The Protein Toxins Ricin and Shiga Toxin as Tools to Explore Cellular Mechanisms of Internalization and Intracellular Transport
Abstract
:1. Introduction
2. Cellular Pathways Exploited by Toxins
2.1. Endocytic Mechanisms and Recycling
2.2. Endosome to Golgi Transport
2.3. Retrograde Toxin Transport from the Golgi to the ER and Translocation to the Cytosol
3. Role of Lipids for Membrane Function and Intracellular Transport
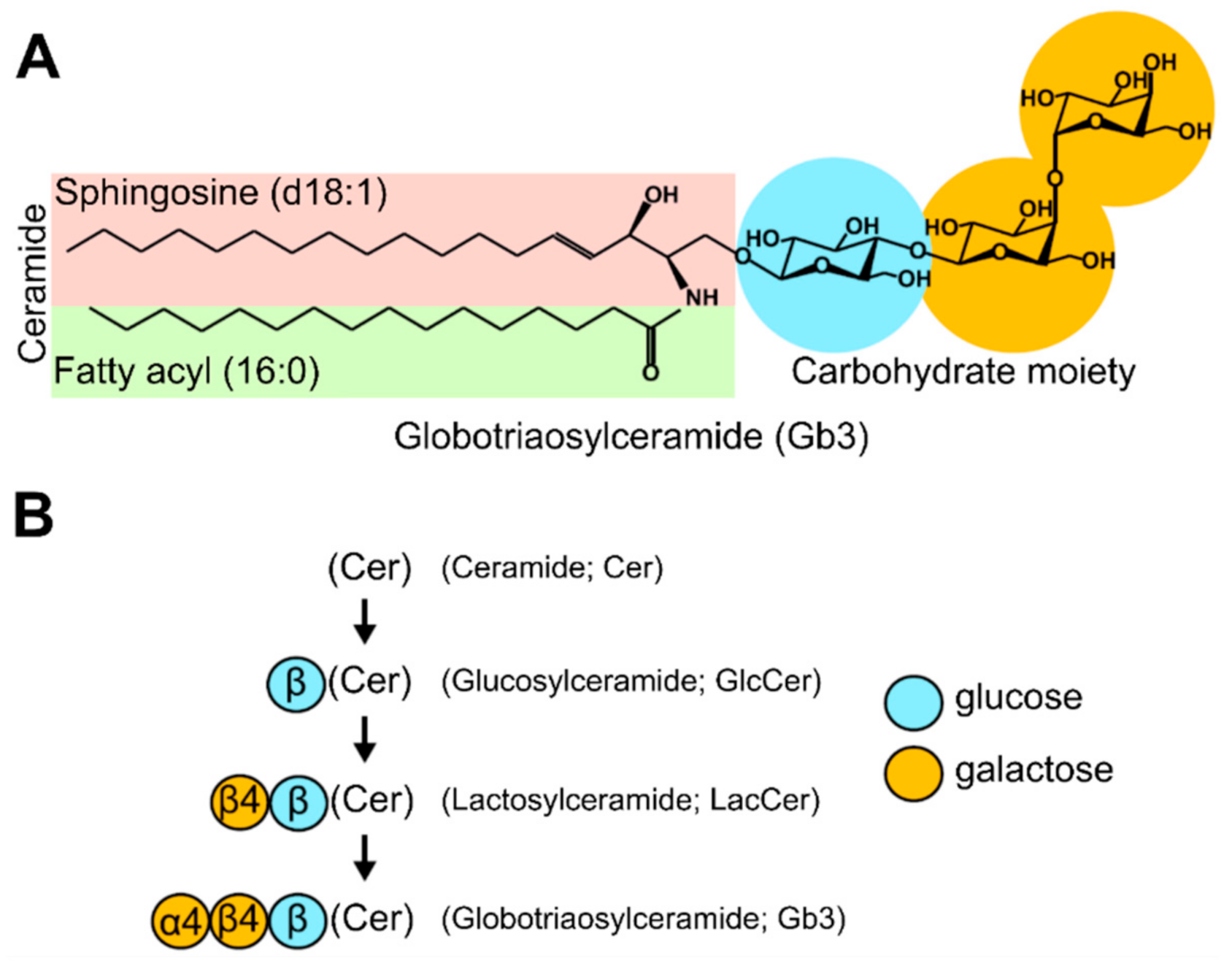
3.1. Lipid Classes and Species
3.2. Interleaflet Coupling
3.3. Methods Used for Quantification of Gb3
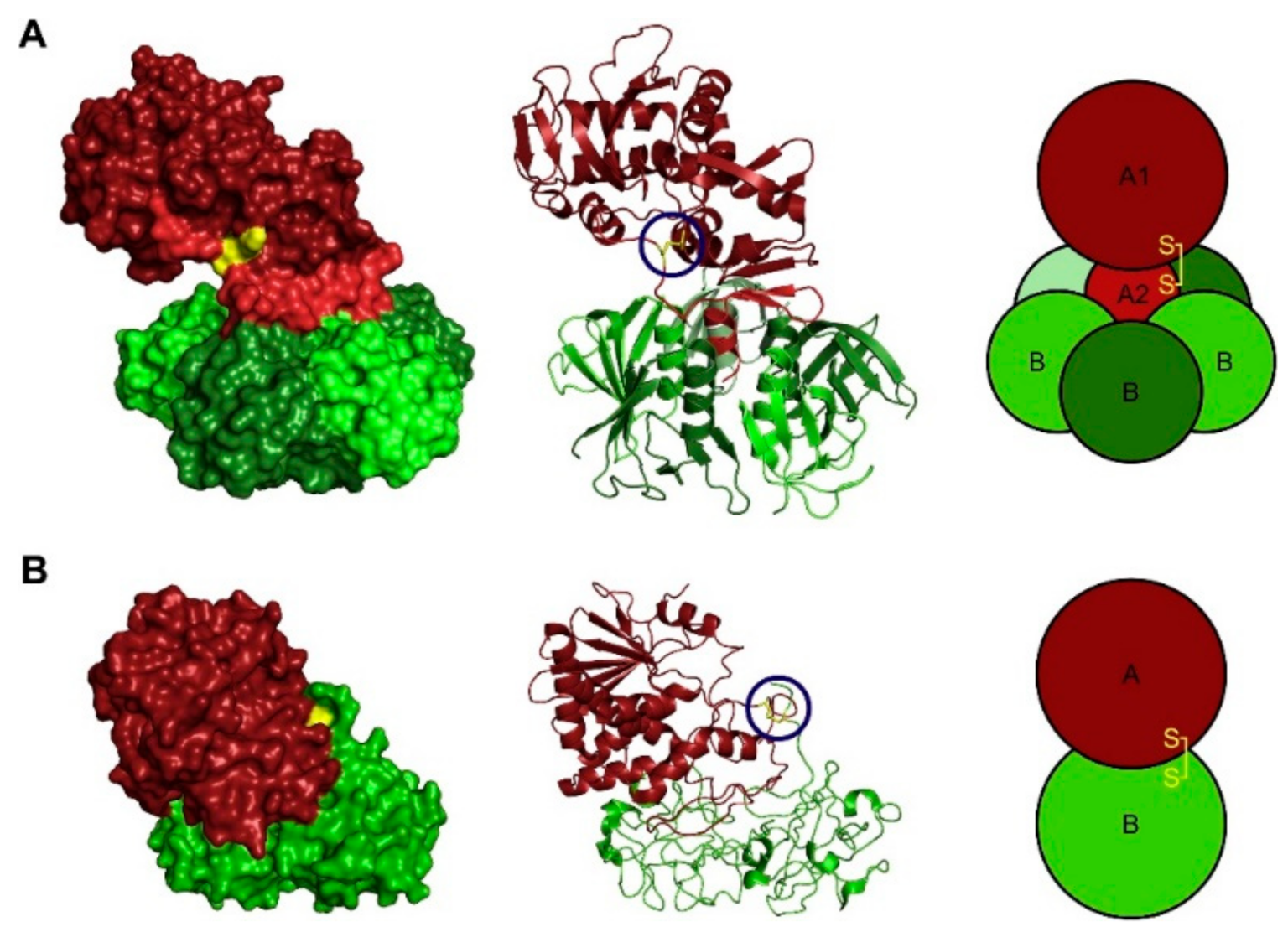
3.4. Binding Sites for Gb3 on Shiga Toxin
3.5. Lipid Rafts
3.6. Signaling into Cells due to Binding of Shiga Toxin to Gb3 in the outer PM Leaflet
3.7. Effect on Endocytosis and/or Retrograde Transport due to Manipulations of the Lipidome
3.7.1. Consequences of Decreasing the Amount of GSLs
3.7.2. Studies Related to Ether Lipids
3.7.3. Effect of Cell Density
3.7.4. Glucose Analogues Induce Protection against Shiga Toxins
3.7.5. Substances Affecting the Membrane Fluidity
3.8. Modifications of the Lipidome: Changes as Expected?
3.9. The Need for Future Studies and a Focus on the Importance of Lipid Species
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sandvig, K.; van Deurs, B. Delivery into cells: Lessons learned from plant and bacterial toxins. Gene Ther. 2005, 12, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Mitsui, K.; Motizuki, M.; Tsurugi, K. The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in the 28 S ribosomal RNA caused by the toxins. J. Biol. Chem. 1987, 262, 5908–5912. [Google Scholar] [CrossRef]
- Endo, Y.; Tsurugi, K.; Yutsudo, T.; Takeda, Y.; Ogasawara, T.; Igarashi, K. Site of action of Vero toxin (VT2) from Escherichia coli 0157:H7 and of Shiga toxin on eukaryotic ribosomes. RNA glycosidase activity of the toxins. Eur. J. Biochem. 1988, 171, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Fraser, M.E.; Chernaia, M.M.; Kozlov, Y.V.; James, M.N. Crystal structure of the holotoxin from Shigella dysenteriae at 2.5 A resolution. Nat. Struct. Biol. 1994, 1, 59–64. [Google Scholar] [CrossRef]
- Rutenber, E.; Katzin, B.J.; Ernst, S.; Collins, E.J.; Mlsna, D.; Ready, M.P.; Robertus, J.D. Crystallographic refinement of ricin to 2.5 Angstroms. Proteins 1991, 10, 240–250. [Google Scholar] [CrossRef]
- Sandvig, K.; Bergan, J.; Kavaliauskiene, S.; Skotland, T. Lipid requirements for entry of protein toxins into cells. Prog. Lipid Res. 2014, 54C, 1–13. [Google Scholar] [CrossRef]
- Pappenheimer, A.M., Jr. Diphtheria toxin. Annu. Rev. Biochem. 1977, 46, 69–94. [Google Scholar] [CrossRef]
- Kim, K.; Groman, N.B. Mode of inhibition of diphtheria toxin by ammonium chloride. J. Bacteriol. 1965, 90, 1557–1562. [Google Scholar] [CrossRef] [Green Version]
- Sandvig, K.; Olsnes, S. Diphtheria toxin entry into cells is facilitated by low pH. J. Cell Biol. 1980, 87, 828–832. [Google Scholar] [CrossRef] [Green Version]
- Draper, R.K.; Simon, M.I. The entry of diphtheria toxin into the mammalian cell cytoplasm: Evidence for lysosomal involvement. J. Cell Biol. 1980, 87, 849–854. [Google Scholar] [CrossRef]
- Sandvig, K.; Olsnes, S. Entry of the toxic proteins abrin, modeccin, ricin, and diphtheria toxin into cells. II. Effect of pH, metabolic inhibitors, and ionophores and evidence for toxin penetration from endocytotic vesicles. J. Biol. Chem. 1982, 257, 7504–7513. [Google Scholar] [CrossRef]
- Sandvig, K.; Garred, Ø.; Prydz, K.; Kozlov, J.V.; Hansen, S.H.; van Deurs, B. Retrograde transport of endocytosed Shiga toxin to the endoplasmic reticulum. Nature 1992, 358, 510–511. [Google Scholar] [CrossRef]
- Rapak, A.; Falnes, P.O.; Olsnes, S. Retrograde transport of mutant ricin to the endoplasmic reticulum with subsequent translocation to cytosol. Proc. Natl. Acad. Sci. USA 1997, 94, 3783–3788. [Google Scholar] [CrossRef] [Green Version]
- Johannes, L.; Tenza, D.; Antony, C.; Goud, B. Retrograde transport of KDEL-bearing B-fragment of Shiga toxin. J. Biol. Chem. 1997, 272, 19554–19561. [Google Scholar] [CrossRef] [Green Version]
- Tekle, C.; Deurs, B.; Sandvig, K.; Iversen, T.G. Cellular trafficking of quantum dot-ligand bioconjugates and their induction of changes in normal routing of unconjugated ligands. Nano. Lett. 2008, 8, 1858–1865. [Google Scholar] [CrossRef]
- Sandvig, K.; Skotland, T.; van Deurs, B.; Klokk, T.I. Retrograde transport of protein toxins through the Golgi apparatus. Histochem. Cell Biol. 2013, 140, 317–326. [Google Scholar] [CrossRef]
- Johannes, L.; Romer, W. Shiga toxins—from cell biology to biomedical applications. Nat. Rev. Microbiol. 2010, 8, 105–116. [Google Scholar] [CrossRef]
- Engedal, N.; Skotland, T.; Torgersen, M.L.; Sandvig, K. Shiga toxin and its use in targeted cancer therapy and imaging. Microbiol. Biotechnol. 2011, 4, 32–46. [Google Scholar] [CrossRef]
- Antignani, A.; Ho, E.C.H.; Bilotta, M.T.; Qiu, R.; Sarnvosky, R.; FitzGerald, D.J. Targeting Receptors on Cancer Cells with Protein Toxins. Biomolecules 2020, 10, 1331. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, S.; Thaker, H.; Dong, M. Shiga Toxins: An Update on Host Factors and Biomedical Applications. Toxins 2021, 13, 222. [Google Scholar] [CrossRef]
- Dhillon, S. Moxetumomab Pasudotox: First Global Approval. Drugs 2018, 78, 1763–1767. [Google Scholar] [CrossRef] [Green Version]
- El Alaoui, A.; Schmidt, F.; Amessou, M.; Sarr, M.; Decaudin, D.; Florent, J.C.; Johannes, L. Shiga toxin-mediated retrograde delivery of a topoisomerase I inhibitor prodrug. Angew. Chem. Int. Ed. Engl. 2007, 46, 6469–6472. [Google Scholar] [CrossRef] [PubMed]
- Kaksonen, M.; Roux, A. Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2018, 19, 313–326. [Google Scholar] [CrossRef]
- Chen, Z.; Schmid, S.L. Evolving models for assembling and shaping clathrin-coated pits. J. Cell Biol. 2020, 219. [Google Scholar] [CrossRef]
- Sathe, M.; Muthukrishnan, G.; Rae, J.; Disanza, A.; Thattai, M.; Scita, G.; Parton, R.G.; Mayor, S. Small GTPases and BAR domain proteins regulate branched actin polymerisation for clathrin and dynamin-independent endocytosis. Nat. Commun. 2018, 9, 1835. [Google Scholar] [CrossRef]
