Abstract
Cholera toxin B-subunit (CTxB) has emerged as one of the most widely utilized tools in membrane biology and biophysics. CTxB is a homopentameric stable protein that binds tightly to up to five GM1 glycosphingolipids. This provides a robust and tractable model for exploring membrane structure and its dynamics including vesicular trafficking and nanodomain assembly. Here, we review important advances in these fields enabled by use of CTxB and its lipid receptor GM1.
Keywords:
cholera toxin B-subunit; membrane nanodomains; endocytosis; retrograde trafficking; membrane curvature; membrane rafts; glycolipids; GM1; GL-Lect hypothesis Key Contribution:
Recent advances in our understanding of membrane biology emerging from studies using cholera toxin B-subunit and synthetic derivatives of its receptor GM1 are highlighted.
1. Introduction
Cholera toxin (CTx) typifies the AB5 bacterial toxins, and it is the essential pathogenic factor that causes the massive secretory diarrhea seen in humans infected with V. cholerae [1,2]. The Vibrio pathogen first secretes the toxin into the intestinal lumen after colonization of the mucosal surface, but CTx is not active in this space. Remarkably, the toxin encodes within its protein structure everything necessary to breach the intestinal epithelial barrier and enter the cytosol of host cells. Here, in the cytosol, a portion of the toxin induces disease by activation of adenylyl cyclase. This alters the physiology of the intestinal epithelium by activating the Cl− channel CFTR and inhibiting the Na+/H+ exchanger NHE3 to cause Cl secretion and Na malabsorption leading to a severe form of secretory watery diarrhea [3].
The toxin accomplishes cytosolic entry by co-opting normal aspects of host cell membrane and organelle biology. It does not induce pathogenic membrane pores, or penetrate cell membranes, or damage the integrity of the mucosal surface in any way. Rather, it traffics into the cell and across the mucosal barrier by riding along endogenous pathways of membrane lipid and protein trafficking, and by engaging different aspects of normal sub-cellular organelle biology. The evolutionarily driven adaptations enabling these processes have rendered the toxin one of the most potent and informative probes of cell and membrane structure and function and mucosal tissue biology. This is the topic of the current review: how CTx has illuminated our understanding of basic membrane and subcellular processes fundamental to cell biology.
To enter the cytosol of host cells, CTx has evolved to bind with high affinity to the oligosaccharide moiety of a raft-associated glycosphingolipid, ganglioside GM1. This is mediated entirely by the toxin’s binding B-subunit, CTxB (Figure 1 [4]). CTxB assembles as a homopentamer of 11 kDa peptide chains and it functions as a lectin with five binding sites for the oligosaccharide head group of GM1 (and other closely related gangliosides [5,6,7]). It is thus capable of clustering up to five glycosphingolipids together [8]. GM1 acts as the vehicle for endocytic uptake and retrograde trafficking of CTx all the way backwards in the secretory pathway into the endoplasmic reticulum (ER) [9,10,11]. The structure of the ceramide moiety of GM1 dictates the trafficking of the toxin-GSL complexes in this pathway [12,13]. Once in the ER, the CTx A-subunit co-opts the mechanics of protein folding quality control to separate from the B subunit and retrotranslocate across the ER-limiting membrane into the cytosol where it induces toxicity by enzymatically ADP-ribosylating Gαs and activating adenylyl cyclase (Figure 2) [14,15]. Separate secondary and lower affinity binding sites for glycoproteins also exist on CTxB that modify toxin action [16,17,18,19,20,21,22,23,24,25,26,27].
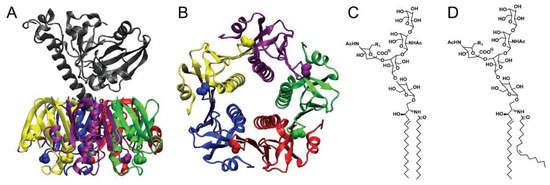
Figure 1.
The molecular structures of CTx and GM1. (A) A side view of CTx is shown with the A subunit in grey and the five B subunits shown in color. (B) A view from the bottom, membrane-binding surface of CTxB. The five G33 amino acids of the GM1 binding pockets are shown as space-filling spheres. (C,D) Structure of the CTx receptor ganglioside GM1. GM1 structures with ceramides containing acyl chains of C16:0 (C) and C16:1 (D) are shown. The crystal structure for CTx was downloaded from the Protein Data Bank 1S5E [4]. CTx structure is from O’Neal, C.J.; Amaya, E.I.; Jobling, M.G.; Holmes, R.K.; Hol, W.G. Crystal structures of an intrinsically active cholera toxin mutant yield insight into the toxin activation mechanism. Biochemistry 2004, 43, 3772–3782. [4].
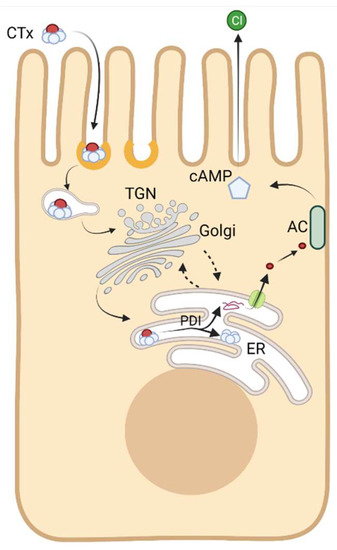
Figure 2.
Intracellular itinerary of CTx trafficking and mechanism of intoxification. The CTx holotoxin binds the plasma membrane via its pentameric membrane binding B subunit. It is subsequently internalized and delivered to endosomes. From there the toxin enters into the retrograde trafficking pathway, leading to its delivery to the endoplasmic reticulum (ER). In the lumen of the ER, the A subunit is released from the B subunit and unfolded by protein disulfide isomerase (PDI), enabling its translocation across the ER membrane into the cytoplasm. The A subunit then refolds and ADP ribosylates Gαs. This leads to activation of adenylate cyclase (AC) and increased cAMP levels. Chloride secretion follows, triggering massive watery diarrhea. See text for further details. Created using Biorender.
Here, we focus in this review on how the CTxB subunit can be used as a non-toxic reporter to probe basic aspects of membrane structure, mechanisms of endocytosis, nanodomain assembly, and membrane trafficking enabled by glycosphingolipid biology.
2. CTxB as a Probe for Membrane Organization
The plasma membrane is thought to contain over 100,000 different lipid species whose distributions within the membrane leaflets are not homogeneous. Instead, these lipids, along with membrane-associated proteins are often organized laterally into domains based on differential physicochemical interactions [28]. One well-studied example of membrane organization is membrane (lipid) rafts, which are regions enriched in sterols, sphingolipids and saturated phospholipids [29,30]. Rafts are defined by having altered membrane miscibility, highly packed and tightly ordered lipid molecules characteristic of lipids in the liquid ordered (Lo) phase, distinct from a more fluid liquid disordered (Ld) membrane environment [31]. The biology of CTx is closely linked to membrane rafts [32,33,34,35], and over the last two decades, CTxB has often been used as a marker for rafts and thereby for deciphering their properties and physiological functions [29,36,37]. In this section, we focus on several recent studies seeking to uncover the mechanisms that control the association of CTxB with rafts and related membrane nanodomains (Figure 3). Membrane rafts are thought to range in size from few to a couple of hundreds of nanometers. Likewise, their lifetimes are also thought to vary from nanoseconds to much longer times under conditions where they are stabilized [29]. Given the inherent difficulty of experimentally elucidating such dynamic and diffraction-limited processes as well as their composition in cells, many of the characteristics of lipid rafts in cells are still under debate [38,39,40,41]. Several fundamental properties of rafts can, however, be studied in model systems such as giant unilamellar vesicles (GUVs) and giant plasma membrane vesicles (GPMVs) [42,43]. Depending on the lipid composition and experimental conditions, membranes in GUVs and GPMVs form co-existing Lo and Ld domains.
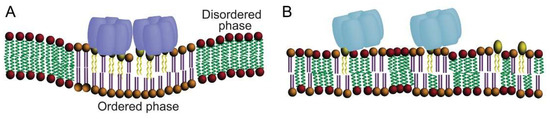
Figure 3.
Multivalent binding of CTxB binding to order-preferring GM1 is required to induce phase separation and stabilize ordered membrane domains. (A) Wild type CTxB can bind to up to 5 GM1s. As a consequence of multivalent binding to GM1, CTxB can generate and sort to order-preferring lipid phases. Multivalent binding also induces membrane curvature (see Figure 4 for further details). (B) A CTxB mutant containing a single binding functional GM1 site associates equally well with ordered and disordered domains. It is also incapable of stabilizing raft domains or inducing membrane curvature. Wild type CTxB is predicted to behave similarly under conditions where it binds to a single GM1, for example at low GM1: CTxB ratios. Note that for simplicity, cholesterol is not depicted in the membrane.
