Gottlieb, D.; Carter, H.E. Filipin, an antifungal antibiotic: Isolation and properties. J. Am. Chem. Soc. 1955, 77, 4799–4801. [Google Scholar] [CrossRef]
- Jundt, L.; Steinmetz, H.; Luger, P.; Weber, M.; Kunze, B.; Reichenbach, H.; Höfle, G. Isolation and structure elucidation of cruentarens A and b—Novel members of the benzolactone class of ATPase inhibitors from the Myxobacterium Byssovorax cruenta. Eur. J. Org. Chem. 2006, 2006, 5036–5044. [Google Scholar] [CrossRef]
- Kunze, B.; Steinmetz, H.; Höfle, G.; Huss, M.; Wieczorek, H.; Reichenbach, H. Cruentaren, a new antifungal salicylate-type macrolide from Byssovorax cruenta (Myxobacteria) with inhibitory effect on mitochondrial ATPase activity. J. Antibiot. 2006, 59, 664–668. [Google Scholar] [CrossRef][Green Version]
- Hąc-Wydro, K.; Dynarowicz-Łątka, P. Interaction between nystatin and natural membrane lipids in Langmuir monolayers—the role of a phospholipid in the mechanism of polyenes mode of action. Biophys. Chem. 2006, 123, 154–161. [Google Scholar] [CrossRef]
- Te Welscher, Y.M.; Hendrik, H.; Balagué, M.M.; Souza, C.M.; Riezman, H.; De Kruijff, B.; Breukink, E. Natamycin blocks fungal growth by binding specifically to ergosterol without permeabilizing the membrane. J. Biol. Chem. 2008, 283, 6393–6401. [Google Scholar] [CrossRef] [PubMed]
- Baginski, M.; Resat, H.; McCammon, J.A. Molecular properties of amphotericin B membrane channel: A molecular dynamics simulation. Mol. Pharmacol. 1997, 52, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Casey, W.M.; Parks, L.W. A role for sterols in the porphyrin mediated photosensitization of yeast. Photochem. Photobiol. 1989, 50, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Bolard, J.; Prasad, R. Emerging role of lipids of Candida albicans, a pathogenic dimorphic yeast. Biochim. Biophys. Acta Lipids Lipid Metab. 1992, 1127, 1–14. [Google Scholar] [CrossRef]
- De Kruijff, B.; Demel, R. Polyene antibiotic-sterol interactions in membranes of Acholeplasma laidlawii cells and lecithin liposomes. III. Molecular structure of the polyene antibiotic-cholesterol complexes. Biochim. Biophys. Acta 1974, 339, 57–70. [Google Scholar] [CrossRef]
- Fisher, P.B.; Bryson, V.; Schaffner, C.P. Polyene macrolide antibiotic cytotoxicity and membrane permeability alterations I. Comparative effects of four classes of polyene macrolides on mammalian cells. J. Cell. Physiol. 1978, 97, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Sokol-Anderson, M.L.; Brajtburg, J.; Medoff, G. Amphotericin B-induced oxidative damage and killing of Candida albicans. J. Infect. Dis. 1986, 154, 76–83. [Google Scholar] [CrossRef]
- Mesa-Arango, A.C.; Trevijano-Contador, N.; Román, E.; Sánchez-Fresneda, R.; Casas, C.; Herrero, E.; Argüelles, J.C.; Pla, J.; Cuenca-Estrella, M.; Zaragoza, O. The production of reactive oxygen species is a universal action mechanism of amphotericin B against pathogenic yeasts and contributes to the fungicidal effect of this drug. Antimicrob. Agents Chemother. 2014, 58, 6627–6638. [Google Scholar] [CrossRef]
- Kunze, B.; Sasse, F.; Wieczorek, H.; Huss, M. Cruentaren A, a highly cytotoxic benzolactone from Myxobacteria is a novel selective inhibitor of mitochondrial F1-ATPases. FEBS Lett. 2007, 581, 3523–3527. [Google Scholar] [CrossRef]
