How Does Bacillus thuringiensis Crystallize Such a Large Diversity of Toxins?
Abstract
:1. Introduction
2. A Diverse Set of Mechanisms to Produce High Quantities of Toxins in Bt
3. Toxins Evolved to Be Crystallization-Prone
4. The (Facultative?) Role of Accessory Proteins
5. Crystallization in Bt Is a Finely-Tuned Multifactorial Process
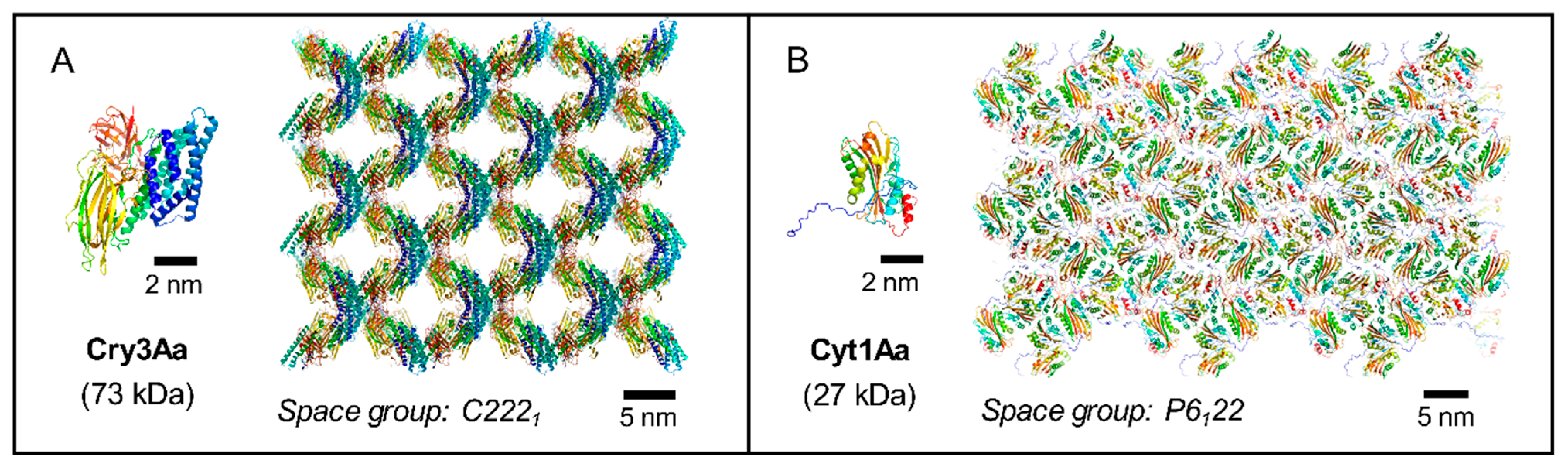
6. The (R)Evolution of Structural Biology Allows the Unraveling of Key Steps in the Crystallization Pathways of Bt Toxins
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raymond, B.; Johnston, P.R.; Nielsen-LeRoux, C.; Lereclus, D.; Crickmore, N. Bacillus thuringiensis: An impotent pathogen? Trends Microbiol. 2010, 18, 189–194. [Google Scholar] [CrossRef]
- Berry, C.; Crickmore, N. Structural classification of insecticidal proteins—Towards an in silico characterisation of novel toxins. J. Invertebr. Pathol. 2017, 142, 16–22. [Google Scholar] [CrossRef]
- Crickmore, N.; Berry, C.; Panneerselvam, S.; Mishra, R.; Connor, T.R.; Bonning, B.C. A structure-based nomenclature for Bacillus thuringiensis and other bacteria-derived pesticidal proteins. J. Invertebr. Pathol. 2020, 107438. [Google Scholar] [CrossRef]
- Xu, C.; Wang, B.C.; Yu, Z.; Sun, M. Structural insights into Bacillus thuringiensis Cry, Cyt and parasporin toxins. Toxins 2014, 6, 2732–2770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurat-Fuentes, J.L.; Crickmore, N. Specificity determinants for Cry insecticidal proteins: Insights from their mode of action. J. Invertebr. Pathol. 2017, 142, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Bravo, A.; Likitvivatanavong, S.; Gill, S.S.; Soberon, M. Bacillus thuringiensis: A story of a successful bioinsecticide. Insect Biochem. Mol. Biol. 2011, 41, 423–431. [Google Scholar] [CrossRef] [Green Version]
- Arantes, O.; Lereclus, D. Construction of cloning vectors for Bacillus thuringiensis. Gene 1991, 108, 115–119. [Google Scholar] [CrossRef]
- Federici, B.A.; Park, H.W.; Bideshi, D.K.; Wirth, M.C.; Johnson, J.J. Recombinant bacteria for mosquito control. J. Exp. Biol. 2003, 206, 3877–3885. [Google Scholar] [CrossRef] [Green Version]
- Federici, B.A.; Park, H.-W.; Bideshi, D.K. Overview of the Basic Biology of Bacillus thuringiensis with Emphasis on Genetic Engineering of Bacterial Larvicides for Mosquito Control. Open Toxinol. J. 2010, 3, 83–100. [Google Scholar] [CrossRef] [Green Version]
- Evdokimov, A.G.; Moshiri, F.; Sturman, E.J.; Rydel, T.J.; Zheng, M.; Seale, J.W.; Franklin, S. Structure of the full-length insecticidal protein Cry1Ac reveals intriguing details of toxin packaging into in vivo formed crystals. Protein Sci. 2014, 23, 1491–1497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tetreau, G.; Banneville, A.S.; Andreeva, E.A.; Brewster, A.S.; Hunter, M.D.; Sierra, R.G.; Teulon, J.M.; Young, I.D.; Burke, N.; Gruenewald, T.A.; et al. Serial femtosecond crystallography on in vivo-grown crystals drives elucidation of mosquitocidal Cyt1Aa bioactivation cascade. Nat. Commun. 2020, 11, 1153. [Google Scholar] [CrossRef] [Green Version]