CTxB associates with the ordered phase of GUVs containing trace amounts of GM1, but this depends on the structure of its ceramide moiety (see further discussion below) (Figure 1C,D) [44]. CTxB also can induce domain formation in single-phase GUVs comprised of lipids close to a demixing point, suggesting that the toxin can actively reorganize the membrane to form domains [45,46]. Typically, CTxB preferentially associates with and stabilizes ordered raft-like domains in GPMVs derived from living cell plasma membranes [12,44,45,47,48,49,50,51,52,53,54]. These findings led to the hypothesis that CTxB assembles stabilized raft domains via its ability to cluster together multiple copies of GM1. We recently tested this idea in GPMVs using a monovalent variant of CTxB capable of only binding to a single GM1 [55]. Consistent with the predictions of this hypothesis, a monovalent mutant CTxB did not bind preferentially to Lo nanodomains—rather it bound equally well to the ordered and disordered phases [55]. Since GPMVs retain the same lipid complexity as biological membranes [54], similar stabilization of rafts likely occurs upon binding of CTxB to the plasma membrane of living cells.
The intrinsic preference of GM1 itself for raft or non-raft domains also influences the phase in which CTxB preferentially resides. GM1 is classically thought to be a raft-associated glycolipid [29,36,43,45]. However, some GM1 species with unsaturated acyl chain lengths do not associate with raft domains. We recently investigated how these key structural features of GM1 influence its phase preference [13,56]. Headgroup-labeled fluorescent GM1 species containing a C16:0 acyl chain partition into the Lo phase, whereas GM1 with a C16:1 acyl chain do not, even when clustered by CTxB [56]. A small library of GM1 species was also recently tested for Lo and Ld phase preference in GPMVs obtained from various cell types. Here, we found that the partitioning between phases depended on the presence or absence of unsaturated cis-double bonds in the acyl chain of the ceramide moiety [13]. Normally in cells, the most prominent acyl chain structures include palmitic, stearic and nervonic acid [57]. How CTxB would behave when bound to multiple but different GM1 species containing different combinations of acyl chains remains to be investigated.
GM1 is also known to form nanoclusters in cell membranes and model membranes [58,59,60]. There is wide discrepancy in the reported size, composition and phase of these clusters in model systems [60]. In cells, GM1 nanoclusters have been reported to form in a cholesterol, actin, and temperature-dependent manner [58,59]. Our recent work has revealed that the ability of GM1 species to form nanoclusters is also controlled by their ceramide structure [56]. As reported by fluorescence anisotropy homoFRET measurements, GM1 containing a C16:0 acyl chain forms nanoclusters in live cell membranes. These nanoclusters are cholesterol, phosphatidylserine, and actin-dependent, suggesting that they share some features previously reported for nanoclusters of GPI-anchored proteins [61]. In contrast, GM1 with a C16:1 acyl chain is predominantly randomly distributed across the cell surface [56]. The addition of CTxB induces higher order clustering within live plasma membranes, leading to the formation of domains which are stable over a timescale of seconds [56]. While an increase in cluster size was observed for both saturated and unsaturated GM1 upon CTxB binding, the nanodomain properties differed depending on GM1′s acyl chain. The function of the GM1 nanodomains is not yet clear, but could represent sites where CTxB initially binds the membrane or sites that dictate lipid sorting through the different recycling, retrograde, and late endosome/lysosome endocytic pathways [13]. They may for example be linked to transport of CTx from the plasma membrane to the Golgi complex, a process already known to be actin dependent [62].
Taken together, these findings emphasize the importance of the structure of both CTxB and GM1 in controlling their association with rafts and cellular nanodomains. This has broader implications for our understanding of how AB5 toxins regulate their association with membrane domains, as well as the general roles that lipid acyl chain structure and protein-mediated lipid clustering events play in membrane organization. They also raise interesting questions about how the structure of CTxB and GM1 controls additional biological activities of the toxin such as its ability to sense and/or induce curvature in cell membranes and influences its endocytic and intracellular trafficking, as discussed further below.
3. CTxB as a Sensor and Inducer of Membrane Curvature
Regulated membrane shapes are critical to diverse cellular processes such as exocytosis/endocytosis, pathogen vulnerability/protection, therapeutic targeting, and organelle morphology [63]. Proteins have shown a diverse capability to sense and generate curvature by a variety of mechanisms depending on both the membrane and protein properties [64,65,66,67]. CTxB has emerged as an important model and tool to understand how proteins affect and are affected by membrane curvature. CTxB exhibits an intrinsic capability to manipulate membrane shapes, as shown by CTxB-induced membrane budding in quasi-one component model lipid bilayers [68,69]. In cells, CTxB localizes to the inside of membrane tubules and vesicles as it is trafficked from the plasma membrane to the ER. Current models suggest that CTxB and related toxin B-subunits such as that of Shiga toxin (STxB) not only prefer to reside in regions of negative membrane curvature like those found inside transport carriers but also induce de novo curvature upon binding to membranes via a cooperative process [36,68,70,71,72,73,74,75,76,77,78]. Thus, curvature sensing and generation are key to the toxin’s biological activities.
To provide mechanistic insights into how CTxB induces membrane curvature, we recently employed polarized localization microscopy (PLM), a form of super-resolution microscopy that detects membrane curvature [69,79]. PLM combines single-molecule localization microscopy with polarized total internal reflection microscopy to reveal membrane curvature. By varying the ratio of GM1: CTxB in model membranes of controlled composition, we found that CTxB requires a stoichiometry of binding with at least two GM1 molecules per CTxB to generate curvature [79,80]. This was confirmed by comparing pentavalent wild type (wt) or a monovalent mutant CTxB (mCTxB) capable of binding only a single GM1 in both cells and model membranes [79]. In all conditions, multivalent binding was critical for CTxB to induce membrane shape changes.
CTxB likely induces membrane curvature by several mechanisms, all of which depend on its ability to cluster multiple GM1s. The first is a direct consequence of the physical shape of CTxB and relative locations of the five GM1 binding pockets. In particular, the GM1 binding pockets on CTxB are located on the perimeter of the homopentamer and elevated above the membrane-binding surface of CTxB. Because of this the membrane must deform to enable binding to more than one GM1 simultaneously (Figure 4). According to this model, upon engagement of the first GM1 CTxB assumes a tilted orientation, minimally perturbing membrane shape [72,81,82,83]. In response to binding of CTxB to two or more GM1s, however, the membrane bends to allow GM1 to reach the peripheral GM1 binding pockets on the CTxB. Multivalent binding of GM1 to CTxB ultimately requires the membrane to wrap around the CTxB [72,77]. The degree of induced curvature is thus directly linked to the GM1:CTxB stoichiometry.
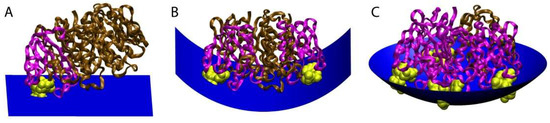
Figure 4.
Model for how the stoichiometry of binding of CTxB to GM1 controls the degree of membrane curvature. The ratio of CTxB subunits bound (pink) and unbound (brown) to GM1 (yellow) affects the shape of the membrane surface (blue). (A) Binding of CTxB to a single GM1 has no effect on membrane curvature. (B) Binding to two GM1s generates cylindrical negative curvature in one dimension. (C) Binding of CTxB to three or more GM1s induces spherical negative membrane curvature in two dimensions. This results in a wrapping of the membrane around the CTxB, such as within an endocytic pit. The schematics were created in Visual Molecular Dynamics [84] by building upon a crystal structure of CTxB [85]. Figure reproduced with permission Kabbani, A.M., Raghunathan, K., Lencer, W.I., Kenworthy, A.K., and Kelly, C.V. Structured clustering of the glycosphingolipid GM1 is required for membrane curvature induced by cholera toxin. Proc. Natl. Acad. Sci. USA 2020, 117, 14978–14986 [79].
The ability of CTxB to induce membrane phase separation in response to GM1 crosslinking also contributes to its membrane bending activity. Local enrichment and crosslinking of GM1 by CTxB creates a local membrane composition enriched in order-preferring gangliosides, which may trigger lipid phase separation. Lipid phase separation can in turn encourage membrane bending by creating a line tension or differential lateral pressure profiles [86,87,88,89]. Coupling between the lipid phase and membrane curvature would thus be expected to initiate a cooperative feedback loop that amplifies membrane curvature [90].