- Groll, A.H.; Rijnders, B.J.A.; Walsh, T.J.; Adler-Moore, J.; Lewis, R.E.; Brüggemann, R.J.M. Clinical Pharmacokinetics, Pharmacodynamics, Safety and Efficacy of Liposomal Amphotericin B. Clin. Infect. Dis. 2019, 68, S260–S274. [Google Scholar] [CrossRef]
- Atkinson, A.J.; Bennett, J.E. Amphotericin B Pharmacokinetics in Humans. Antimicrob. Agents Chemother. 1978, 13, 271–276. [Google Scholar] [CrossRef]
- Craven, P.C.; Ludden, T.M.; Drutz, D.J.; Rogers, W.; Haegele, K.A.; Skrdlant, H.B. Excretion Pathways of Amphotericin B. J. Infect. Dis. 1979, 140, 329–341. [Google Scholar] [CrossRef]
- Mesa-Arango, A.C.; Scorzoni, L.; Zaragoza, O. It only takes one to do many jobs: Amphotericin B as antifungal and immunomodulatory drug. Front. Microbiol. 2012, 3, 286. [Google Scholar] [CrossRef]
- Laniado-Laborín, R.; Cabrales-Vargas, M.N. Amphotericin B: Side effects and toxicity. Rev. Iberoam. Micol. 2009, 26, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Grela, E.; Piet, M.; Luchowski, R.; Grudzinski, W.; Paduch, R.; Gruszecki, W.I. Imaging of human cells exposed to an antifungal antibiotic amphotericin B reveals the mechanisms associated with the drug toxicity and cell defence. Sci. Rep. 2018, 8, 14067. [Google Scholar] [CrossRef]
- Kagan, S.; Ickowicz, D.; Shmuel, M.; Altschuler, Y.; Sionov, E.; Pitusi, M.; Weiss, A.; Farber, S.; Domb, A.J.; Polacheck, I. Toxicity mechanisms of amphotericin B and its neutralization by conjugation with arabinogalactan. Antimicrob. Agents Chemother. 2012, 56, 5603–5611. [Google Scholar] [CrossRef] [PubMed]
- Bhamra, R.; Sa’ad, A.; Bolcsak, L.E.; Janoff, A.S.; Swenson, C.E. Behavior of amphotericin B lipid complex in plasma in vitro and in the circulation of rats. Antimicrob. Agents Chemother. 1997, 41, 886–892. [Google Scholar] [CrossRef][Green Version]
- Semis, R.; Kagan, S.; Berdicevsky, I.; Polacheck, I.; Segal, E. Mechanism of activity and toxicity of Nystatin-Intralipid. Med. Mycol. 2013, 51, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Mrowietz, U. Macrolide immunosuppressants. Eur. J. Dermatol. 1999, 9, 346–351. [Google Scholar] [PubMed]
- Hatanaka, H.; Iwami, M.; Kino, T.; Goto, T.; Okuhara, M. FR-900520 and FR-900523, Novel Immunosuppressants Isolated from A Streptomyces. Taxonomy of the Producing Strain. J. Antibiot. 1988, 41, 1586–1591. [Google Scholar] [CrossRef]
- Jacobson, P.; Uberti, J.; Davis, W.; Ratanatharathorn, V. Tacrolimus: A new agent for the prevention of graft-versus-host disease in hematopoietic stem cell transplantation. Bone Marrow Transplant. 1998, 22, 217–225. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nghiem, P.; Pearson, G.; Langley, R. Tacrolimus and pimecrolimus: From clever prokaryotes to inhibiting calcineurin and treating atopic dermatitis. J. Am. Acad. Dermatol. 2002, 26, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Wallemacq, P.E.; Verbeeck, R.K. Comparative Clinical Pharmacokinetics of Tacrolimus in Paediatric and Adult Patients. Clin. Pharmacokinet. 2001, 40, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Grassberger, M.; Steinhoff, M.; Schneider, D.; Luger, T.A. Pimecrolimus—An anti-inflammatory drug targeting the skin. Exp. Dermatol. 2004, 13, 721–730. [Google Scholar] [CrossRef]