- Casamento, A.; Boucrot, E. Molecular mechanism of Fast Endophilin-Mediated Endocytosis. Biochem. J. 2020, 477, 2327–2345. [Google Scholar] [CrossRef]
- Sandvig, K.; Kavaliauskiene, S.; Skotland, T. Clathrin-independent endocytosis: An increasing degree of complexity. Histochem. Cell Biol. 2018, 150, 107–118. [Google Scholar] [CrossRef] [Green Version]
- Sandvig, K.; Olsnes, S.; Pihl, A. Kinetics of binding of the toxic lectins abrin and ricin to surface receptors of human cells. J. Biol. Chem. 1976, 251, 3977–3984. [Google Scholar] [CrossRef]
- Sandvig, K.; Olsnes, S. Effect of temperature on the uptake, excretion and degradation of abrin and ricin by HeLa cells. Exp. Cell Res. 1979, 121, 15–25. [Google Scholar] [CrossRef]
- Sandvig, K. Cell density affects the binding of the toxic lectin abrin to HeLa cells in monolayer cultures. FEBS. Lett. 1978, 89, 233–236. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, J. Cell contact induces an increase in pinocytotic rate in cultured epithelial cells. Nature 1976, 263, 596–597. [Google Scholar] [CrossRef] [PubMed]
- Snijder, B.; Sacher, R.; Ramo, P.; Damm, E.M.; Liberali, P.; Pelkmans, L. Population context determines cell-to-cell variability in endocytosis and virus infection. Nature 2009, 461, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Kavaliauskiene, S.; Nymark, C.M.; Bergan, J.; Simm, R.; Sylvänne, T.; Simolin, H.; Ekroos, K.; Skotland, T.; Sandvig, K. Cell density induced changes in lipid composition and intracellular trafficking. Cell. Mol. Life Sci. 2014, 71, 1097–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frechin, M.; Stoeger, T.; Daetwyler, S.; Gehin, C.; Battich, N.; Damm, E.M.; Stergiou, L.; Riezman, H.; Pelkmans, L. Cell-intrinsic adaptation of lipid composition to local crowding drives social behaviour. Nature 2015, 523, 88–91. [Google Scholar] [CrossRef]
- Sandvig, K.; Olsnes, S.; Pihl, A. Binding, uptake and degradation of the toxic proteins abrin and ricin by toxin-resistant cell variants. Eur. J. Biochem. 1978, 82, 13–23. [Google Scholar] [CrossRef]
- Weeratunga, S.; Paul, B.; Collins, B.M. Recognising the signals for endosomal trafficking. Curr. Opin. Cell Biol. 2020, 65, 17–27. [Google Scholar] [CrossRef]
- Montesano, R.; Roth, J.; Robert, A.; Orci, L. Non-coated membrane invaginations are involved in binding internalization of cholera and tetanus toxins. Nature 1982, 296, 651–653. [Google Scholar] [CrossRef]
- Parton, R.G.; McMahon, K.A.; Wu, Y. Caveolae: Formation, dynamics, and function. Curr. Opin. Cell Biol. 2020, 65, 8–16. [Google Scholar] [CrossRef]
- Dixon, S.J.; Stewart, D.; Grinstein, S.; Spiegel, S. Transmembrane signaling by the B subunit of cholera toxin: Increased cytoplasmic free calcium in rat lymphocytes. J. Cell Biol. 1987, 105, 1153–1161. [Google Scholar] [CrossRef] [Green Version]
- Gouy, H.; Deterre, P.; Debre, P.; Bismuth, G. Cell calcium signaling via GM1 cell surface gangliosides in the human Jurkat T cell line. J. Immunol. 1994, 152, 3271–3281. [Google Scholar]
- Klokk, T.I.; Kavaliauskiene, S.; Sandvig, K. Cross-linking of glycosphingolipids at the plasma membrane: Consequences for intracellular signaling and traffic. Cell. Mol. Life Sci. 2016, 73, 1301–1316. [Google Scholar] [CrossRef]
- Torgersen, M.L.; Skretting, G.; van Deurs, B.; Sandvig, K. Internalization of cholera toxin by different endocytic mechanisms. J. Cell Sci. 2001, 114, 3737–3747. [Google Scholar] [CrossRef]
- Nichols, B.J.; Kenworthy, A.K.; Polishchuk, R.S.; Lodge, R.; Roberts, T.H.; Hirschberg, K.; Phair, R.D.; Lippincott-Schwartz, J. Rapid Cycling of Lipid Raft Markers between the Cell Surface and Golgi Complex. J. Cell Biol. 2001, 153, 529–542. [Google Scholar] [CrossRef]
- Shogomori, H.; Futerman, A.H. Cholera Toxin Is Found in Detergent-insoluble Rafts/Domains at the Cell Surface of Hippocampal Neurons but Is Internalized via a Raft-independent Mechanism. J. Biol. Chem. 2001, 276, 9182–9188. [Google Scholar] [CrossRef] [Green Version]
- Hommelgaard, A.M.; Roepstorff, K.; Vilhardt, F.; Torgersen, M.L.; Sandvig, K.; van Deurs, B. Caveolae: Stable membrane domains with a potential for internalization. Traffic 2005, 6, 720–724. [Google Scholar] [CrossRef]
- Pelkmans, L.; Kartenbeck, J.; Helenius, A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 2001, 3, 473–483. [Google Scholar] [CrossRef]
- Hayer, A.; Stoeber, M.; Ritz, D.; Engel, S.; Meyer, H.H.; Helenius, A. Caveolin-1 is ubiquitinated and targeted to intralumenal vesicles in endolysosomes for degradation. J. Cell Biol. 2010, 191, 615–629. [Google Scholar] [CrossRef] [Green Version]
- Parton, R.G.; Howes, M.T. Revisiting caveolin trafficking: The end of the caveosome. J. Cell Biol. 2010, 191, 439–441. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, Y.; Wang, M.; Zhang, C.; Zhang, T.; Zhou, H.; Zhao, W.; Zhao, W.; Xia, G.; Shao, R. Endocytosis and Organelle Targeting of Nanomedicines in Cancer Therapy. Int. J. Nanomed. 2020, 15, 9447–9467. [Google Scholar] [CrossRef]
- Pelkmans, L.; Puntener, D.; Helenius, A. Local Actin Polymerization and Dynamin Recruitment in SV40-Induced Internalization of Caveolae. Science 2002, 296, 535–539. [Google Scholar] [CrossRef]
- Damm, E.M.; Pelkmans, L.; Kartenbeck, J.; Mezzacasa, A.; Kurzchalia, T.; Helenius, A. Clathrin- and caveolin-1-independent endocytosis: Entry of simian virus 40 into cells devoid of caveolae. J. Cell Biol. 2005, 168, 477–488. [Google Scholar] [CrossRef]
- Lajoie, P.; Nabi, I.R. Lipid rafts, caveolae, and their endocytosis. Int. Rev. Cell Mol. Biol. 2010, 282, 135–163. [Google Scholar] [CrossRef]
- Del Pozo, M.A.; Lolo, F.N.; Echarri, A. Caveolae: Mechanosensing and mechanotransduction devices linking membrane trafficking to mechanoadaptation. Curr. Opin. Cell Biol. 2021, 68, 113–123. [Google Scholar] [CrossRef]
- Larkin, J.M.; Brown, M.S.; Goldstein, J.L.; Anderson, R.G.W. Depletion of intracellular potassium arrests coated pit formation and receptor-mediated endocytosis in fibroblasts. Cell 1983, 33, 273–285. [Google Scholar] [CrossRef]