Crosslinking of GM1 by CTxB may also induce local lipid compression and membrane tension changes, similar to that observed upon crosslinking of Gb3 by STxB [78]. This is important because membrane tension is key to membrane shape regulation [67]. In model membranes consisting primarily of POPC, CTxB induces similar membrane curvature when bound to GM1s that differ in the length and saturation of their acyl chains [79]. This implies that curvature generation is independent of the acyl chain composition of GM1 and that lipid phase separation is not required for CTxB to induce membrane shape changes [79]. Since both CTxB and STxB induce negative membrane curvature and are localized to the outer leaflet of the plasma membrane, it is unlikely that toxin crowding generates steric pressure since this would be counterproductive to endocytosis [91]. Instead, toxin binding may induce asymmetric membrane tension via compression of glycolipids in the outer leaflet to foster the negative membrane curvature. These local tension variations across the plasma membrane may in turn facilitate toxin internalization [92].
CTxB molecules must also act cooperatively to facilitate large-scale membrane shape changes [93]. The multivalent binding of CTxB to GM1 may also play an important role by facilitating the formation of local membrane hotspots of lipid phase separation and curvature [69,94]. One potential mechanism that could contribute to the local accumulation of CTxB is membrane-mediated attractive forces extending >20 nm between CTxBs [93,95,96]. These forces would be expected to be further amplified by membrane shape undulations [97,98]. The collective action of multiple CTxBs to bend cell membranes likely employs a three-dimensional scaffolding to generate membrane topographies orders-of-magnitude larger than a single CTxB [99].
In summary, CTxB induces and senses membrane curvature through a complex interplay of physical factors resulting from its molecular shape and lipid crosslinking. Recent results have demonstrated that CTxB locally accumulates and induces membrane bending though the collective result of lipid phase separation, compression, and crosslinking inherent in the multivalent binding of GM1 by CTxB. Ongoing efforts will focus on the precise mechanisms by which local physical changes to the membrane composition and shape recruit the downstream endocytic and intracellular trafficking machinery, for which WT and mutant CTxB are likely to be key tools for research.
4. CTxB as a Reporter of Clathrin-Independent Endocytosis
Endocytosis- a processes by which a plethora of molecules such as nutrients, extracellular cargoes, and activated membrane proteins/receptors are internalized into the cell- is critical for physiological proceedings such as nutrient uptake and intracellular signaling driving cellular homeostasis (reviewed in [100,101,102,103]). Endocytosis also drives cellular infection induced by bacterial and viral proteins and/or toxins (selected reviews include [104,105,106,107]). The process of endocytosis occurs at plasma membrane. Initiation of endocytic events induces biophysical modifications of the plasma membrane, leading to membrane invagination and scission of endocytic carriers to facilitate the uptake of molecules/cargoes into the interior of the cell.
Mechanistically, endocytosis can be broadly classified into clathrin-dependent and clathrin-independent pathways (extensively reviewed in [108,109,110]). Driven predominantly by the presence and activity of clathrin, clathrin mediated endocytosis (CME) is well-studied and characterized at molecular level [111,112,113]. In contrast to CME, the molecular determinants and mechanisms of clathrin independent endocytosis (CIE) differ depending upon the morphology of the endocytic carriers, cargoes and physiological need of the cells (reviewed extensively in [114,115,116,117,118,119,120,121,122]). Furthermore, a unique or a universal molecular player for CIE is yet to be defined and our knowledge about CIE machinery is continuing to evolve.
Studies on the uptake of cholera toxin and in particular its B subunit CTxB have not only enhanced our understanding of the endocytic itinerary and pathophysiology induced by bacterial toxins but also enlightened us about the biophysical modifications that the plasma membrane undergoes in preparation for endocytosis. In this section, we will briefly discuss about different routes of clathrin independent internalization of CTxB. Further, we will also touch upon the utility of CTxB in delineating bacterial toxin internalization pathways that in turn has broadened our knowledge about modalities and machineries driving CIE pathway in general.
Early on, it was recognized that CTxB can enter cells via multiple mechanisms, including both canonical clathrin-dependent endocytosis and clathrin-independent mechanisms [123,124,125] (Figure 5). For example, CTxB can be internalized via flask-shaped plasma membrane invaginations known as caveolae [126,127,128]. It is also taken up into cells via the CLathrin-Independent Carriers (CLICs) and GPI-Enriched Endocytic Compartments (GEECs) pathway, a dynamin-independent endocytic pathway responsible for the uptake of a variety of raft-associated proteins including GPI-anchored proteins [125,129,130,131,132,133]. Another dynamin- and caveolin-independent CIE mechanism utilized by CTxB is Arf6-mediated endocytosis, a pathway that internalizes similar cargoes as the CLIC/GEEC pathway [121,124,134,135].

Figure 5.
Overview of the intracellular trafficking of CTxB. The biological function of CTxB is to carry the enzymatically active A subunit of cholera toxin into cells. To do so, CTxB must first bind GM1 on the host cell membrane. The subsequent entry of CTxB into cells depends importantly on its ability to cluster multiple GM1s. This enables CTxB to induce and/or sort into areas of negative membrane curvature and to enter cells via raft-dependent, clathrin-independent endocytic pathways (CIE). It can also be internalized via clathrin coated pits (CCP) and caveolae (CAV). Following endocytosis, CTxB is trafficked to endosomal compartments such as early endosomes (EE) and recycling endosomes (RE). To induce cellular intoxification, CTxB must undergo additional retrograde trafficking steps to the trans-Golgi network (TGN) and endoplasmic reticulum (ER). Which intracellular trafficking pathways CTxB ultimately follows is controlled in part by the structure of the ceramide moiety of its receptor GM1.
More recent work has revealed that cholera toxin activates and is internalized by a clathrin-independent pathway dubbed fast-endophilin mediated endocytosis or FEME [116,118,136,137,138,139]. Driven by the N-BAR domain protein endophilin A2 (endoA2), the FEME pathway is activated at the leading edge of migrating cells in response to ligand binding to receptor tyrosine kinases and G-coupled receptors (GPCR), which are subsequently taken up by the pathway [137,138,139]. Endophilin plays several important roles in this pathway, including capture of transmembrane receptor cargo, generation of membrane curvature, and scission of tubular endocytic carriers in cooperation with dynamin and actin via a friction driven process [136,137,140]. Microtubules and dynein play important roles in the FEME pathway as well, contributing to membrane tubulation and scission [136,141,142]. Interestingly, CTxB is capable of activating FEME: in response to CTxB binding, endoA2 is recruited to the plasma membrane, resulting in uptake of CTxB into endoA2-positive carriers. Internalization of CTxB is reduced upon knock down of EndoA2, further implicating FEME as a mechanism that controls toxin uptake [136,143]. Additional machinery that regulates the FEME pathway is continuing to emerge [142,143].
An important question raised by these findings is how toxin binding is sensed by the cell and translated into a signal that triggers endocytosis. One hypothesis is that clustering of multiple copies of GM1 upon CTxB itself serves as a signal. According to this model, the ability of the toxin to bind multiple copies of GM1, as well as structured clustering of GM1 by CTxB, functionally regulate toxin internalization. In support of this idea, toxin variants engineered to contain as few as one GM1 binding site exhibit strongly attenuated internalization, although they are still capable of completing the intoxification pathway [144,145]. One major consequence of multivalent glycolipid binding, as discussed above, is induction of membrane curvature [79]. This principle was first identified for the case of STxB and represents an example of a broader mechanism whereby lectins generate membrane curvature to drive endocytosis by binding to multiple glycolipids or glycoproteins (the GL-Lect hypothesis) [146]. Membrane curvature created by the extracellular CTxB could potentially lead to the recruitment of intracellular curvature-sensing proteins, in turn controlling the local membrane composition [147,148]. However, CTxB mutants capable of binding to only a single copy of GM1 can sort into preformed clathrin-independent endocytic structures, suggesting glycolipid clustering-induced curvature generation is dispensable for its uptake into at least a subset of CIE carriers [141].
As discussed above, the binding of CTxB to multiple copies of GM1 also regulates its association with ordered domains and the ability of the toxin to stabilize raft domains [55]. Glycolipid crosslinking initiated by cell surface binding of cholera toxin and other AB5 family toxins such as STx also initiates signaling events [149]. For example, binding of STx upregulates the formation of clathrin-coated pits, and modulates microtubule dynamics [150,151]. It seems likely that these raft-stabilizing activities and signaling capacities of CTxB, combined with local curvature generation that drives the recruitment of the intracellular endocytic machinery, contribute to its endocytic uptake by generating a “curvature-signaling hub”. However, the exact mechanisms by which these processes are coupled remain to be determined.
In conclusion, internalization of CTxB occurs via multiple mechanisms and depends on a variety of molecular players operating at the plasma membrane on different time scales. CTxB actively regulates several of these pathways by inducing changes in membrane organization and intracellular signaling in response to toxin binding. These activities are enhanced by CTxB’s ability to cluster multiple GM1s, but glycolipid clustering is not essential for internalization of CTxB or the ability of CTx to cause cellular intoxification. However, many open questions remain about how these events are orchestrated. It is thus clear that CTxB will continue to be a critically important tool to advance our knowledge of bacterial toxin internalization as well as to further illuminate our understanding of mechanisms of CIE.