- Kelker, M.S.; Berry, C.; Evans, S.L.; Pai, R.; McCaskill, D.G.; Wang, N.X.; Russell, J.C.; Baker, M.D.; Yang, C.; Pflugrath, J.W.; et al. Structural and Biophysical Characterization of Bacillus thuringiensis Insecticidal Proteins Cry34Ab1 and Cry35Ab1. PLoS ONE 2014, 9, e112555. [Google Scholar] [CrossRef]
- Xu, C.; Chinte, U.; Chen, L.; Yao, Q.; Meng, Y.; Zhou, D.; Bi, L.-J.; Rose, J.; Adang, M.J.; Wang, B.-C.; et al. Crystal structure of Cry51Aa1: A potential novel insecticidal aerolysin-type β-pore-forming toxin from Bacillus thuringiensis. Biochem. Biophys. Res. Commun. 2015, 462, 184–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dementiev, A.; Board, J.; Sitaram, A.; Hey, T.; Kelker, M.S.; Xu, X.; Hu, Y.; Vidal-Quist, C.; Chikwana, V.; Griffin, S.; et al. The pesticidal Cry6Aa toxin from Bacillus thuringiensis is structurally similar to HlyE-family alpha pore-forming toxins. BMC Biol. 2016, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crickmore, N.; Zeigler, D.R.; Feitelson, J.; Schnepf, E.; Van Rie, J.; Lereclus, D.; Baum, J.; Dean, D.H. Revision of the nomenclature for the Bacillus thuringiensis pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 1998, 62, 807–813. [Google Scholar] [CrossRef] [Green Version]
- Vachon, V.; Laprade, R.; Schwartz, J.L. Current models of the mode of action of Bacillus thuringiensis insecticidal crystal proteins: A critical review. J. Invertebr. Pathol. 2012, 111, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Albeck, S.; Ben-Dov, E.; Cahan, R.; Firer, M.; Zaritsky, A.; Dym, O. Cyt1Aa Toxin: Crystal Structure Reveals Implications for Its Membrane-Perforating Function. J. Mol. Biol. 2011, 413, 804–814. [Google Scholar] [CrossRef]
- Adalat, R.; Saleem, F.; Crickmore, N.; Naz, S.; Shakoori, A.R. In Vivo Crystallization of Three-Domain Cry Toxins. Toxins 2017, 9, 80. [Google Scholar] [CrossRef]
- Agaisse, H.; Lereclus, D. How does Bacillus thuringiensis produce so much insecticidal crystal protein? J. Bacteriol. 1995, 177, 6027–6032. [Google Scholar] [CrossRef] [Green Version]
- Deng, C.; Peng, Q.; Song, F.; Lereclus, D. Regulation of cry gene expression in Bacillus thuringiensis. Toxins 2014, 6, 2194–2209. [Google Scholar] [CrossRef] [Green Version]
- Schnepf, E.; Crickmore, N.; Van Rie, J.; Lereclus, D.; Baum, J.; Feitelson, J.; Zeigler, D.R.; Dean, D.H. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 1998, 62, 775–806. [Google Scholar] [CrossRef] [Green Version]
- Federici, B.A.; Park, H.-W.; Sakano, Y. Insecticidal Protein Crystals of Bacillus thuringiensis. In Inclusions in Prokaryotes; Shively, J.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 195–236. [Google Scholar] [CrossRef]
- Lereclus, D.; Agaisse, H.; Grandvalet, C.; Salamitou, S.; Gominet, M. Regulation of toxin and virulence gene transcription in Bacillus thuringiensis. Int. J. Med. Microbiol. 2000, 290, 295–299. [Google Scholar] [CrossRef]
- Stein, C.; Jones, G.W.; Chalmers, T.; Berry, C. Transcriptional analysis of the toxin-coding plasmid pBtoxis from Bacillus thuringiensis subsp. israelensis. Appl. Environ. Microbiol. 2006, 72, 1771–1776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Mei, H.; Zheng, C.; Qian, H.; Cui, C.; Fu, Y.; Su, J.; Liu, Z.; Yu, Z.; He, J. The metabolic regulation of sporulation and parasporal crystal formation in Bacillus thuringiensis revealed by transcriptomics and proteomics. Mol. Cell. Proteom. 2013, 12, 1363–1376. [Google Scholar] [CrossRef] [Green Version]