- Moya, M.; Dautry-Varsat, A.; Goud, B.; Louvard, D.; Boquet, P. Inhibition of coated pit formation in Hep2 cells blocks the cytotoxicity of diphtheria toxin but not that of ricin. J. Cell Biol. 1985, 101, 548–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandvig, K.; Olsnes, S.; Petersen, O.W.; van Deurs, B. Acidification of the cytosol inhibits endocytosis from coated pits. J. Cell Biol. 1987, 105, 679–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doxsey, S.J.; Brodsky, F.M.; Blank, G.S.; Helenius, A. Inhibition of endocytosis by anti-clathrin antibodies. Cell 1987, 50, 453–463. [Google Scholar] [CrossRef]
- Hansen, S.H.; Sandvig, K.; van Deurs, B. The preendosomal compartment comprises distinct coated and noncoated endocytic vesicle populations. J. Cell Biol. 1991, 113, 731–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damke, H.; Baba, T.; van der Bliek, A.M.; Schmid, S.L. Clathrin-independent pinocytosis is induced in cells overexpressing a temperature-sensitive mutant of dynamin. J. Cell Biol. 1995, 131, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Damke, H.; Baba, T.; Warnock, D.E.; Schmid, S.L. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol. 1994, 127, 915–934. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.J.; Singh, R.D.; Holicky, E.L.; Wheatley, C.L.; Marks, D.L.; Pagano, R.E. Co-regulation of caveolar and Cdc42-dependent fluid phase endocytosis by phosphocaveolin-1. J. Biol. Chem. 2010, 285, 15119–15125. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, N.; Gomez, G.A.; Howes, M.T.; Lo, H.P.; McMahon, K.A.; Rae, J.A.; Schieber, N.L.; Hill, M.M.; Gaus, K.; Yap, A.S.; et al. Endocytic crosstalk: Cavins, caveolins, and caveolae regulate clathrin-independent endocytosis. PLoS Biol. 2014, 12, e1001832. [Google Scholar] [CrossRef] [Green Version]
- Renard, H.F.; Boucrot, E. Unconventional endocytic mechanisms. Curr. Opin. Cell Biol. 2021, 71, 120–129. [Google Scholar] [CrossRef]
- Rothberg, K.G.; Ying, Y.S.; Kamen, B.A.; Anderson, R.G. Cholesterol controls the clustering of glycosphingolipid-anchored membrane receptor for 5-methyltetrahydrofolate. J. Cell Biol. 1990, 111, 2931–2938. [Google Scholar] [CrossRef] [Green Version]
- Klein, U.; Gimpl, G.; Fahrenholz, F. Alteration of the myometrial plasma membrane cholesterol content with beta-cyclodextrin modulates the binding affinity of the oxytocin receptor. Biochemistry 1995, 34, 13784–13793. [Google Scholar] [CrossRef]
- Grimmer, S.; van Deurs, B.; Sandvig, K. Membrane ruffling and macropinocytosis require cholesterol. J. Cell Sci. 2002, 115, 2953–2962. [Google Scholar] [CrossRef]
- Rodal, S.K.; Skretting, G.; Garred, Ø.; Vilhardt, F.; van Deurs, B.; Sandvig, K. Extraction of cholesterol with methyl-b-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol. Biol. Cell 1999, 10, 961–974. [Google Scholar] [CrossRef]
- Subtil, A.; Gaidarov, I.; Kobylarz, K.; Lampson, M.A.; Keen, J.H.; McGraw, T.E. Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc. Natl. Acad. Sci. USA 1999, 96, 6775–6780. [Google Scholar] [CrossRef] [Green Version]
- Watkins, E.B.; Majewski, J.; Chi, E.Y.; Gao, H.; Florent, J.C.; Johannes, L. Shiga Toxin Induces Lipid Compression: A Mechanism for Generating Membrane Curvature. Nano Lett. 2019, 19, 7365–7369. [Google Scholar] [CrossRef]
- Johannes, L. Shiga Toxin-A Model for Glycolipid-Dependent and Lectin-Driven Endocytosis. Toxins 2017, 9, 340. [Google Scholar] [CrossRef]
- Bergan, J.; Dyve Lingelem, A.B.; Simm, R.; Skotland, T.; Sandvig, K. Shiga toxins. Toxicon 2012, 60, 1085–1107. [Google Scholar] [CrossRef]
- Kavaliauskiene, S.; Dyve Lingelem, A.B.; Skotland, T.; Sandvig, K. Protection against Shiga Toxins. Toxins 2017, 9, 44. [Google Scholar] [CrossRef]
- Sandvig, K.; Olsnes, S.; Brown, J.E.; Petersen, O.W.; van Deurs, B. Endocytosis from coated pits of Shiga toxin: A glycolipid- binding protein from Shigella dysenteriae 1. J. Cell Biol. 1989, 108, 1331–1343. [Google Scholar] [CrossRef] [Green Version]
- Schapiro, F.B.; Lingwood, C.; Furuya, W.; Grinstein, S. pH-independent retrograde targeting of glycolipids to the Golgi complex. Am. J. Physiol. 1998, 274, C319–C332. [Google Scholar] [CrossRef]
- Lauvrak, S.U.; Torgersen, M.L.; Sandvig, K. Efficient endosome-to-Golgi transport of Shiga toxin is dependent on dynamin and clathrin. J. Cell Sci. 2004, 117, 2321–2331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauvrak, S.U.; Wälchli, S.; Slagsvold, H.H.; Torgersen, M.L.; Spilsberg, B.; Sandvig, K. Shiga toxin regulates its entry in a Syk-dependent manner. Mol. Biol. Cell 2006, 17, 1096–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katagiri, Y.U.; Mori, T.; Nakajima, H.; Katagiri, C.; Taguchi, T.; Takeda, T.; Kiyokawa, N.; Fujimoto, J. Activation of Src family kinase yes induced by Shiga toxin binding to globotriaosyl ceramide (Gb3/CD77) in low density, detergent-insoluble microdomains. J. Biol. Chem. 1999, 274, 35278–35282. [Google Scholar] [CrossRef] [Green Version]
- Mori, T.; Kiyokawa, N.; Katagiri, Y.U.; Taguchi, T.; Suzuki, T.; Sekino, T.; Sato, N.; Ohmi, K.; Nakajima, H.; Takeda, T.; et al. Globotriaosyl ceramide (CD77/Gb3) in the glycolipid-enriched membrane domain participates in B-cell receptor-mediated apoptosis by regulating lyn kinase activity in human B cells. Exp. Hematol. 2000, 28, 1260–1268. [Google Scholar] [CrossRef]
- Utskarpen, A.; Massol, R.; van Deurs, B.; Lauvrak, S.U.; Kirchhausen, T.; Sandvig, K. Shiga toxin increases formation of clathrin-coated pits through Syk kinase. PLoS ONE 2010, 5, e70944. [Google Scholar] [CrossRef] [Green Version]
- Torgersen, M.L.; Lauvrak, S.U.; Sandvig, K. Shiga toxin A-chain stimulates clathrin-dependent uptake of the toxin. FEBS J. 2005, 272, 4103–4113. [Google Scholar] [CrossRef]
- Pascolutti, R.; Algisi, V.; Conte, A.; Raimondi, A.; Pasham, M.; Upadhyayula, S.; Gaudin, R.; Maritzen, T.; Barbieri, E.; Caldieri, G.; et al. Molecularly Distinct Clathrin-Coated Pits Differentially Impact EGFR Fate and Signaling. Cell Rep. 2019, 27, 3049–3061.e3046. [Google Scholar] [CrossRef] [Green Version]
- Skotland, T.; Sandvig, K. The role of PS 18:0/18:1 in membrane function. Nat. Commun. 2019, 10, 2752. [Google Scholar] [CrossRef]
- Varga, K.; Jiang, Z.J.; Gong, L.W. Phosphatidylserine is critical for vesicle fission during clathrin-mediated endocytosis. J. Neurochem. 2020, 152, 48–60. [Google Scholar] [CrossRef]