5. CTxB as a Probe of Retrograde Trafficking Mechanisms
The ultimate destination of internalized CTxB is the ER. The endocytic network of all cells includes a pathway from the plasma membrane retrograde to the trans-Golgi network (TGN)—and for the glycosphingolipids all the way backwards in the secretory pathway to the ER [152]. For example, the pathway operates to regulate recycling of the mannose-6-phosphate receptor, the endosome protease furin, and the endogenous glycosphingolipids. Trafficking of CTxB and the other enteric AB5 toxins from cell surface all the way into the ER requires binding to membrane glycosphingolipids of the host cell, followed by endocytosis, endosomal sorting, and transport of the CTxB-GM1 complex into the retrograde pathway (Figure 5) [153,154]. The retrograde pathway links the cell surface with the trans-Golgi complex and the ER by vesicular trafficking. This pathway was first discovered in studies on STx by Sandvig and van Deurs using thin-section electron microscopy [155]. This marked a fundamental turning point in our understanding of how these toxins entered host cells to cause disease.
STx, CTx and other AB5-toxins have since provided robust tools to study the mechanisms and components responsible for endosomal sorting and membrane trafficking in the retrograde pathway (summarized before in [11,77,152]). Toxin trafficking was measured by immune or direct labeling of the toxins with fluorophores or nano-gold particles or biochemically by tagging the toxins with N-glycosylation motifs that became glycosylated when the toxins entered the ER lumen [33,156]. These studies were highly informative, but none were able to measure retrograde trafficking in real time or quantitatively. In addition, both the imaging and biochemical approaches were technically demanding, which prevented their application to high content and high throughput unbiased genetic or chemical screens.
To address these problems, we modified the new split fluorescent protein technologies to link a small fragment of GFP to CTx (via fusion to the A2-chain, termed CTB-mNG211) [10,157]. The approach led to the development of a novel quantitative and near real-time single-cell flow cytometry assay for retrograde membrane transport driven by CTxB binding to GM1 [10]. Retrograde trafficking to either the TGN or ER was monitored in cells stably expressing the GFP acceptor fragment (mNG21–10 GFP) fused to TGN or ER targeting sequence by quantifying the evolution of a fluorescence signal upon binding of CTB-mNG211 and mNG21–10 GFP. The assay led to the discovery that perturbations of the sheet and tubular morphology of the ER affects the retrograde trafficking pathway. Moving forward, this approach should be fully amenable to high throughput studies on the underlying biology of membrane trafficking.
In a second approach, we have directly visualized the trafficking of GM1 itself through the use of fluorescent headgroup-labeled forms of GM1 [12,13,158]. These lipids enabled us to monitor retrograde trafficking of GM1 in the presence and absence of bound CTxB and dissect the role of ceramide structure in dictating the trafficking pathways utilized by GM1. These are important questions given that lipids themselves are sorted in endosomal pathways [159] and reports that trafficking of other AB5 toxins are affected by the structure of their glycolipid receptors [160]. We discovered that retrograde sorting of the CTxB-GM1 complex depends importantly on the structure of the GM1 ceramide moiety [12,13]. Ceramide structure, driven by the length of the ceramide acyl chain and position of any cis double bonds if present [13], enables sorting of the toxin (and the glycosphingolipid itself) into the narrow and highly curved sorting tubules emerging from the early sorting endosome compartment. These sorting tubules feed the recycling, retrograde, and (in polarized cells) transcytotic pathways. Fully saturated ceramide acyl chains drive the lipid away from sorting tubules causing retention of GM1 in the body of the sorting endosome and maturation into late endosomes and lysosomes. Notably, sorting of the different GM1 species among these pathways correlates with the ability of the different ceramide structures to regulate the association with cholesterol-dependent membrane nanodomains [13]. As GM1 acts as the trafficking receptor for the 84 kDa CTx, we believe these discoveries might be harnessed for clinical applications that require transport of therapeutic peptides and proteins into the various sub-cellular compartments of cells and even across mucosal barriers by transcytosis [158,161,162,163,164].
Altogether, these recent advances highlight the continued importance of CTx and GM1 as reporters of retrograde trafficking pathways. The tools described here should ultimately help develop a deeper understanding of mechanisms controlling the intracellular transport of CTx and related toxins, uncover principles that govern both protein and lipid sorting at multiple sites within cells, and design new strategies for delivery of therapeutics.
6. Alternative Membrane Glycoprotein Receptors Affecting CTx Biology
CTxB binds the ganglioside GM1 with high affinity, and GM1 has been shown with the greatest clarity to act as the functional receptor leading to CTx entry into the ER of host cells and the induction of toxicity [12,13,144,165,166,167,168,169,170,171,172]. However, it has long been known that CTx will also bind other glycosphingolipids [7,167,173,174,175,176,177,178,179,180,181]—and even glycosylated proteins, including the histo-blood group antigens [24,25]. The site of (low affinity) binding to the histo-blood group antigens was recently elucidated and found to occur on the side of the B-pentamer separate from the site where the B-subunit binds the glycosphingolipids [26]. Some reports show evidence consistent with the idea that these glycoproteins can act on their own, like the glycosphingolipids, to enable endocytic uptake and retrograde trafficking of CTx into the ER required for the induction of toxicity [27].
The evidence for secondary receptors, though in many ways compelling, is largely circumstantial and could have alternative explanations. The glycoprotein receptors do lead to endocytic uptake, for example [182]. However, for our part, we do not think binding to the glycoproteins function in the retrograde pathway. First, none of the implicated glycoproteins have been shown to traffic retrograde all the way into the ER; this is a very unusual pathway for plasma membrane proteins. Additionally, more unambiguously, point mutations in the primary binding site for the glycosphingolipids fully inactivate toxicity [85,168]. Thus, binding to the glycosphingolipids is essential. The secondary glycoprotein receptors for CTx do, however, modify toxin action, and the histo-blood group antigens for example are known modifiers of disease [16,17,18,19,20,21,22,23]. As originally proposed by Heim et al. [26], we believe the highly prevalent glycoproteins act as low-affinity binding site receptors for CTx influencing toxin action by enabling the initial binding of toxin to the intestinal cell surface. This precedes and likely enables toxin binding to the much more sparsely prevalent membrane glycosphingolipids, which act as the functional trafficking receptors enabling toxicity.
7. Take-Homes and Open Questions
In this review, we highlighted recent advances in our understanding of membrane biology and biophysics obtained through the use of CTxB. One important conclusion that emerges from these studies is that CTxB is not simply a passive reporter. It can drive phase separation, induce membrane curvature, stabilize rafts, stimulate its own internalization into cells, and re-direct GM1 into different intracellular trafficking pathways. This is not a new message, but it is one worth repeating given CTxB is still sometimes assumed to represent a benign raft and endocytic marker in the literature.
It is thus essential to exercise caution when using CTxB as a probe for membrane organization and trafficking mechanisms, especially in poorly defined systems.
It is also becoming increasingly clear that not all GM1s are created equal. Depending on its acyl chain structure and degree of saturation, GM1 can associate with ordered or disordered membrane phases in model systems, and form nanoclusters- or not- in cell membranes. CTxB itself also behaves very differently depending on whether it is bound to one or more copies of GM1, as well as their structural features. This can have critical consequences for CTxB’s ability to associate with rafts, bend membranes, and trigger endocytosis. Even changing the GM1/ CTxB ratio is sufficient to evoke some of these changes. Finally, it is important to recognize that lower affinity receptors for CTxB also exist, including other glycolipids and fucosylated secondary receptors. The membrane remodeling activities of CTxB are thus highly context-dependent.
The recent pandemic has highlighted the importance of understanding the varied mechanisms by which pathogens gain entry into cells. Many of the membrane remodeling activites of CTxB are shared with other members of the AB5 toxin family and some viruses [68,76,183]. For example, binding of certain viruses to cells via glycolipid receptors is thought to trigger similar mechanisms that facilitate their endocytic uptake [68,183]. The GL-Lect hypothesis suggests an intriguing mechanism by which this might occur [77,116,118,146,184,185]. Understanding how these events are orchestrated could provide essential insights into how the uptake of multiple classes of pathogens could be blocked- or how the internalization of specific pathogens could be inhibited while leaving endogenous endocytic pathways intact. Finally, the pathways uncovered through studies of CTxB hold the potential to be targeted for the delivery of large drug molecules [161]. Thus, the tricks developed by CTxB to enable CTx to enter cells may ultimately be exploited for pharmacological purposes.