- Aronson, A. Sporulation and Delta-Endotoxin Synthesis by Bacillus Thuringiensis. Cell. Mol. Life Sci. 2002, 59, 417–425. [Google Scholar] [CrossRef]
- Ma, J.; Campbell, A.; Karlin, S. Correlations between Shine-Dalgarno Sequences and Gene Features Such as Predicted Expression Levels and Operon Structures. J. Bacteriol. 2002, 184, 5733–5745. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, S.; Niimura, Y.; Miura, K.i.; Gojobori, T. Dynamic evolution of translation initiation mechanisms in prokaryotes. Proc. Natl. Acad. Sci. USA 2010, 107, 6382–6387. [Google Scholar] [CrossRef] [Green Version]
- Adalat, R.; Saleem, F.; Bashir, A.; Ahmad, M.; Zulfiqar, S.; Shakoori, A.R. Multiple upstream start codons (AUG) in 5′ untranslated region enhance translation efficiency of cry2Ac11 without helper protein. J. Cell. Biochem. 2018, 120, 2236–2250. [Google Scholar] [CrossRef]
- Glatron, M.F.; Rapoport, G. Biosynthesis of the parasporal inclusion of Bacillus thuringiensis: Half-life of its corresponding messenger RNA. Biochimie 1972, 54, 1291–1301. [Google Scholar] [CrossRef]
- Laalami, S.; Zig, L.; Putzer, H. Initiation of mRNA decay in bacteria. Cell. Mol. Life Sci. 2013, 71, 1799–1828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Condon, C. RNA Processing and Degradation in Bacillus subtilis. Microbiol. Mol. Biol. Rev. 2003, 67, 157–174. [Google Scholar] [CrossRef] [Green Version]
- Sakano, Y.; Park, H.W.; Bideshi, D.K.; Ge, B.; Federici, B.A. Contributions of 5’-UTR and 3’-UTR cis elements to Cyt1Aa synthesis in Bacillus thuringiensis subsp. israelensis. J. Invertebr. Pathol. 2017, 149, 66–75. [Google Scholar] [CrossRef]
- Agaisse, H.; Lereclus, D. STAB-SD: A Shine-Dalgarno sequence in the 5’ untranslated region is a determinant of mRNA stability. Mol. Microbiol. 1996, 20, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Jin, S.; Wu, W. Regulation of bacterial gene expression by ribosome stalling and rescuing. Curr. Genet. 2015, 62, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, P.; Fahnert, B.; Lilie, H.; Villaverde, A. Protein Inclusion Bodies in Recombinant Bacteria. In Inclusions in Prokaryotes; Shively, J.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 237–292. [Google Scholar] [CrossRef]
- Sawaya, M.R.; Cascio, D.; Gingery, M.; Rodriguez, J.; Goldschmidt, L.; Colletier, J.-P.; Messerschmidt, M.M.; Boutet, S.; Koglin, J.E.; Williams, G.J.; et al. Protein crystal structure obtained at 2.9 Å resolution from injecting bacterial cells into an X-ray free-electron laser beam. Proc. Natl. Acad. Sci. USA 2014, 111, 12769–12774. [Google Scholar] [CrossRef] [Green Version]
- Li, J.D.; Carroll, J.; Ellar, D.J. Crystal structure of insecticidal delta-endotoxin from Bacillus thuringiensis at 2.5 A resolution. Nature 1991, 353, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Henderson, R.; Carroll, J.; Ellar, D. X-ray analysis of the crystalline parasporal inclusion in Bacillus thuringiensis var. tenebrionis. J. Mol. Biol. 1988, 199, 543–544. [Google Scholar] [CrossRef]