- Römer, W.; Berland, L.; Chambon, V.; Gaus, K.; Windschiegl, B.; Tenza, D.; Aly, M.R.; Fraisier, V.; Florent, J.C.; Perrais, D.; et al. Shiga toxin induces tubular membrane invaginations for its uptake into cells. Nature 2007, 450, 670–675. [Google Scholar] [CrossRef]
- Lippincott-Schwartz, J.; Yuan, L.C.; Bonifacino, J.S.; Klausner, R.D. Rapid redistribution of Golgi proteins into the ER in cells treated with Brefeldin A: Evidence for membrane cycling from Golgi to ER. Cell 1989, 56, 801–813. [Google Scholar] [CrossRef]
- Fujiwara, T.; Oda, K.; Yokota, S.; Takatsuki, A.; Ikehara, Y. Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J. Biol. Chem. 1988, 263, 18545–18552. [Google Scholar] [CrossRef]
- Doms, R.W.; Russ, G.; Yewdell, J.W. Brefeldin A redistributes resident and itinerant Golgi proteins to the endoplasmic reticulum. J. Cell Biol. 1989, 109, 61–72. [Google Scholar] [CrossRef]
- Hunziker, W.; Whitney, J.A.; Mellman, I. Selective inhibition of transcytosis by brefeldin A in MDCK cells. Cell 1991, 67, 617–627. [Google Scholar] [CrossRef]
- Ktistakis, N.T.; Roth, M.G.; Bloom, G.S. PtK1 cells contain a nondiffusible, dominant factor that makes the Golgi apparatus resistant to brefeldin A. J. Cell Biol. 1991, 113, 1009–1023. [Google Scholar] [CrossRef] [Green Version]
- Sandvig, K.; Prydz, K.; Hansen, S.H.; van Deurs, B. Ricin transport in brefeldin A treated cells: Correlation between Golgi structure and toxic effect. J. Cell Biol. 1991, 115, 971–981. [Google Scholar] [CrossRef]
- Iversen, T.-G.; Skretting, G.; Llorente, A.; Nicoziani, P.; van Deurs, B.; Sandvig, K. Endosome to Golgi transport of ricin is independent of clathrin and of the Rab9- and Rab11-GTPases. Mol. Biol. Cell 2001, 12, 2099–2107. [Google Scholar] [CrossRef] [Green Version]
- Mallard, F.; Antony, C.; Tenza, D.; Salamero, J.; Goud, B.; Johannes, L. Direct pathway from early/recycling endosomes to the Golgi apparatus revealed through the study of shiga toxin B-fragment transport. J. Cell Biol. 1998, 143, 973–990. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Linstedt, A.D. Manganese blocks intracellular trafficking of Shiga toxin and protects against Shiga toxicosis. Science 2012, 335, 332–335. [Google Scholar] [CrossRef] [Green Version]
- Mukhopadhyay, S.; Redler, B.; Linstedt, A.D. Shiga toxin-binding site for host cell receptor GPP130 reveals unexpected divergence in toxin-trafficking mechanisms. Mol. Biol. Cell 2013, 24, 2311–2318. [Google Scholar] [CrossRef]
- Li, D.; Selyunin, A.; Mukhopadhyay, S. Targeting the Early Endosome-to-Golgi Transport of Shiga Toxins as a Therapeutic Strategy. Toxins 2020, 12, 342. [Google Scholar] [CrossRef]
- Sowa-Rogozinska, N.; Sominka, H.; Nowakowska-Golacka, J.; Sandvig, K.; Slominska-Wojewodzka, M. Intracellular Transport and Cytotoxicity of the Protein Toxin Ricin. Toxins 2019, 11, 350. [Google Scholar] [CrossRef] [Green Version]
- Mallard, F.; Tang, B.L.; Galli, T.; Tenza, D.; Saint-Pol, A.; Yue, X.; Antony, C.; Hong, W.; Goud, B.; Johannes, L. Early/recycling endosomes-to-TGN transport involves two SNARE complexes and a Rab6 isoform. J. Cell Biol. 2002, 156, 653–664. [Google Scholar] [CrossRef] [Green Version]
- del Nery, E.; Miserey-Lenkei, S.; Falguieres, T.; Nizak, C.; Johannes, L.; Perez, F.; Goud, B. Rab6A and Rab6A′ GTPases play non-overlapping roles in membrane trafficking. Traffic 2006, 7, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Utskarpen, A.; Slagsvold, H.H.; Iversen, T.-G.; Wälchli, S.; Sandvig, K. Retrograde transport of ricin is regulated by Rab6A/A′ in a sequential manner. Traffic 2006, 7, 663–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauvrak, S.U.; Llorente, A.; Iversen, T.-G.; Sandvig, K. Selective regulation of the Rab9-independent transport of ricin to the Golgi apparatus by calcium. J. Cell Sci. 2002, 115, 3449–3456. [Google Scholar] [CrossRef] [PubMed]
- Skånland, S.S.; Wälchli, S.; Wandinger-Ness, A.; Sandvig, K. Phosphoinositide-regulated retrograde transport of ricin: Crosstalk between hVps34 and sorting nexins. Traffic 2007, 8, 297–309. [Google Scholar] [CrossRef] [Green Version]
- Utskarpen, A.; Slagsvold, H.H.; Dyve, A.B.; Skanland, S.S.; Sandvig, K. SNX1 and SNX2 mediate retrograde transport of Shiga toxin. Biochem. Biophys. Res. Comm. 2007, 358, 566–570. [Google Scholar] [CrossRef]
- Bujny, M.V.; Popoff, V.; Johannes, L.; Cullen, P.J. The retromer component sorting nexin-1 is required for efficient retrograde transport of Shiga toxin from early endosome to the trans Golgi network. J. Cell Sci. 2007, 120, 2010–2021. [Google Scholar] [CrossRef] [Green Version]
- Popoff, V.; Mardones, G.A.; Tenza, D.; Rojas, R.; Lamaze, C.; Bonifacino, J.S.; Raposo, G.; Johannes, L. The retromer complex and clathrin define an early endosomal retrograde exit site. J. Cell Sci. 2007, 120, 2022–2031. [Google Scholar] [CrossRef] [Green Version]
- Lieu, Z.Z.; Gleeson, P.A. Identification of different itineraries and retromer components for endosome-to-Golgi transport of TGN38 and Shiga toxin. Eur. J. Cell Biol. 2010, 89, 379–393. [Google Scholar] [CrossRef]
- McKenzie, J.E.; Raisley, B.; Zhou, X.; Naslavsky, N.; Taguchi, T.; Caplan, S.; Sheff, D. Retromer guides STxB and CD8-M6PR from early to recycling endosomes, EHD1 guides STxB from recycling endosome to Golgi. Traffic 2012, 13, 1140–1159. [Google Scholar] [CrossRef] [Green Version]
- Jing, J.; Junutula, J.R.; Wu, C.; Burden, J.; Matern, H.; Peden, A.A.; Prekeris, R. FIP1/RCP binding to Golgin-97 regulates retrograde transport from recycling endosomes to the trans-Golgi network. Mol. Biol. Cell 2010, 21, 3041–3053. [Google Scholar] [CrossRef] [Green Version]
- Lieu, Z.Z.; Derby, M.C.; Teasdale, R.D.; Hart, C.; Gunn, P.; Gleeson, P.A. The golgin GCC88 is required for efficient retrograde transport of cargo from the early endosomes to the trans-Golgi network. Mol. Biol. Cell 2007, 18, 4979–4991. [Google Scholar] [CrossRef] [Green Version]
- Arakel, E.C.; Schwappach, B. Formation of COPI-coated vesicles at a glance. J. Cell Sci. 2018, 131. [Google Scholar] [CrossRef] [Green Version]