Author Contributions
Conceptualization, A.K.K., C.V.K. and W.I.L.; writing—original draft preparation, A.K.K., S.S.S., K.R., A.T., T.W., C.V.K. and W.I.L.; writing—review and editing, A.K.K., S.S.S., K.R., A.T., T.W., C.V.K. and W.I.L.; funding acquisition, A.K.K., C.V.K., W.I.L. All authors have read and agreed to the published version of the manuscript.
Funding
This material is based upon work supported by the National Science Foundation under Grant No. DMR-1652316 (C.V.K.), National Institutes of Health R37 DK048106 (W.I.L.); National Institutes of Health RO1 DK104868 (W.I.L.); Harrington Scholar Innovator Award (W.I.L); National Institutes of Health P30 DK034854; The Harvard Digestive Diseases Center (W.I.L.), and National Institutes of Health R01 GM106720 (A.K.K.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the writing of the manuscript or in the decision to publish it.
References
- De, S.N. Enterotoxicity of bacteria-free culture-filtrate of Vibrio cholerae. Nature 1959, 183, 1533–1534. [Google Scholar] [CrossRef]
- Herrington, D.A.; Hall, R.H.; Losonsky, G.; Mekalanos, J.J.; Taylor, R.K.; Levine, M.M. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J. Exp. Med. 1988, 168, 1487–1492. [Google Scholar] [CrossRef]
- Bharati, K.; Ganguly, N.K. Cholera toxin: A paradigm of a multifunctional protein. Indian J. Med. Res. 2011, 133, 179–187. [Google Scholar]
- O’Neal, C.J.; Amaya, E.I.; Jobling, M.G.; Holmes, R.K.; Hol, W.G. Crystal structures of an intrinsically active cholera toxin mutant yield insight into the toxin activation mechanism. Biochemistry 2004, 43, 3772–3782. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, W.B.; Precious, B.L.; Homans, S.W. Dissecting the cholera toxin-ganglioside GM1 interaction by isothermal titration calorimetry. J. Am. Chem. Soc. 2004, 126, 1047–1054. [Google Scholar] [CrossRef]
- Krishnan, P.; Singla, A.; Lee, C.A.; Weatherston, J.D.; Worstell, N.C.; Wu, H.J. Hetero-multivalent binding of cholera toxin subunit B with glycolipid mixtures. Colloids Surf. B Biointerfaces 2017, 160, 281–288. [Google Scholar] [CrossRef]
- Chiricozzi, E.; Mauri, L.; Ciampa, M.G.; Prinetti, A.; Sonnino, S. On the use of cholera toxin. Glycoconj. J. 2018, 35, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Merritt, E.A.; Sarfaty, S.; van den Akker, F.; L’Hoir, C.; Martial, J.A.; Hol, W.G. Crystal structure of cholera toxin B-pentamer bound to receptor GM1 pentasaccharide. Protein Sci. 1994, 3, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Lencer, W.I. Retrograde transport of cholera toxin into the ER of host cells. Int. J. Med. Microbiol. 2004, 293, 491–494. [Google Scholar] [CrossRef]
- Luong, P.; Li, Q.; Chen, P.F.; Wrighton, P.J.; Chang, D.; Dwyer, S.; Bayer, M.T.; Snapper, S.B.; Hansen, S.H.; Thiagarajah, J.R.; et al. A quantitative single-cell assay for retrograde membrane traffic enables rapid detection of defects in cellular organization. Mol. Biol. Cell 2020, 31, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Spooner, R.A.; Smith, D.C.; Easton, A.J.; Roberts, L.M.; Lord, J.M. Retrograde transport pathways utilised by viruses and protein toxins. Virol. J. 2006, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Chinnapen, D.J.; Hsieh, W.T.; te Welscher, Y.M.; Saslowsky, D.E.; Kaoutzani, L.; Brandsma, E.; D’Auria, L.; Park, H.; Wagner, J.S.; Drake, K.R.; et al. Lipid sorting by ceramide structure from plasma membrane to ER for the cholera toxin receptor ganglioside GM1. Dev. Cell 2012, 23, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Schmieder, S.S.; Tatituri, R.; Anderson, A.; Kelly, K.; Lencer, W.I. Structural basis for acyl chain control over glycosphingolipid sorting and vesicular trafficking. bioRxiv 2021. [Google Scholar] [CrossRef]
- Wernick, N.L.; Chinnapen, D.J.; Cho, J.A.; Lencer, W.I. Cholera toxin: An intracellular journey into the cytosol by way of the endoplasmic reticulum. Toxins 2010, 2, 310–325. [Google Scholar] [CrossRef]
- Lencer, W.I.; Tsai, B. The intracellular voyage of cholera toxin: Going retro. Trends Biochem. Sci. 2003, 28, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.; DasAdhikary, C.R. Possible role of blood-group secretory substances in the aetiology of cholera. Trans. R. Soc. Trop. Med. Hyg. 1978, 72, 664–665. [Google Scholar] [CrossRef]
- Harris, J.B.; LaRocque, R.C. Cholera and ABO blood group: Understanding an ancient association. Am. J. Trop. Med. Hyg. 2016, 95, 263–264. [Google Scholar] [CrossRef]
- Heggelund, J.E.; Haugen, E.; Lygren, B.; Mackenzie, A.; Holmner, A.; Vasile, F.; Reina, J.J.; Bernardi, A.; Krengel, U. Both El Tor and classical cholera toxin bind blood group determinants. Biochem. Biophys. Res. Commun. 2012, 418, 731–735. [Google Scholar] [CrossRef]
- Kuhlmann, F.M.; Santhanam, S.; Kumar, P.; Luo, Q.; Ciorba, M.A.; Fleckenstein, J.M. Blood Group O-Dependent Cellular Responses to Cholera Toxin: Parallel Clinical and Epidemiological Links to Severe Cholera. Am. J. Trop. Med. Hyg. 2016, 95, 440–443. [Google Scholar] [CrossRef][Green Version]
- Mandal, P.K.; Branson, T.R.; Hayes, E.D.; Ross, J.F.; Gavin, J.A.; Daranas, A.H.; Turnbull, W.B. Towards a structural basis for the relationship between blood group and the severity of El Tor cholera. Angew. Chem. Int. Ed. Engl. 2012, 51, 5143–5146. [Google Scholar] [CrossRef]
- Sircar, B.K.; Dutta, P.; De, S.P.; Sikdar, S.N.; Deb, B.C.; Pal, S.C.; Mitra, S.S. ABO blood group distributions in diarrhoea cases including cholera in Calcutta. Ann. Hum. Biol. 1981, 8, 289–291. [Google Scholar] [CrossRef]
- Swerdlow, D.L.; Mintz, E.D.; Rodriguez, M.; Tejada, E.; Ocampo, C.; Espejo, L.; Barrett, T.J.; Petzelt, J.; Bean, N.H.; Seminario, L.; et al. Severe life-threatening cholera associated with blood group O in Peru: Implications for the Latin American epidemic. J. Infect. Dis. 1994, 170, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Vasile, F.; Reina, J.J.; Potenza, D.; Heggelund, J.E.; Mackenzie, A.; Krengel, U.; Bernardi, A. Comprehensive analysis of blood group antigen binding to classical and El Tor cholera toxin B-pentamers by NMR. Glycobiology 2014, 24, 766–778. [Google Scholar] [CrossRef]
- Wands, A.M.; Fujita, A.; McCombs, J.E.; Cervin, J.; Dedic, B.; Rodriguez, A.C.; Nischan, N.; Bond, M.R.; Mettlen, M.; Trudgian, D.C.; et al. Fucosylation and protein glycosylation create functional receptors for cholera toxin. eLife 2015, 4, e09545. [Google Scholar] [CrossRef] [PubMed]
- Heggelund, J.E.; Burschowsky, D.; Bjornestad, V.A.; Hodnik, V.; Anderluh, G.; Krengel, U. High-resolution crystal structures elucidate the molecular basis of cholera blood group dependence. PLoS Pathog. 2016, 12, e1005567. [Google Scholar] [CrossRef] [PubMed]
- Heim, J.B.; Hodnik, V.; Heggelund, J.E.; Anderluh, G.; Krengel, U. Crystal structures of cholera toxin in complex with fucosylated receptors point to importance of secondary binding site. Sci. Rep. 2019, 9, 12243. [Google Scholar] [CrossRef]
- Cervin, J.; Wands, A.M.; Casselbrant, A.; Wu, H.; Krishnamurthy, S.; Cvjetkovic, A.; Estelius, J.; Dedic, B.; Sethi, A.; Wallom, K.L.; et al. GM1 ganglioside-independent intoxication by cholera toxin. PLoS Pathog. 2018, 14, e1006862. [Google Scholar] [CrossRef]