- Bennett, M.J.; Choe, S.; Eisenberg, D. Domain swapping: Entangling alliances between proteins. Proc. Natl. Acad. Sci. USA 1994, 91, 3127–3131. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Koni, P.A.; Ellar, D.J. Structure of the mosquitocidal delta-endotoxin CytB from Bacillus thuringiensis sp kyushuensis and implications for membrane pore formation. J. Mol. Biol. 1996, 257, 129–152. [Google Scholar] [CrossRef]
- Bietlot, H.P.; Vishnubhatla, I.; Carey, P.R.; Pozsgay, M.; Kaplan, H. Characterization of the cysteine residues and disulphide linkages in the protein crystal of Bacillus thuringiensis. Biochem. J. 1990, 267, 309–315. [Google Scholar] [CrossRef] [Green Version]
- Holmes, K.C.; Monro, R.E. Studies on the structure of parasporal inclusions from Bacillus thuringiensis. J. Mol. Biol. 1965, 14, 572–581. [Google Scholar] [CrossRef]
- Diaz-Mendoza, M.; Bideshi, D.K.; Ortego, F.; Farinós, G.P.; Federici, B.A. The 20-kDa chaperone-like protein of Bacillus thuringiensis ssp. israelensis enhances yield, crystal size and solubility of Cry3A. Lett. Appl. Microbiol. 2012, 54, 88–95. [Google Scholar] [CrossRef]
- Rang, C.; Bes, M.; Lullien-Pellerin, V.; Wu, D.; Federici, B.A.; Frutos, R. Influence of the 20-kDa protein from Bacillus thuringiensis ssp. israelensis on the rate of production of truncated Cry1C proteins. FEMS Microbiol. Lett. 1996, 141, 261–264. [Google Scholar] [CrossRef]
- Shao, Z.; Liu, Z.; Yu, Z. Effects of the 20-Kilodalton Helper Protein on Cry1Ac Production and Spore Formation in Bacillus thuringiensis. Appl. Environ. Microbiol. 2001, 67, 5362–5369. [Google Scholar] [CrossRef] [Green Version]
- Koni, P.A.; Ellar, D.J. Cloning and characterization of a novel Bacillus thuringiensis cytolytic delta-endotoxin. J. Mol. Biol. 1993, 229, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Manasherob, R.; Zaritsky, A.; Ben-Dov, E.; Saxena, D.; Barak, Z.e.; Einav, M. Effect of Accessory Proteins P19 and P20 on Cytolytic Activity of Cyt1Aa from Bacillus thuringiensis subsp. israelensis in Escherichia coli. Curr. Microbiol. 2014, 43, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Barboza-Corona, J.E.; Park, H.W.; Bideshi, D.K.; Federici, B.A. The 60-kilodalton protein encoded by orf2 in the cry19A operon of Bacillus thuringiensis subsp. jegathesan functions like a C-terminal crystallization domain. Appl. Environ. Microbiol. 2012, 78, 2005–2012. [Google Scholar] [CrossRef] [Green Version]
- Widner, W.R.; Whiteley, H.R. Two highly related insecticidal crystal proteins of Bacillus thuringiensis subsp. kurstaki possess different host range specificities. J. Bacteriol. 1989, 171, 965–974. [Google Scholar] [CrossRef] [Green Version]
- Ge, B.; Bideshi, D.; Moar, W.J.; Federici, B.A. Differential effects of helper proteins encoded by the cry2A and cry11A operons on the formation of Cry2A inclusions in Bacillus thuringiensis. FEMS Microbiol. Lett. 1998, 165, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crickmore, N.; Ellar, D.J. Involvement of a possible chaperonin in the efficient expression of a cloned CryllA δ-endotoxin gene in Bacillus thuringiensis. Mol. Microbiol. 1992, 6, 1533–1537. [Google Scholar] [CrossRef]