- Luo, P.M.; Boyce, M. Directing Traffic: Regulation of COPI Transport by Post-translational Modifications. Front. Cell Dev. Biol. 2019, 7, 190. [Google Scholar] [CrossRef]
- Girod, A.; Storrie, B.; Simpson, J.C.; Johannes, L.; Goud, B.; Roberts, L.M.; Lord, J.M.; Nilsson, T.; Pepperkok, R. Evidence for a COP-I-independent transport route from the Golgi complex to the endoplasmic reticulum. Nature Cell Biol. 1999, 1, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.E.; Simpson, J.C.; Girod, A.; Pepperkok, R.; Roberts, L.M.; Lord, J.M. The KDEL retrieval system is exploited by Pseudomonas exotoxin A, but not by Shiga-like toxin-1, during retrograde transport from the Golgi complex to the endoplasmic reticulum. J. Cell Sci. 1999, 112, 467–475. [Google Scholar] [CrossRef] [PubMed]
- White, J.; Johannes, L.; Mallard, F.; Girod, A.; Grill, S.; Reinsch, S.; Keller, P.; Tzschaschel, B.; Echard, A.; Goud, B.; et al. Rab6 coordinates a novel Golgi to ER retrograde transport pathway in live cells. J. Cell Biol. 1999, 147, 743–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassik, M.C.; Kampmann, M.; Lebbink, R.J.; Wang, S.; Hein, M.Y.; Poser, I.; Weibezahn, J.; Horlbeck, M.A.; Chen, S.; Mann, M.; et al. A systematic Mammalian genetic interaction map reveals pathways underlying ricin susceptibility. Cell 2013, 152, 909–922. [Google Scholar] [CrossRef] [Green Version]
- Moreau, D.; Kumar, P.; Wang, S.C.; Chaumet, A.; Chew, S.Y.; Chevalley, H.; Bard, F. Genome-wide RNAi screens identify genes required for Ricin and PE intoxications. Dev. Cell 2011, 21, 231–244. [Google Scholar] [CrossRef] [Green Version]
- Garred, Ø.; van Deurs, B.; Sandvig, K. Furin-induced cleavage and activation of Shiga toxin. J. Biol. Chem. 1995, 270, 10817–10821. [Google Scholar] [CrossRef] [Green Version]
- Llorente, A.; Lauvrak, S.U.; van Deurs, B.; Sandvig, K. Induction of direct endosome to endoplasmic reticulum transport in Chinese hamster ovary (CHO) cells (LdlF) with a temperature-sensitive defect in epsilon-coatomer protein (epsilon-COP). J. Biol. Chem. 2003, 278, 35850–35855. [Google Scholar] [CrossRef] [Green Version]
- Wesche, J.; Rapak, A.; Olsnes, S. Dependence of ricin toxicity on translocation of the toxin A-chain from the endoplasmic reticulum to the cytosol. J. Biol. Chem. 1999, 274, 3443–3449. [Google Scholar] [CrossRef] [Green Version]
- Simpson, J.C.; Roberts, L.M.; Römisch, K.; Davey, J.; Wolf, D.H.; Lord, J.M. Ricin A chain utilises the endoplasmic reticulum-associated protein degradation pathway to enter the cytosol of yeast. FEBS Lett. 1999, 459, 80–84. [Google Scholar] [CrossRef] [Green Version]
- Slominska-Wojewodzka, M.; Gregers, T.F.; Wälchli, S.; Sandvig, K. EDEM is involved in translocation of ricin from the endoplasmic reticulum to the cytosol. Mol. Biol. Cell 2006, 17, 1664–1675. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Haslam, D.B. Shiga toxin is transported from the endoplasmic reticulum following interaction with the luminal chaperone HEDJ/ERdj3. Infect. Immun. 2005, 73, 2524–2532. [Google Scholar] [CrossRef] [Green Version]
- Forrester, A.; Rathjen, S.J.; Daniela Garcia-Castillo, M.; Bachert, C.; Couhert, A.; Tepshi, L.; Pichard, S.; Martinez, J.; Munier, M.; Sierocki, R.; et al. Functional dissection of the retrograde Shiga toxin trafficking inhibitor Retro-2. Nat. Chem. Biol. 2020, 16, 327–336. [Google Scholar] [CrossRef]
- Stechmann, B.; Bai, S.K.; Gobbo, E.; Lopez, R.; Merer, G.; Pinchard, S.; Panigai, L.; Tenza, D.; Raposo, G.; Beaumelle, B.; et al. Inhibition of retrograde transport protects mice from lethal ricin challenge. Cell 2010, 141, 231–242. [Google Scholar] [CrossRef]
- Morgens, D.W.; Chan, C.; Kane, A.J.; Weir, N.R.; Li, A.; Dubreuil, M.M.; Tsui, C.K.; Hess, G.T.; Lavertu, A.; Han, K.; et al. Retro-2 protects cells from ricin toxicity by inhibiting ASNA1-mediated ER targeting and insertion of tail-anchored proteins. Elife 2019, 8. [Google Scholar] [CrossRef]
- Norlin, S.; Parekh, V.S.; Naredi, P.; Edlund, H. Asna1/TRC40 Controls beta-Cell Function and Endoplasmic Reticulum Homeostasis by Ensuring Retrograde Transport. Diabetes 2016, 65, 110–119. [Google Scholar] [CrossRef] [Green Version]
- Simpson, J.C.; Dascher, C.; Roberts, L.M.; Lord, J.M.; Balch, W.E. Ricin cytotoxicity is sensitive to recycling between the endoplasmic reticulum and the Golgi complex. J. Biol. Chem. 1995, 270, 20078–20083. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, A.; Capalbo, A.; Iyengar, N.R.; Rizzo, R.; di Campli, A.; Di Martino, R.; Lo Monte, M.; Beccari, A.R.; Yerudkar, A.; Del Vecchio, C.; et al. Auto-regulation of Secretory Flux by Sensing and Responding to the Folded Cargo Protein Load in the Endoplasmic Reticulum. Cell 2019, 176, 1461–1476.e23. [Google Scholar] [CrossRef] [Green Version]
- Shevchenko, A.; Simons, K. Lipidomics: Coming to grips with lipid diversity. Nat. Rev. Mol. Cell Biol. 2010, 11, 593–598. [Google Scholar] [CrossRef]
- Jung, H.R.; Sylvanne, T.; Koistinen, K.M.; Tarasov, K.; Kauhanen, D.; Ekroos, K. High throughput quantitative molecular lipidomics. Biochim. Biophys. Acta 2011, 1811, 925–934. [Google Scholar] [CrossRef]
- Harayama, T.; Riezman, H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol. 2018, 19, 281–296. [Google Scholar] [CrossRef]
- Hanada, K. Lipid transfer proteins rectify inter-organelle flux and accurately deliver lipids at membrane contact sites. J. Lipid Res. 2018, 59, 1341–1366. [Google Scholar] [CrossRef] [Green Version]
- Skotland, T.; Kavaliauskiene, S.; Sandvig, K. The role of lipid species in membranes and cancer-related changes. Cancer Metastasis Rev. 2020, 39, 343–360. [Google Scholar] [CrossRef] [Green Version]
- Merrill, A.H., Jr. Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chem. Rev. 2011, 111, 6387–6422. [Google Scholar] [CrossRef]
- Park, J.W.; Park, W.J.; Futerman, A.H. Ceramide synthases as potential targets for therapeutic intervention in human diseases. Biochim. Biophys. Acta 2014, 1841, 671–681. [Google Scholar] [CrossRef]
- Nilsson, O.; Svennerholm, L. Characterization and quantitative determination of gangliosides and neutral glycosphingolipids in human liver. J. Lipid Res. 1982, 23, 327–334. [Google Scholar] [CrossRef]