- Lingwood, D.; Simons, K. Lipid rafts as a membrane-organizing principle. Science 2010, 327, 46–50. [Google Scholar] [CrossRef]
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–41. [Google Scholar] [CrossRef]
- Simons, K.; Sampaio, J.L. Membrane organization and lipid rafts. Cold Spring Harb. Perspect. Biol. 2011, 3, a004697. [Google Scholar] [CrossRef]
- Badizadegan, K.; Wolf, A.A.; Rodighiero, C.; Jobling, M.; Hirst, T.R.; Holmes, R.K.; Lencer, W.I. Floating cholera toxin into epithelial cells: Functional association with caveolae-like detergent-insoluble membrane microdomains. Int. J. Med. Microbiol. 2000, 290, 403–408. [Google Scholar] [CrossRef]
- Fujinaga, Y.; Wolf, A.A.; Rodighiero, C.; Wheeler, H.; Tsai, B.; Allen, L.; Jobling, M.G.; Rapoport, T.; Holmes, R.K.; Lencer, W.I. Gangliosides that associate with lipid rafts mediate transport of cholera and related toxins from the plasma membrane to endoplasmic reticulm. Mol. Biol. Cell 2003, 14, 4783–4793. [Google Scholar] [CrossRef] [PubMed]
- Lencer, W.I.; Saslowsky, D. Raft trafficking of AB5 subunit bacterial toxins. Biochim. Biophys. Acta 2005, 1746, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Chinnapen, D.J.; Chinnapen, H.; Saslowsky, D.; Lencer, W.I. Rafting with cholera toxin: Endocytosis and trafficking from plasma membrane to ER. FEMS Microbiol. Lett. 2007, 266, 129–137. [Google Scholar] [CrossRef]
- Day, C.A.; Kenworthy, A.K. Functions of cholera toxin B-subunit as a raft cross-linker. Essays Biochem. 2015, 57, 135–145. [Google Scholar] [PubMed]
- Raghunathan, K.; Kenworthy, A.K. Dynamic pattern generation in cell membranes: Current insights into membrane organization. Biochim. Biophys. Acta Biomembr. 2018, 1860, 2018–2031. [Google Scholar] [CrossRef]
- Levental, I.; Levental, K.R.; Heberle, F.A. Lipid rafts: Controversies resolved, mysteries remain. Trends Cell Biol. 2020, 30, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Klotzsch, E.; Schutz, G.J. A critical survey of methods to detect plasma membrane rafts. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20120033. [Google Scholar] [CrossRef] [PubMed]
- Sevcsik, E.; Schutz, G.J. With or without rafts? Alternative views on cell membranes. Bioessays 2016, 38, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.M.; Fairn, G.D. Mesoscale organization of domains in the plasma membrane—Beyond the lipid raft. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 192–207. [Google Scholar] [CrossRef] [PubMed]
- Wesolowska, O.; Michalak, K.; Maniewska, J.; Hendrich, A.B. Giant unilamellar vesicles—A perfect tool to visualize phase separation and lipid rafts in model systems. Acta Biochim. Pol. 2009, 56, 33–39. [Google Scholar] [CrossRef]
- Sezgin, E.; Kaiser, H.J.; Baumgart, T.; Schwille, P.; Simons, K.; Levental, I. Elucidating membrane structure and protein behavior using giant plasma membrane vesicles. Nat. Protoc. 2012, 7, 1042–1051. [Google Scholar] [CrossRef]
- Bacia, K.; Schwille, P.; Kurzchalia, T. Sterol structure determines the separation of phases and the curvature of the liquid-ordered phase in model membranes. Proc. Natl. Acad. Sci. USA 2005, 102, 3272–3277. [Google Scholar] [CrossRef] [PubMed]
- Hammond, A.T.; Heberle, F.A.; Baumgart, T.; Holowka, D.; Baird, B.; Feigenson, G.W. Crosslinking a lipid raft component triggers liquid ordered-liquid disordered phase separation in model plasma membranes. Proc. Natl. Acad. Sci. USA 2005, 102, 6320–6325. [Google Scholar] [CrossRef] [PubMed]
- Lingwood, D.; Ries, J.; Schwille, P.; Simons, K. Plasma membranes are poised for activation of raft phase coalescence at physiological temperature. Proc. Natl. Acad. Sci. USA 2008, 105, 10005–10010. [Google Scholar] [CrossRef]
- Sezgin, E.; Levental, I.; Grzybek, M.; Schwarzmann, G.; Mueller, V.; Honigmann, A.; Belov, V.N.; Eggeling, C.; Coskun, U.; Simons, K.; et al. Partitioning, diffusion, and ligand binding of raft lipid analogs in model and cellular plasma membranes. Biochim. Biophys. Acta 2012, 1818, 1777–1784. [Google Scholar] [CrossRef]
- Sengupta, P.; Baird, B.; Holowka, D. Lipid rafts, fluid/fluid phase separation, and their relevance to plasma membrane structure and function. Semin. Cell Dev. Biol. 2007, 18, 583–590. [Google Scholar] [CrossRef]
- Johnson, S.A.; Stinson, B.M.; Go, M.S.; Carmona, L.M.; Reminick, J.I.; Fang, X.; Baumgart, T. Temperature-dependent phase behavior and protein partitioning in giant plasma membrane vesicles. Biochim. Biophys. Acta 2010, 1798, 1427–1435. [Google Scholar] [CrossRef]
- Kaiser, H.J.; Lingwood, D.; Levental, I.; Sampaio, J.L.; Kalvodova, L.; Rajendran, L.; Simons, K. Order of lipid phases in model and plasma membranes. Proc. Natl. Acad. Sci. USA 2009, 106, 16645–16650. [Google Scholar] [CrossRef]
- Levental, I.; Lingwood, D.; Grzybek, M.; Coskun, U.; Simons, K. Palmitoylation regulates raft affinity for the majority of integral raft proteins. Proc. Natl. Acad. Sci. USA 2010, 107, 22050–22054. [Google Scholar] [CrossRef] [PubMed]
- Levental, I.; Byfield, F.J.; Chowdhury, P.; Gai, F.; Baumgart, T.; Janmey, P.A. Cholesterol-dependent phase separation in cell-derived giant plasma-membrane vesicles. Biochem. J. 2009, 424, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Kahya, N.; Scherfeld, D.; Bacia, K.; Poolman, B.; Schwille, P. Probing lipid mobility of raft-exhibiting model membranes by fluorescence correlation spectroscopy. J. Biol. Chem. 2003, 278, 28109–28115. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, T.; Hammond, A.T.; Sengupta, P.; Hess, S.T.; Holowka, D.A.; Baird, B.A.; Webb, W.W. Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles. Proc. Natl. Acad. Sci. USA 2007, 104, 3165–3170. [Google Scholar] [CrossRef]
- Raghunathan, K.; Wong, T.H.; Chinnapen, D.J.; Lencer, W.I.; Jobling, M.G.; Kenworthy, A.K. Glycolipid crosslinking is required for cholera toxin to partition into and stabilize ordered domains. Biophys. J. 2016, 111, 2547–2550. [Google Scholar] [CrossRef][Green Version]
- Arumugam, S.; Schmieder, S.; Pezeshkian, W.; Becken, U.; Wunder, C.; Chinnapen, D.; Ipsen, J.H.; Kenworthy, A.K.; Lencer, W.; Mayor, S.; et al. Ceramide structure dictates glycosphingolipid nanodomain assembly and function. Nat. Commun. 2021, 12, 3675. [Google Scholar] [CrossRef]
- Schumann, J.; Leichtle, A.; Thiery, J.; Fuhrmann, H. Fatty acid and peptide profiles in plasma membrane and membrane rafts of PUFA supplemented RAW264.7 macrophages. PLoS ONE 2011, 6, e24066. [Google Scholar] [CrossRef]
- Fujita, A.; Cheng, J.; Hirakawa, M.; Furukawa, K.; Kusunoki, S.; Fujimoto, T. Gangliosides GM1 and GM3 in the living cell membrane form clusters susceptible to cholesterol depletion and chilling. Mol. Biol. Cell 2007, 18, 2112–2122. [Google Scholar] [CrossRef]
- Fujita, A.; Cheng, J.; Fujimoto, T. Segregation of GM1 and GM3 clusters in the cell membrane depends on the intact actin cytoskeleton. Biochim. Biophys. Acta 2009, 1791, 388–396. [Google Scholar] [CrossRef]
- Sarmento, M.J.; Ricardo, J.C.; Amaro, M.; Sachl, R. Organization of gangliosides into membrane nanodomains. FEBS Lett. 2020, 594, 3668–3697. [Google Scholar] [CrossRef]
- Raghupathy, R.; Anilkumar, A.A.; Polley, A.; Singh, P.P.; Yadav, M.; Johnson, C.; Suryawanshi, S.; Saikam, V.; Sawant, S.D.; Panda, A.; et al. Transbilayer lipid interactions mediate nanoclustering of lipid-anchored proteins. Cell 2015, 161, 581–594. [Google Scholar] [CrossRef]