- Wu, D.; Federici, B.A. A 20-kilodalton protein preserves cell viability and promotes CytA crystal formation during sporulation in Bacillus thuringiensis. J. Bacteriol. 1993, 175, 5276–5280. [Google Scholar] [CrossRef] [Green Version]
- Berry, C.; O’Neil, S.; Ben-Dov, E.; Jones, A.F.; Murphy, L.; Quail, M.A.; Holden, M.T.G.; Harris, D.; Zaritsky, A.; Parkhill, J. Complete sequence and organization of pBtoxis, the toxin-coding plasmid of Bacillus thuringiensis subsp. israelensis. Appl. Environ. Microbiol. 2002, 68, 5082–5095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, C.; Board, J. The use of structural modelling to infer structure and function in biocontrol agents. J. Invertebr. Pathol. 2017, 142, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Visick, J.E.; Whiteley, H.R. Effect of a 20-kilodalton protein from Bacillus thuringiensis subsp. israelensis on production of the CytA protein by Escherichia coli. J. Bacteriol. 1991, 173, 1748–1756. [Google Scholar] [CrossRef] [Green Version]
- McMurray, M.A. Coupling de novo protein folding with subunit exchange into pre-formed oligomeric protein complexes: The ‘heritable template’ hypothesis. Biomol. Concepts 2016, 7. [Google Scholar] [CrossRef]
- Juarez Perez, V.; Guerchicoff, A.; Rubinstein, C.; Delecluse, A. Characterization of Cyt2Bc Toxin from Bacillus thuringiensis subsp. medellin. Appl. Environ. Microbiol. 2002, 68, 1228–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshisue, H.; Yoshida, K.; Sen, K.; Sakai, H.; Komano, T. Effects of Bacillus thuringiensis var. israelensis 20-kDa protein on production of the Bti 130-kDa crystal protein in Escherichia coli. Biosci. Biotechnol. Biochem. 1992, 56, 1429–1433. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Federici, B.A. Improved production of the insecticidal CryIVD protein in Bacillus-thuringiensis using CryLA(c) promoters to express the gene for an associated 20-KDa protein. Appl. Microbiol. Biotechnol. 1995, 42, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Martin, P.A.; Nickerson, K.W. Comparison of Disulfide Contents and Solubility at Alkaline pH of Insecticidal and Noninsecticidal Bacillus thuringiensis Protein Crystals. Appl. Environ. Microbiol. 1994, 60, 3847–3853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.W.; Ge, B.; Bauer, L.S.; Federici, B.A. Optimization of Cry3A yields in Bacillus thuringiensis by use of sporulation-dependent promoters in combination with the STAB-SD mRNA sequence. Appl. Environ. Microbiol. 1998, 64, 3932–3938. [Google Scholar] [CrossRef] [Green Version]
- Park, H.-W.; Bideshi, D.K.; Johnson, J.J.; Federici, B.A. Differential enhancement of Cry2A versus Cry11A yields in Bacillus thuringiensis by use of the cry3A STAB mRNA sequence. FEMS Microbiol. Lett. 1999, 181, 319–327. [Google Scholar] [CrossRef] [Green Version]