- Brandel, A.; Aigal, S.; Lagies, S.; Schlimpert, M.; Melendez, A.V.; Xu, M.; Lehmann, A.; Hummel, D.; Fisch, D.; Madl, J.; et al. The Gb3-enriched CD59/flotillin plasma membrane domain regulates host cell invasion by Pseudomonas aeruginosa. Cell. Mol. Life Sci. 2021. [Google Scholar] [CrossRef]
- Devaux, P.F.; Morris, R. Transmembrane asymmetry and lateral domains in biological membranes. Traffic 2004, 5, 241–246. [Google Scholar] [CrossRef]
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Steck, T.L.; Lange, Y. Transverse distribution of plasma membrane bilayer cholesterol: Picking sides. Traffic 2018, 19, 750–760. [Google Scholar] [CrossRef] [Green Version]
- Skotland, T.; Sagini, K.; Sandvig, K.; Llorente, A. An emerging focus on lipids in extracellular vesicles. Adv. Drug Deliv. Rev. 2020, 159, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Rog, T.; Orlowski, A.; Llorente, A.; Skotland, T.; Sylvanne, T.; Kauhanen, D.; Ekroos, K.; Sandvig, K.; Vattulainen, I. Interdigitation of long-chain sphingomyelin induces coupling of membrane leaflets in a cholesterol dependent manner. Biochim. Biophys. Acta 2016, 1858, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, T.; Parmryd, I. Interleaflet Coupling, Pinning, and Leaflet Asymmetry-Major Players in Plasma Membrane Nanodomain Formation. Front. Cell Dev. Biol. 2017, 4, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rog, T.; Vattulainen, I. Cholesterol, sphingolipids, and glycolipids: What do we know about their role in raft-like membranes? Chem. Phys. Lipids 2014, 184, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Nickels, J.D.; Smith, J.C.; Cheng, X. Lateral organization, bilayer asymmetry, and inter-leaflet coupling of biological membranes. Chem. Phys. Lipids 2015, 192, 87–99. [Google Scholar] [CrossRef] [Green Version]
- Lingwood, D.; Binnington, B.; Rog, T.; Vattulainen, I.; Grzybek, M.; Coskun, U.; Lingwood, C.A.; Simons, K. Cholesterol modulates glycolipid conformation and receptor activity. Nat. Chem. Biol. 2011, 7, 260–262. [Google Scholar] [CrossRef]
- Yahi, N.; Aulas, A.; Fantini, J. How cholesterol constrains glycolipid conformation for optimal recognition of Alzheimer’s beta amyloid peptide (Abeta1-40). PLoS ONE 2010, 5, e9079. [Google Scholar] [CrossRef]
- Ling, H.; Boodhoo, A.; Hazes, B.; Cummings, M.D.; Armstrong, G.D.; Brunton, J.L.; Read, R.J. Structure of the shiga-like toxin I B-pentamer complexed with an analogue of its receptor Gb3. Biochemistry 1998, 37, 1777–1788. [Google Scholar] [CrossRef]
- Sandvig, K.; Lingelem, A.B.D.; Skotland, T.; Bergan, J. Shiga toxins: Properties and action on cells. In The Comprehensive Sourcebook of Bacterial Protein Toxins, 4th ed.; Alouf, J., Ladant, D., Popoff, M.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 267–286. [Google Scholar]
- Pike, L.J.; Han, X.; Gross, R.W. Epidermal growth factor receptors are localized to lipid rafts that contain a balance of inner and outer leaflet lipids: A shotgun lipidomics study. J. Biol. Chem. 2005, 280, 26796–26804. [Google Scholar] [CrossRef] [Green Version]
- Legros, N.; Pohlentz, G.; Runde, J.; Dusny, S.; Humpf, H.U.; Karch, H.; Muthing, J. Colocalization of receptors for Shiga toxins with lipid rafts in primary human renal glomerular endothelial cells and influence of D-PDMP on synthesis and distribution of glycosphingolipid receptors. Glycobiology 2017, 27, 947–965. [Google Scholar] [CrossRef] [Green Version]
- Skotland, T.; Sandvig, K.; Llorente, A. Lipids in exosomes: Current knowledge and the way forward. Prog. Lipid Res. 2017, 66, 30–41. [Google Scholar] [CrossRef]
- Lingwood, C.A.; Manis, A.; Mahfoud, R.; Khan, F.; Binnington, B.; Mylvaganam, M. New aspects of the regulation of glycosphingolipid receptor function. Chem. Phys. Lipids 2010, 163, 27–35. [Google Scholar] [CrossRef]
- Falguieres, T.; Romer, W.; Amessou, M.; Afonso, C.; Wolf, C.; Tabet, J.C.; Lamaze, C.; Johannes, L. Functionally different pools of Shiga toxin receptor, globotriaosyl ceramide, in HeLa cells. FEBS J. 2006, 273, 5205–5218. [Google Scholar] [CrossRef]
- Falguieres, T.; Mallard, F.; Baron, C.; Hanau, D.; Lingwood, C.; Goud, B.; Salamero, J.; Johannes, L. Targeting of shiga toxin b-subunit to retrograde transport route in association with detergent-resistant membranes. Mol. Biol. Cell 2001, 12, 2453–2468. [Google Scholar] [CrossRef] [Green Version]
- Takenouchi, H.; Kiyokawa, N.; Taguchi, T.; Matsui, J.; Katagiri, Y.U.; Okita, H.; Okuda, K.; Fujimoto, J. Shiga toxin binding to globotriaosyl ceramide induces intracellular signals that mediate cytoskeleton remodeling in human renal carcinoma-derived cells. J. Cell Sci. 2004, 117, 3911–3922. [Google Scholar] [CrossRef] [Green Version]
- Walchli, S.; Skanland, S.S.; Gregers, T.F.; Lauvrak, S.U.; Torgersen, M.L.; Ying, M.; Kuroda, S.; Maturana, A.; Sandvig, K. The Mitogen-activated Protein Kinase p38 Links Shiga Toxin-dependent Signaling and Trafficking. Mol. Biol. Cell 2008, 19, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Spiegel, S.; Fishman, P.H.; Weber, R.J. Direct evidence that endogenous GM1 ganglioside can mediate thymocyte proliferation. Science 1985, 230, 1285–1287. [Google Scholar] [CrossRef]
- Schnitzler, A.C.; Burke, J.M.; Wetzler, L.M. Induction of cell signaling events by the cholera toxin B subunit in antigen-presenting cells. Infect. Immun. 2007, 75, 3150–3159. [Google Scholar] [CrossRef] [Green Version]
- Ravichandra, B.; Joshi, P.G. Regulation of transmembrane signaling by ganglioside GM1: Interaction of anti-GM1 with Neuro2a cells. J. Neurochem. 1999, 73, 557–567. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Z.H.; Gabius, H.J.; Rohowsky-Kochan, C.; Ledeen, R.W.; Wu, G. Cross-linking of GM1 ganglioside by galectin-1 mediates regulatory T cell activity involving TRPC5 channel activation: Possible role in suppressing experimental autoimmune encephalomyelitis. J. Immunol. 2009, 182, 4036–4045. [Google Scholar] [CrossRef] [Green Version]
- Wands, A.M.; Fujita, A.; McCombs, J.E.; Cervin, J.; Dedic, B.; Rodriguez, A.C.; Nischan, N.; Bond, M.R.; Mettlen, M.; Trudgian, D.C.; et al. Fucosylation and protein glycosylation create functional receptors for cholera toxin. Elife 2015, 4, e09545. [Google Scholar] [CrossRef]