- Badizadegan, K.; Wheeler, H.E.; Fujinaga, Y.; Lencer, W.I. Trafficking of cholera toxin-ganglioside GM1 complex into Golgi and induction of toxicity depend on actin cytoskeleton. Am. J. Physiol. Cell Physiol. 2004, 287, C1453–C1462. [Google Scholar] [CrossRef] [PubMed]
- McMahon, H.T.; Boucrot, E. Membrane curvature at a glance. J. Cell Sci. 2015, 128, 1065–1070. [Google Scholar] [CrossRef]
- Zimmerberg, J.; Kozlov, M.M. How proteins produce cellular membrane curvature. Nat. Rev. Mol. Cell Biol. 2006, 7, 9–19. [Google Scholar] [CrossRef]
- Stachowiak, J.C.; Brodsky, F.M.; Miller, E.A. A cost-benefit analysis of the physical mechanisms of membrane curvature. Nat. Cell Biol. 2013, 15, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, T.; Capraro, B.R.; Zhu, C.; Das, S.L. Thermodynamics and mechanics of membrane curvature generation and sensing by proteins and lipids. Annu. Rev. Phys. Chem. 2011, 62, 483–506. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Baumgart, T. Membrane tension and peripheral protein density mediate membrane shape transitions. Nat. Commun. 2015, 6, 5974. [Google Scholar] [CrossRef] [PubMed]
- Ewers, H.; Römer, W.; Smith, A.E.; Bacia, K.; Dmitrieff, S.; Chai, W.; Mancini, R.; Kartenbeck, J.; Chambon, V.; Berland, L.; et al. GM1 structure determines SV40-induced membrane invagination and infection. Nat. Cell Biol. 2010, 12, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Kabbani, A.M.; Kelly, C.V. Nanoscale membrane budding induced by CTxB and detected via polarized localization microscopy. Biophys. J. 2017, 113, 1795–1806. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, W.T.; Hsu, C.J.; Capraro, B.R.; Wu, T.; Chen, C.M.; Yang, S.; Baumgart, T. Curvature sorting of peripheral proteins on solid-supported wavy membranes. Langmuir 2012, 28, 12838–12843. [Google Scholar] [CrossRef]
- Groza, R.; Ewers, H. Membrane deformation by the cholera toxin beta subunit requires more than one binding site. Proc. Natl. Acad. Sci. USA 2020, 117, 17467–17469. [Google Scholar] [CrossRef]
- Pezeshkian, W.; Nabo, L.J.; Ipsen, J.H. Cholera toxin B subunit induces local curvature on lipid bilayers. FEBS Open Bio 2017, 7, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Tian, A.; Baumgart, T. Sorting of lipids and proteins in membrane curvature gradients. Biophys. J. 2009, 96, 2676–2688. [Google Scholar] [CrossRef] [PubMed]
- Sens, P.; Johannes, L.; Bassereau, P. Biophysical approaches to protein-induced membrane deformations in trafficking. Curr. Opin Cell Biol. 2008, 20, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Römer, W.; Berland, L.; Chambon, V.; Gaus, K.; Windschiegl, B.; Tenza, D.; Aly, M.R.; Fraisier, V.; Florent, J.C.; Perrais, D.; et al. Shiga toxin induces tubular membrane invaginations for its uptake into cells. Nature 2007, 450, 670–675. [Google Scholar] [CrossRef]
- Pezeshkian, W.; Hansen, A.G.; Johannes, L.; Khandelia, H.; Shillcock, J.C.; Kumar, P.B.; Ipsen, J.H. Membrane invagination induced by Shiga toxin B-subunit: From molecular structure to tube formation. Soft Matter 2016, 12, 5164–5171. [Google Scholar] [CrossRef]
- Johannes, L. Shiga toxin-a model for glycolipid-dependent and lectin-driven endocytosis. Toxins 2017, 9, 340. [Google Scholar] [CrossRef]
- Watkins, E.B.; Majewski, J.; Chi, E.Y.; Gao, H.; Florent, J.C.; Johannes, L. Shiga toxin induces lipid compression: A mechanism for generating membrane curvature. Nano Lett. 2019, 19, 7365–7369. [Google Scholar] [CrossRef]
- Kabbani, A.M.; Raghunathan, K.; Lencer, W.I.; Kenworthy, A.K.; Kelly, C.V. Structured clustering of the glycosphingolipid GM1 is required for membrane curvature induced by cholera toxin. Proc. Natl. Acad. Sci. USA 2020, 117, 14978–14986. [Google Scholar] [CrossRef] [PubMed]
- Kabbani, A.M.; Kelly, C.V. The detection of nanoscale membrane bending with polarized localization microscopy. Biophys. J. 2017, 113, 1782–1794. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, A.; Kumar, A.; Dasmahapatra, A.K. Multi-scale molecular dynamics study of cholera pentamer binding to a GM1-phospholipid membrane. J. Mol. Graph. Model. 2016, 68, 236–251. [Google Scholar] [CrossRef] [PubMed]
- Basu, I.; Mukhopadhyay, C. Insights into binding of cholera toxin to GM1 containing membrane. Langmuir 2014, 30, 15244–15252. [Google Scholar] [CrossRef]
- Rissanen, S.; Grzybek, M.; Orlowski, A.; Rog, T.; Cramariuc, O.; Levental, I.; Eggeling, C.; Sezgin, E.; Vattulainen, I. Phase partitioning of GM1 and its Bodipy-labeled analog determine their different binding to cholera toxin. Front. Physiol. 2017, 8, 252. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Merritt, E.A.; Sarfaty, S.; Jobling, M.G.; Chang, T.; Holmes, R.K.; Hirst, T.R.; Hol, W.G. Structural studies of receptor binding by cholera toxin mutants. Protein Sci. 1997, 6, 1516–1528. [Google Scholar] [CrossRef]
- Kuzmin, P.I.; Akimov, S.A.; Chizmadzhev, Y.A.; Zimmerberg, J.; Cohen, F.S. Line tension and interaction energies of membrane rafts calculated from lipid splay and tilt. Biophys. J. 2005, 88, 1120–1133. [Google Scholar] [CrossRef]
- Rozycki, B.; Lipowsky, R. Spontaneous curvature of bilayer membranes from molecular simulations: Asymmetric lipid densities and asymmetric adsorption. J. Chem. Phys. 2015, 142, 054101. [Google Scholar] [CrossRef]
- Yang, S.T.; Kiessling, V.; Tamm, L.K. Line tension at lipid phase boundaries as driving force for HIV fusion peptide-mediated fusion. Nat. Commun. 2016, 7, 11401. [Google Scholar] [CrossRef] [PubMed]
- Vind-Kezunovic, D.; Nielsen, C.H.; Wojewodzka, U.; Gniadecki, R. Line tension at lipid phase boundaries regulates formation of membrane vesicles in living cells. Biochim. Biophys. Acta 2008, 1778, 2480–2486. [Google Scholar] [CrossRef] [PubMed]
- Woodward, X.; Kelly, C.V. Coexisting lipid phases alter lipid dynamics and sort on nanoscale membrane curvature. bioRxiv 2020. [Google Scholar] [CrossRef]
- Stachowiak, J.C.; Hayden, C.C.; Sasaki, D.Y. Steric confinement of proteins on lipid membranes can drive curvature and tubulation. Proc. Natl. Acad. Sci. USA 2010, 107, 7781–7786. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.E.; Shi, Z. Do Cell Membranes Flow Like Honey or Jiggle Like Jello? Bioessays 2020, 42, e1900142. [Google Scholar] [CrossRef] [PubMed]
- Reynwar, B.J.; Illya, G.; Harmandaris, V.A.; Muller, M.M.; Kremer, K.; Deserno, M. Aggregation and vesiculation of membrane proteins by curvature-mediated interactions. Nature 2007, 447, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.S.; Lee, I.H.; Suh, J.H.; Park, S.C.; Oh, S.; Jordan, L.R.; Wittenberg, N.J.; Oh, S.H.; Jeon, N.L.; Lee, B.; et al. Reconstituting ring-rafts in bud-mimicking topography of model membranes. Nat. Commun. 2014, 5, 4507. [Google Scholar] [CrossRef]
- Simunovic, M.; Voth, G.A. Membrane tension controls the assembly of curvature-generating proteins. Nat. Commun. 2015, 6, 7219. [Google Scholar] [CrossRef]
- Muller, M.M.; Deserno, M.; Guven, J. Interface-mediated interactions between particles: A geometrical approach. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2005, 72, 061407. [Google Scholar] [CrossRef]