- Gupta, A.K.; Chow, M. Pimecrolimus: A review. J. Eur. Dermatol. Venereol. 2003, 17, 493–503. [Google Scholar] [CrossRef]
- MacDonald, A.; Scarola, J.; Burke, J.T.; Zimmerman, J.J. Clinical Pharmacokinetics and Therapeutic Drug Monitoring of Sirolimus. Clin. Ther. 2000, 22, B101–B121. [Google Scholar] [CrossRef]
- Li, J.; Kim, S.G.; Blenis, J. Rapamycin: One Drug, Many Effects. Cell Metab. 2014, 19, 373–379. [Google Scholar] [CrossRef]
- Sehgal, S.N. Sirolimus: Its Discovery, Biological Properties, and Mechanism of Action. Transplant. Proc. 2003, 35, 7S–14S. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mukherjee, U. A Comprehensive Review of Immunosuppression Used for Liver Transplantation. J. Transplant. 2009, 2009, 701464. [Google Scholar] [CrossRef]
- Seto, B. Rapamycin and mTOR: A serendipitous discovery and implications for breast cancer. Clin. Transl. Med. 2012, 1. [Google Scholar] [CrossRef]
- Stenton, S.B.; Partovi, N.; Ensom, M.H.H. Sirolimus: The Evidence for Clinical Pharmacokinetic Monitoring. Clin. Pharmacokinet. 2005, 44, 769–786. [Google Scholar] [CrossRef] [PubMed]
- Billich, A.; Heinrich, A.; Aszódi, A.; Stuetz, A. Percutaneous absorption of drugs used in atopic eczema: Pimecrolimus permeates less through skin than corticosteroids and tacrolimus. Int. J. Pharm. 2004, 269, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Mahalati, K.; Kahan, B. Clinical Pharmacokinetics of Sirolimus. Clin. Pharmacokinet. 2001, 40, 573–585. [Google Scholar] [CrossRef]
- Liu, J.; Farmer, J.D.; Lane, W.S.; Friedman, J.; Weissman, I.; Schreiber, S.L. Calcineurin Is a Common Target of Cyclophilin-Cyclosporin A and FKBP-FK506 Complexes. Cell 1991, 66, 807–815. [Google Scholar] [CrossRef]
- Plager, D.A.; Henke, S.A.; Matsuwaki, Y.; Madaan, A.; Squillace, D.L.; Dierkhising, R.A.; Kita, H. Pimecrolimus Reduces Eosinophil Activation Associated with Calcium Mobilization. Int. Arch. Allergy. Immunol. 2009, 149, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Peters, D.H.; Fitton, A.; Plosker, G.L.; Faulds, D. Tacrolimus: A Review of its Pharmacology, and Therapeutic Potential in Hepatic and Renal Transplantation. Drugs 1993, 46, 746–794. [Google Scholar] [CrossRef]
- Spencer, C.M.; Goa, K.L.; Gillis, J.C. Tacrolimus: An Update of its Pharmacology and Clinical Efficacy in the Management of Organ Transplantation. Drugs 1997, 54, 925–975. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.; Osborne, S.A.; Greig, G.; Hartmann, S.; Ebelin, M.; Burtin, P.; Rappersberger, K.; Komar, M.; Wolff, K. Pharmacokinetics of Pimecrolimus, a Novel Nonsteroid Anti-Inflammatory Drug, After Single and Multiple Oral Administration. Clin. Pharmacokinet. 2003, 42, 1305–1314. [Google Scholar] [CrossRef]
- Zollinger, M.; Waldmeier, F.; Hartmann, S.; Zenke, G.; Zimmerlin, A.G.; Glaenzel, U.; Baldeck, J.-P.; Schweitzer, A.; Berthier, S.; Moenius, T.; et al. Pimecrolimus: Absorption, distribution, metabolism, and excretion in healthy volunteers after a single oral dose and supplementary investigations in vitro. Drug Metab. Dispos. 2006, 34, 765–774. [Google Scholar] [CrossRef]
| Macrolide Class | Compounds | Source * | Example Structure |
|---|---|---|---|