- Cervin, J.; Wands, A.M.; Casselbrant, A.; Wu, H.; Krishnamurthy, S.; Cvjetkovic, A.; Estelius, J.; Dedic, B.; Sethi, A.; Wallom, K.L.; et al. GM1 ganglioside-independent intoxication by Cholera toxin. PLoS Pathog. 2018, 14, e1006862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monferran, C.G.; Roth, G.A.; Cumar, F.A. Inhibition of cholera toxin binding to membrane receptors by pig gastric mucin-derived glycopeptides: Differential effect depending on the ABO blood group antigenic determinants. Infect. Immun. 1990, 58, 3966–3972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raa, H.A.; Grimmer, S.; Schwudke, D.; Bergan, J.; Wälchli, S.; Skotland, T.; Shevchenko, A.; Sandvig, K. Glycosphingolipid requirements for endosome-to-Golgi transport of Shiga toxin. Traffic 2009, 10, 868–882. [Google Scholar] [CrossRef] [PubMed]
- Grimmer, S.; Spilsberg, B.; Hanada, K.; Sandvig, K. Depletion of sphingolipids facilitates endosome to Golgi transport of ricin. Traffic 2006, 7, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Spilsberg, B.; van Meer, G.; Sandvig, K. Role of lipids in the retrograde pathway of ricin intoxication. Traffic 2003, 4, 544–552. [Google Scholar] [CrossRef]
- Pike, L.J.; Han, X.; Chung, K.N.; Gross, R.W. Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: A quantitative electrospray ionization/mass spectrometric analysis. Biochemistry 2002, 41, 2075–2088. [Google Scholar] [CrossRef]
- Wallner, S.; Schmitz, G. Plasmalogens the neglected regulatory and scavenging lipid species. Chem. Phys. Lipids 2011, 164, 573–589. [Google Scholar] [CrossRef]
- Braverman, N.E.; Moser, A.B. Functions of plasmalogen lipids in health and disease. Biochim. Biophys. Acta 2012, 1822, 1442–1452. [Google Scholar] [CrossRef] [Green Version]
- Phuyal, S.; Skotland, T.; Hessvik, N.P.; Simolin, H.; Overbye, A.; Brech, A.; Parton, R.G.; Ekroos, K.; Sandvig, K.; Llorente, A. The Ether Lipid Precursor Hexadecylglycerol Stimulates the Release and Changes the Composition of Exosomes Derived from PC-3 Cells. J. Biol. Chem. 2015, 290, 4225–4237. [Google Scholar] [CrossRef] [Green Version]
- Torgersen, M.L.; Klokk, T.I.; Kavaliauskiene, S.; Klose, C.; Simons, K.; Skotland, T.; Sandvig, K. The anti-tumor drug 2-hydroxyoleic acid (Minerval) stimulates signaling and retrograde transport. Oncotarget 2016, 7, 86871–86888. [Google Scholar] [CrossRef] [Green Version]
- Rother, N.; Yanginlar, C.; Lindeboom, R.G.H.; Bekkering, S.; van Leent, M.M.T.; Buijsers, B.; Jonkman, I.; de Graaf, M.; Baltissen, M.; Lamers, L.A.; et al. Hydroxychloroquine Inhibits the Trained Innate Immune Response to Interferons. Cell Rep. Med. 2020, 1, 100146. [Google Scholar] [CrossRef]
- Jimenez-Rojo, N.; Riezman, H. On the road to unraveling the molecular functions of ether lipids. FEBS Lett. 2019, 593, 2378–2389. [Google Scholar] [CrossRef] [Green Version]
- Fontaine, D.; Figiel, S.; Felix, R.; Kouba, S.; Fromont, G.; Maheo, K.; Potier-Cartereau, M.; Chantome, A.; Vandier, C. Roles of endogenous ether lipids and associated PUFAs in the regulation of ion channels and their relevance for disease. J. Lipid Res. 2020, 61, 840–858. [Google Scholar] [CrossRef] [Green Version]
- Dean, J.M.; Lodhi, I.J. Structural and functional roles of ether lipids. Protein Cell 2018, 9, 196–206. [Google Scholar] [CrossRef]
- Bergan, J.; Skotland, T.; Sylvänne, T.; Simolin, H.; Ekroos, K.; Sandvig, K. The ether lipid precurson hexadecylglycerol causes major changes in the lipidome of HEp-2 cells. PLoS ONE 2013, 8, e75904. [Google Scholar] [CrossRef] [Green Version]
- Bergan, J.; Skotland, T.; Dyve Lingelem, A.B.; Simm, R.; Spilsberg, B.; Lindback, T.; Sylvänne, T.; Simolin, H.; Ekroos, K.; Sandvig, K. The ether lipid precursor hexadecylglycerol protects against Shiga toxins. Cell. Mol. Life Sci. 2014, 71, 4285–4300. [Google Scholar] [CrossRef] [PubMed]
- Kavaliauskiene, S.; Skotland, T.; Sylvänne, T.; Simolin, H.; Klokk, T.I.; Torgersen, M.L.; Lingelem, A.B.D.; Simm, R.; Ekroos, K.; Sandvig, K. Novel actions of 2-deoxyglucose: Protection against Shiga toxins and changes in cellular lipids. Biochem. J. 2015, 470, 23–37. [Google Scholar] [CrossRef]
- Kavaliauskiene, S.; Torgersen, M.L.; Lingelem, A.B.; Klokk, T.I.; Lintonen, T.; Simolin, H.; Ekroos, K.; Skotland, T.; Sandvig, K. Cellular effects of fluorodeoxyglucose: Global changes in the lipidome and alteration in intracellular transport. Oncotarget 2016, 7, 79885–79900. [Google Scholar] [CrossRef] [Green Version]
- Ailte, I.; Lingelem, A.B.; Kavaliauskiene, S.; Bergan, J.; Kvalvaag, A.S.; Myrann, A.G.; Skotland, T.; Sandvig, K. Addition of lysophospholipids with large head groups to cells inhibits Shiga toxin binding. Sci. Rep. 2016, 6, 30336. [Google Scholar] [CrossRef] [Green Version]
- Ailte, I.; Lingelem, A.B.; Kvalvaag, A.S.; Kavaliauskiene, S.; Brech, A.; Koster, G.; Dommersnes, P.G.; Bergan, J.; Skotland, T.; Sandvig, K. Exogenous lysophospholipids with large head groups perturb clathrin-mediated endocytosis. Traffic 2017, 18, 176–191. [Google Scholar] [CrossRef] [Green Version]
- Spilsberg, B.; Llorente, A.; Sandvig, K. Polyunsaturated fatty acids regulate Shiga toxin transport. Biochem. Biophys. Res. Commun. 2007, 364, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Lingelem, A.B.D.; Kavaliauskiene, S.; Halsne, R.; Klokk, T.I.; Surma, M.A.; Klose, C.; Skotland, T.; Sandvig, K. Diacylglycerol kinase and phospholipase D inhibitors alter the cellular lipidome and endosomal sorting towards the Golgi apparatus. Cell. Mol. Life Sci. 2021, 78, 985–1009. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Rojo, N.; Leonetti, M.D.; Zoni, V.; Colom, A.; Feng, S.; Iyengar, N.R.; Matile, S.; Roux, A.; Vanni, S.; Weissman, J.S.; et al. Conserved Functions of Ether Lipids and Sphingolipids in the Early Secretory Pathway. Curr. Biol. 2020, 30, 3775–3787.e3777. [Google Scholar] [CrossRef] [PubMed]
- Han, X.L.; Gross, R.W. Plasmenylcholine and phosphatidylcholine membrane bilayers possess distinct conformational motifs. Biochemistry 1990, 29, 4992–4996. [Google Scholar] [CrossRef]