- Park, H.-W.; Delécluse, A.; Federici, B.A. Construction and Characterization of a Recombinant Bacillus thuringiensis subsp. israelensis Strain That Produces Cry11B. J. Invertebr. Pathol. 2001, 78, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhao, Q.; Xia, L.; Ding, X.; Hu, Q.; Federici, B.A.; Park, H.-W. Identification and Characterization of Three Previously Undescribed Crystal Proteins from Bacillus thuringiensis subsp. jegathesan. Appl. Environ. Microbiol. 2013, 79, 3364. [Google Scholar] [CrossRef] [Green Version]
- Thompson, M.C.; Yeates, T.O.; Rodriguez, J.A. Advances in methods for atomic resolution macromolecular structure determination. F1000Research 2020, 9, 667. [Google Scholar] [CrossRef] [PubMed]
- Garman, E.F.; Weik, M. Radiation Damage in Macromolecular Crystallography. Methods Mol. Biol. 2017, 1607, 467–489. [Google Scholar] [CrossRef]
- Garman, E.F.; Weik, M. X-ray radiation damage to biological samples: Recent progress. J. Synchrotron Radiat. 2019, 26, 907–911. [Google Scholar] [CrossRef]
- Holton, J.M.; Frankel, K.A. The minimum crystal size needed for a complete diffraction data set. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 393–408. [Google Scholar] [CrossRef] [Green Version]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palma, L.; Berry, C. Understanding the structure and function of Bacillus thuringiensis toxins. Toxicon 2016, 109, 1–3. [Google Scholar] [CrossRef]
- Quan, Y.; Ferré, J. Structural Domains of the Bacillus thuringiensis Vip3Af Protein Unraveled by Tryptic Digestion of Alanine Mutants. Toxins 2019, 11, 368. [Google Scholar] [CrossRef] [Green Version]
- Ward, E.S.; Ellar, D.J.; Chilcott, C.N. Single amino-acid changes in the Bacillus-thuringiensis var israelensis delta-endotoxin affect the toxicity and expression of the protein. J. Mol. Biol. 1988, 202, 527–535. [Google Scholar] [CrossRef]
- Promdonkoy, B.; Ellar, D.J. Structure-function relationships of a membrane pore forming toxin revealed by reversion mutagenesis. Mol. Membr. Biol. 2005, 22, 327–337. [Google Scholar] [CrossRef]
- Gutierrez, P.; Alzate, O.; Orduz, S. A theoretical model of the tridimensional structure of Bacillus thuringiensis subsp. medellin Cry 11Bb toxin deduced by homology modelling. Mem. Inst. Oswaldo Cruz 2001, 96, 357–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angsuthanasombat, C.; Uawithya, P.; Leetachewa, S.; Pornwiroon, W.; Ounjai, P.; Kerdcharoen, T.; Katzenmeier, G.; Panyim, S. Bacillus thuringiensis Cry4A and Cry4B mosquito-larvicidal proteins: Homology-based 3D model and implications for toxin activity. J. Biochem. Mol. Biol. 2004, 37, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Cartlidge, E. European XFEL to shine as brightest, fastest x-ray source. Science 2016, 354, 22–23. [Google Scholar] [CrossRef]
- Colletier, J.P.; Sawaya, M.R.; Gingery, M.; Rodriguez, J.A.; Cascio, D.; Brewster, A.S.; Michels-Clark, T.; Hice, R.H.; Coquelle, N.; Boutet, S.; et al. De novo phasing with X-ray laser reveals mosquito larvicide BinAB structure. Nature 2016, 539, 43–47. [Google Scholar] [CrossRef] [Green Version]