| Trisoxazole-ring-containing macrolides | Kabiramides Ulapualides Halichondramide Jaspisamides Mycalolides | Hexabranchus sp. Hexabranchus sanguineus Chondrosia corticata Jaspis sp. Mycale sp. | Kabiramide C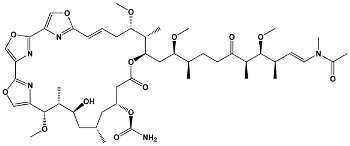 |
| Macrodiolides | Swinholides Ankaraholides | Theonella swinhoei Geitlerinema sp. | Swinholide A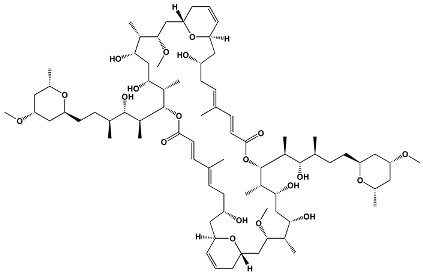 |
| Diterpene macrolactones | Reidispongiolides Sphinxolides | Reidispongia coreulea Neosiphonia superstes | Reidispongiolide A |
| Other macrolides | Jasplakinolides Latrunculins Spongistatin Aplyronine Venturicidins Mycolactone Halichondrins | Jaspis johnstoni Latrunculia magnifica Hyrtios erecta Aplysia kurodai Streptomyces sp. Mycobacterium ulcerans Halichondria okadai | Jasplakinolide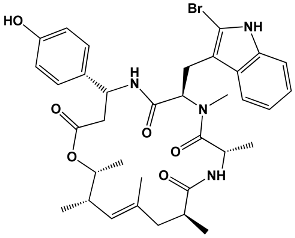 |
| Members in Ring | Compounds | Source | Example Structure |
|---|---|---|---|
| 12 | Methymycin | Streptomyces venezuelae | Methymycin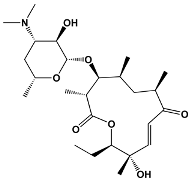 |
| 14 | Erythromycin Pikromycin Oleandomycin | Saccharopolyspora erythraea Streptomyces venezuelae Streptomyces antibioticus | Erythromycin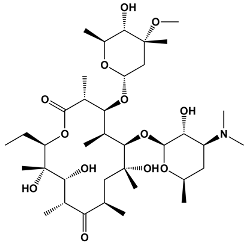 |
| 15 | Azithromycin | Erythromycin derivative | Azithromycin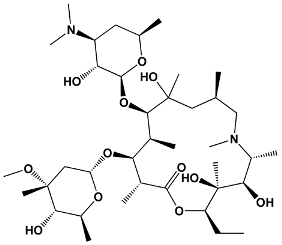 |
| 16 | Spiramycin Tylosin Josamycin | Streptomyces ambofaciens Streptomyces fradiae Streptomyces narbonensis | Josamycin |
| Macrolide Class | Compounds | Source | Example Structure |
|---|---|---|---|
| Avermectins | Ivermectin Selamectin Monoxidectin Abamectin | Avermectins are produced by Streptomyces avermitilis; specific compounds are derivatives | 22,23-dihydroavermectin B1a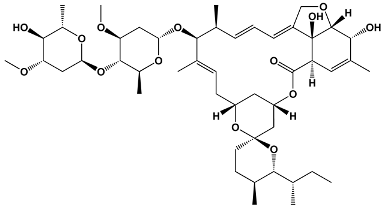 |
| Plecomacrolides | Balticolid | Ascomycetes spp. | Balticolid |
| Oxygenated macrocyclic lactones | Bryostatins | Bugula neritina | Bryostatin 1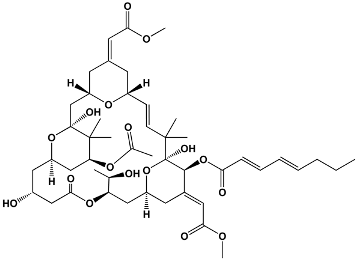 |
| Macrolide Class | Compounds | Source | Example Structure |
|---|---|---|---|
| Polyene | Amphotericin B Nystatin Natamycin Filipin | Streptomyces nodosus Streptomyces noursei Streptomyces natalensis Streptomyces filipinesis | Amphotericin B |
| Salicylate | Cruentaren A | Byssovorax cruenta | Cruentaren A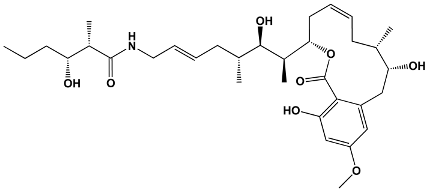 |
| Members in Ring | Compounds | Source | Example Structure |
|---|---|---|---|
| 23 | Tacrolimus Pimecrolimus | Streptomyces tsukabaensis Ascomycin derivative | Tacrolimus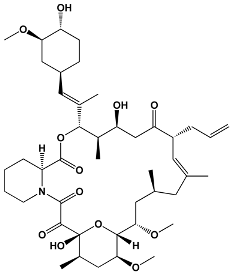 |
| 31 | Sirolimus (Rapamycin) | Streptomyces hygroscopicus | Sirolimus (Rapamycin)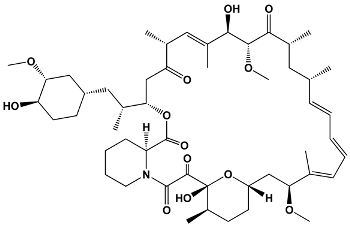 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenz, K.D.; Klosterman, K.E.; Mukundan, H.; Kubicek-Sutherland, J.Z. Macrolides: From Toxins to Therapeutics. Toxins 2021, 13, 347. https://doi.org/10.3390/toxins13050347
Lenz KD, Klosterman KE, Mukundan H, Kubicek-Sutherland JZ. Macrolides: From Toxins to Therapeutics. Toxins. 2021; 13(5):347. https://doi.org/10.3390/toxins13050347
Chicago/Turabian StyleLenz, Kiersten D., Katja E. Klosterman, Harshini Mukundan, and Jessica Z. Kubicek-Sutherland. 2021. "Macrolides: From Toxins to Therapeutics" Toxins 13, no. 5: 347. https://doi.org/10.3390/toxins13050347
APA StyleLenz, K. D., Klosterman, K. E., Mukundan, H., & Kubicek-Sutherland, J. Z. (2021). Macrolides: From Toxins to Therapeutics. Toxins, 13(5), 347. https://doi.org/10.3390/toxins13050347