- Rog, T.; Koivuniemi, A. The biophysical properties of ethanolamine plasmalogens revealed by atomistic molecular dynamics simulations. Biochim. Biophys. Acta 2016, 1858, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Kinoshita, T.; Fujita, M. Biosynthesis of GPI-anchored proteins: Special emphasis on GPI lipid remodeling. J. Lipid Res. 2016, 57, 6–24. [Google Scholar] [CrossRef] [Green Version]
- Obrig, T.G.; Del Vecchio, P.J.; Brown, J.E.; Moran, T.P.; Rowland, B.M.; Judge, T.K.; Rothman, S.W. Direct cytotoxic action of Shiga toxin on human vascular endothelial cells. Infect. Immun. 1988, 56, 2373–2378. [Google Scholar] [CrossRef] [Green Version]
- Kelloff, G.J.; Krohn, K.A.; Larson, S.M.; Weissleder, R.; Mankoff, D.A.; Hoffman, J.M.; Link, J.M.; Guyton, K.Z.; Eckelman, W.C.; Scher, H.I.; et al. The progress and promise of molecular imaging probes in oncologic drug development. Clin. Cancer Res. 2005, 11, 7967–7985. [Google Scholar] [CrossRef] [Green Version]
- Hofman, M.S.; Hicks, R.J. How We Read Oncologic FDG PET/CT. Cancer Imaging 2016, 16, 35. [Google Scholar] [CrossRef] [Green Version]
- Boulant, S.; Kural, C.; Zeeh, J.C.; Ubelmann, F.; Kirchhausen, T. Actin dynamics counteract membrane tension during clathrin-mediated endocytosis. Nat. Cell Biol. 2011, 13, 1124–1131. [Google Scholar] [CrossRef] [Green Version]
- Melero, A.; Chiaruttini, N.; Karashima, T.; Riezman, I.; Funato, K.; Barlowe, C.; Riezman, H.; Roux, A. Lysophospholipids Facilitate COPII Vesicle Formation. Curr. Biol. 2018, 28, 1950–1958.e56. [Google Scholar] [CrossRef] [Green Version]
- Binnington, B.; Nguyen, L.; Kamani, M.; Hossain, D.; Marks, D.L.; Budani, M.; Lingwood, C.A. Inhibition of Rab prenylation by statins induces cellular glycosphingolipid remodeling. Glycobiology 2016, 26, 166–180. [Google Scholar] [CrossRef] [Green Version]
- Cole, S.L.; Grudzien, A.; Manhart, I.O.; Kelly, B.L.; Oakley, H.; Vassar, R. Statins cause intracellular accumulation of amyloid precursor protein, beta-secretase-cleaved fragments, and amyloid beta-peptide via an isoprenoid-dependent mechanism. J. Biol. Chem. 2005, 280, 18755–18770. [Google Scholar] [CrossRef] [Green Version]
- Sheng, R.; Chen, Y.; Yung Gee, H.; Stec, E.; Melowic, H.R.; Blatner, N.R.; Tun, M.P.; Kim, Y.; Kallberg, M.; Fujiwara, T.K.; et al. Cholesterol modulates cell signaling and protein networking by specifically interacting with PDZ domain-containing scaffold proteins. Nat. Commun. 2012, 3, 1249. [Google Scholar] [CrossRef] [Green Version]
- Ikonen, E. Mechanisms of cellular cholesterol compartmentalization: Recent insights. Curr. Opin. Cell Biol. 2018, 53, 77–83. [Google Scholar] [CrossRef]
- Bos, K.; Wraight, C.; Stanley, K.K. TGN38 is maintained in the trans-Golgi network by a tyrosine-containing motif in the cytoplasmic domain. EMBO J. 1993, 12, 2219–2228. [Google Scholar] [CrossRef]
- Stace, C.L.; Ktistakis, N.T. Phosphatidic acid- and phosphatidylserine-binding proteins. Biochim. Biophys. Acta 2006, 1761, 913–926. [Google Scholar] [CrossRef]
- Jungmichel, S.; Sylvestersen, K.B.; Choudhary, C.; Nguyen, S.; Mann, M.; Nielsen, M.L. Specificity and commonality of the phosphoinositide-binding proteome analyzed by quantitative mass spectrometry. Cell Rep. 2014, 6, 578–591. [Google Scholar] [CrossRef] [Green Version]
- Shinzawa-Itoh, K.; Aoyama, H.; Muramoto, K.; Terada, H.; Kurauchi, T.; Tadehara, Y.; Yamasaki, A.; Sugimura, T.; Kurono, S.; Tsujimoto, K.; et al. Structures and physiological roles of 13 integral lipids of bovine heart cytochrome c oxidase. EMBO J. 2007, 26, 1713–1725. [Google Scholar] [CrossRef]
- Contreras, F.X.; Ernst, A.M.; Haberkant, P.; Bjorkholm, P.; Lindahl, E.; Gonen, B.; Tischer, C.; Elofsson, A.; von, H.G.; Thiele, C.; et al. Molecular recognition of a single sphingolipid species by a protein’s transmembrane domain. Nature 2012, 481, 525–529. [Google Scholar] [CrossRef] [Green Version]






| Treatment | Binding | Uptake 1 | Endo → Golgi | Golgi → ER | Toxicity | Cer | GlcCer LacCer | Gb3 | Acyl PL | Ether PL | Other Information |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fumonisin 2 | Stx ↓↓ | ~ | Stx ↓↓↓ | Stx ↓↓↓ | ↓↓ | ↓↓ | ↓↓ | PE ↑↑ | PE ↑↑ PC ↓ | No effect on ricin | |
| PDMP 3 | Stx ↓↓ | ~ | Stx ↓↓↓ | Stx ↓↓ | ~ | ↓↓ | ↓↓ | ~ | ~ | No effect on ricin | |
| HG 4 | Stx ↓ | ~ | ~ | Stx ↓↓ | Stx ↓↓↓ | ~ (↑) | ↓↓ | ↓↓ | PI ↑↑ LPI ↑↑↑ | PE ↑↑ PC ↑↑ | No effect on ricin. See also 4 |
| Cell density 5 | Stx ↓↓ | ~ | ~ | ~ | Stx ↓↓ | ↑ | ↑ | ↑ | PA↑↑ PI + PE ↓ | PE ↓ PC ↓ | No effect on diphtheria toxin |
| 2-DG 6 | ~ | ~ | ~ | ~ | Stx ↓(↓) | ~ (↑) | Cont. 3% 2-DG | ↓ 1% 2-DG | Inhibits release of Shiga toxin A1 in ER | ||
| FDG 7 | Stx ↓ | ~ | ~ | Stx ↓ | Stx ↓↓↓ | ~ | ↓↓↓ | ↓↓↓ | PI ↑ | Inhibits GlcCer synth. | |
| Lyso PL 8 (LPI 18:0) | Stx ↓↓ | Stx ↓↓↓ | Stx ↓↓↓ | PM lipid packing ↓ | |||||||
| Polyunsaturated FA 9 | Stx ↓ | Stx ↓ | Stx ↓ | Stx ↓↓↓ | Varying effect on other toxins (see 9) | ||||||
| OHOA 10 | ~ | ~ | Ricin ↑↑ | ~ | Ricin ↑↑ | ~ | ~ | ~ (see 11) | ~ (see 11) | PM lipid packing ↓ | |
| DAG kinase and PLD 11 | ~ | Ricin ↑↑ | ~ | Ricin ↑ | ~ | ~ | ~ (most) | ~ | See text for DAG, PA and PG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandvig, K.; Kavaliauskiene, S.; Skotland, T. The Protein Toxins Ricin and Shiga Toxin as Tools to Explore Cellular Mechanisms of Internalization and Intracellular Transport. Toxins 2021, 13, 377. https://doi.org/10.3390/toxins13060377
Sandvig K, Kavaliauskiene S, Skotland T. The Protein Toxins Ricin and Shiga Toxin as Tools to Explore Cellular Mechanisms of Internalization and Intracellular Transport. Toxins. 2021; 13(6):377. https://doi.org/10.3390/toxins13060377
Chicago/Turabian StyleSandvig, Kirsten, Simona Kavaliauskiene, and Tore Skotland. 2021. "The Protein Toxins Ricin and Shiga Toxin as Tools to Explore Cellular Mechanisms of Internalization and Intracellular Transport" Toxins 13, no. 6: 377. https://doi.org/10.3390/toxins13060377
APA StyleSandvig, K., Kavaliauskiene, S., & Skotland, T. (2021). The Protein Toxins Ricin and Shiga Toxin as Tools to Explore Cellular Mechanisms of Internalization and Intracellular Transport. Toxins, 13(6), 377. https://doi.org/10.3390/toxins13060377