- Gati, C.; Bourenkov, G.; Klinge, M.; Rehders, D.; Stellato, F.; Oberthur, D.; Yefanov, O.; Sommer, B.P.; Mogk, S.; Duszenko, M.; et al. Serial crystallography on in vivo grown microcrystals using synchrotron radiation. IUCrJ 2014, 1, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Coquelle, N.; Brewster, A.S.; Kapp, U.; Shilova, A.; Weinhausen, B.; Burghammer, M.; Colletier, J.-P. Raster-scanning serial protein crystallography using micro- and nano-focused synchrotron beams. Acta Crystallogr. Sect. D 2015, 71, 1184–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owen, R.L.; Axford, D.; Sherrell, D.A.; Kuo, A.; Ernst, O.P.; Schulz, E.C.; Miller, R.J.D.; Mueller-Werkmeister, H.M. Low-dose fixed-target serial synchrotron crystallography. Acta Crystallogr. Sect. D Struct. Biol. 2017, 73, 373–378. [Google Scholar] [CrossRef]
- Jaeger, K.; Dworkowski, F.; Nogly, P.; Milne, C.; Wang, M.; Standfuss, J. Serial Millisecond Crystallography of Membrane Proteins. Adv. Exp. Med. Biol. 2016, 922, 137–149. [Google Scholar] [CrossRef]
- Botha, S.; Nass, K.; Barends, T.R.M.; Kabsch, W.; Latz, B.; Dworkowski, F.; Foucar, L.; Panepucci, E.; Wang, M.; Shoeman, R.L.; et al. Room-temperature serial crystallography at synchrotron X-ray sources using slowly flowing free-standing high-viscosity microstreams. Acta Crystallogr. Sect. D Biol. Crystallogr. 2015, 71, 387–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, M. Time-Resolved Macromolecular Crystallography at Modern X-Ray Sources. Methods Mol. Biol. 2017, 1607, 273–294. [Google Scholar] [CrossRef]
- Wang, H.W.; Wang, J.W. How cryo-electron microscopy and X-ray crystallography complement each other. Protein Sci. A Publ. Protein Soc. 2017, 26, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Núñez-Ramírez, R.; Huesa, J.; Bel, Y.; Ferré, J.; Casino, P.; Arias-Palomo, E. Molecular architecture and activation of the insecticidal protein Vip3Aa from Bacillus thuringiensis. Nat. Commun. 2020, 11, 3974. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.J.; Iadanza, M.G.; Perez, M.A.; Maskell, D.P.; George, R.M.; Hesketh, E.L.; Beales, P.A.; Zack, M.D.; Berry, C.; Thompson, R.F. Cryo-EM structures of an insecticidal Bt toxin reveal its mechanism of action on the membrane. Nat. Commun. 2021, 12, 2791. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tetreau, G.; Andreeva, E.A.; Banneville, A.-S.; De Zitter, E.; Colletier, J.-P. How Does Bacillus thuringiensis Crystallize Such a Large Diversity of Toxins? Toxins 2021, 13, 443. https://doi.org/10.3390/toxins13070443
Tetreau G, Andreeva EA, Banneville A-S, De Zitter E, Colletier J-P. How Does Bacillus thuringiensis Crystallize Such a Large Diversity of Toxins? Toxins. 2021; 13(7):443. https://doi.org/10.3390/toxins13070443
Chicago/Turabian StyleTetreau, Guillaume, Elena A. Andreeva, Anne-Sophie Banneville, Elke De Zitter, and Jacques-Philippe Colletier. 2021. "How Does Bacillus thuringiensis Crystallize Such a Large Diversity of Toxins?" Toxins 13, no. 7: 443. https://doi.org/10.3390/toxins13070443
APA StyleTetreau, G., Andreeva, E. A., Banneville, A.-S., De Zitter, E., & Colletier, J.-P. (2021). How Does Bacillus thuringiensis Crystallize Such a Large Diversity of Toxins? Toxins, 13(7), 443. https://doi.org/10.3390/toxins13070443