- Bradley, R.P.; Radhakrishnan, R. Curvature-undulation coupling as a basis for curvature sensing and generation in bilayer membranes. Proc. Natl. Acad. Sci. USA 2016, 113, E5117–E5124. [Google Scholar] [CrossRef] [PubMed]
- Johannes, L.; Pezeshkian, W.; Ipsen, J.H.; Shillcock, J.C. Clustering on membranes: Fluctuations and more. Trends Cell Biol. 2018, 28, 405–415. [Google Scholar] [CrossRef]
- Simunovic, M.; Evergren, E.; Golushko, I.; Prevost, C.; Renard, H.F.; Johannes, L.; McMahon, H.T.; Lorman, V.; Voth, G.A.; Bassereau, P. How curvature-generating proteins build scaffolds on membrane nanotubes. Proc. Natl. Acad. Sci. USA 2016, 113, 11226–11231. [Google Scholar] [CrossRef] [PubMed]
- Sigismund, S.; Lanzetti, L.; Scita, G.; Di Fiore, P.P. Endocytosis in the context-dependent regulation of individual and collective cell properties. Nat. Rev. Mol. Cell Biol. 2021. [Google Scholar] [CrossRef]
- Antonescu, C.N.; McGraw, T.E.; Klip, A. Reciprocal regulation of endocytosis and metabolism. Cold Spring Harb. Perspect. Biol. 2014, 6, a016964. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, P.P.; von Zastrow, M. Endocytosis, signaling, and beyond. Cold Spring Harb. Perspect. Biol. 2014, 6, a016865. [Google Scholar] [CrossRef]
- Barbieri, E.; Di Fiore, P.P.; Sigismund, S. Endocytic control of signaling at the plasma membrane. Curr. Opin Cell Biol. 2016, 39, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Cossart, P.; Helenius, A. Endocytosis of viruses and bacteria. Cold Spring Harb. Perspect. Biol. 2014, 6, a016972. [Google Scholar] [CrossRef]
- Bonazzi, M.; Cossart, P. Bacterial entry into cells: A role for the endocytic machinery. FEBS Lett. 2006, 580, 2962–2967. [Google Scholar] [CrossRef]
- Barrow, E.; Nicola, A.V.; Liu, J. Multiscale perspectives of virus entry via endocytosis. Virol. J. 2013, 10, 177. [Google Scholar] [CrossRef] [PubMed]
- Veiga, E.; Cossart, P. The role of clathrin-dependent endocytosis in bacterial internalization. Trends Cell Biol. 2006, 16, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Thottacherry, J.J.; Sathe, M.; Prabhakara, C.; Mayor, S. Spoiled for choice: Diverse endocytic pathways function at the cell surface. Annu. Rev. Cell Dev. Biol. 2019, 35, 55–84. [Google Scholar] [CrossRef] [PubMed]
- Doherty, G.J.; McMahon, H.T. Mechanisms of endocytosis. Annu. Rev. Biochem. 2009, 78, 857–902. [Google Scholar] [CrossRef]
- Conner, S.D.; Schmid, S.L. Regulated portals of entry into the cell. Nature 2003, 422, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Kaksonen, M.; Roux, A. Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2018, 19, 313–326. [Google Scholar] [CrossRef]
- McMahon, H.T.; Boucrot, E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2011, 12, 517–533. [Google Scholar] [CrossRef] [PubMed]
- Mettlen, M.; Chen, P.H.; Srinivasan, S.; Danuser, G.; Schmid, S.L. Regulation of clathrin-mediated endocytosis. Annu. Rev. Biochem. 2018, 87, 871–896. [Google Scholar] [CrossRef]
- Johannes, L.; Parton, R.G.; Bassereau, P.; Mayor, S. Building endocytic pits without clathrin. Nat. Rev. Mol. Cell Biol. 2015, 16, 311–321. [Google Scholar] [CrossRef]
- Shafaq-Zadah, M.; Dransart, E.; Johannes, L. Clathrin-independent endocytosis, retrograde trafficking, and cell polarity. Curr. Opin Cell Biol. 2020, 65, 112–121. [Google Scholar] [CrossRef]
- Renard, H.F.; Boucrot, E. Unconventional endocytic mechanisms. Curr. Opin Cell Biol. 2021, 71, 120–129. [Google Scholar] [CrossRef]
- Sandvig, K.; Kavaliauskiene, S.; Skotland, T. Clathrin-independent endocytosis: An increasing degree of complexity. Histochem. Cell Biol. 2018, 150, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.P.A.; Boucrot, E. Mechanisms of carrier formation during clathrin-independent endocytosis. Trends Cell Biol. 2018, 28, 188–200. [Google Scholar] [CrossRef]
- Hemalatha, A.; Mayor, S. Recent advances in clathrin-independent endocytosis. F1000Research 2019, 8, 138. [Google Scholar] [CrossRef]
- Howes, M.T.; Mayor, S.; Parton, R.G. Molecules, mechanisms, and cellular roles of clathrin-independent endocytosis. Curr. Opin. Cell Biol. 2010, 22, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Mayor, S.; Parton, R.G.; Donaldson, J.G. Clathrin-independent pathways of endocytosis. Cold Spring Harb. Perspect. Biol. 2014, 6, a016758. [Google Scholar] [CrossRef]
- Mayor, S.; Pagano, R.E. Pathways of clathrin-independent endocytosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Torgersen, M.L.; Skretting, G.; van Deurs, B.; Sandvig, K. Internalization of cholera toxin by different endocytic mechanisms. J. Cell Sci. 2001, 114, 3737–3747. [Google Scholar] [CrossRef] [PubMed]
- Massol, R.H.; Larsen, J.E.; Fujinaga, Y.; Lencer, W.I.; Kirchhausen, T. Cholera toxin toxicity does not require functional Arf6- and dynamin-dependent endocytic pathways. Mol. Biol. Cell 2004, 15, 3631–3641. [Google Scholar] [CrossRef]
- Kirkham, M.; Fujita, A.; Chadda, R.; Nixon, S.J.; Kurzchalia, T.V.; Sharma, D.K.; Pagano, R.E.; Hancock, J.F.; Mayor, S.; Parton, R.G. Ultrastructural identification of uncoated caveolin-independent early endocytic vehicles. J. Cell Biol. 2005, 168, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Pelkmans, L.; Kartenbeck, J.; Helenius, A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 2001, 3, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Pelkmans, L.; Zerial, M. Kinase-regulated quantal assemblies and kiss-and-run recycling of caveolae. Nature 2005, 436, 128–133. [Google Scholar] [CrossRef]
- Pelkmans, L.; Burli, T.; Zerial, M.; Helenius, A. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell 2004, 118, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Lundmark, R.; Doherty, G.J.; Howes, M.T.; Cortese, K.; Vallis, Y.; Parton, R.G.; McMahon, H.T. The GTPase-activating protein GRAF1 regulates the CLIC/GEEC endocytic pathway. Curr. Biol. 2008, 18, 1802–1808. [Google Scholar] [CrossRef] [PubMed]
- Thottacherry, J.J.; Kosmalska, A.J.; Kumar, A.; Vishen, A.S.; Elosegui-Artola, A.; Pradhan, S.; Sharma, S.; Singh, P.P.; Guadamillas, M.C.; Chaudhary, N.; et al. Mechanochemical feedback control of dynamin independent endocytosis modulates membrane tension in adherent cells. Nat. Commun. 2018, 9, 4217. [Google Scholar] [CrossRef] [PubMed]
- Howes, M.T.; Kirkham, M.; Riches, J.; Cortese, K.; Walser, P.J.; Simpson, F.; Hill, M.M.; Jones, A.; Lundmark, R.; Lindsay, M.R.; et al. Clathrin-independent carriers form a high capacity endocytic sorting system at the leading edge of migrating cells. J. Cell Biol. 2010, 190, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Kalia, M.; Kumari, S.; Chadda, R.; Hill, M.M.; Parton, R.G.; Mayor, S. Arf6-independent GPI-anchored protein-enriched early endosomal compartments fuse with sorting endosomes via a Rab5/phosphatidylinositol-3’-kinase-dependent machinery. Mol. Biol. Cell 2006, 17, 3689–3704. [Google Scholar] [CrossRef]
- Nonnenmacher, M.; Weber, T. Adeno-associated virus 2 infection requires endocytosis through the CLIC/GEEC pathway. Cell Host Microbe 2011, 10, 563–576. [Google Scholar] [CrossRef]
- Radhakrishna, H.; Donaldson, J.G. ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J. Cell Biol. 1997, 139, 49–61. [Google Scholar] [CrossRef]
- Naslavsky, N.; Weigert, R.; Donaldson, J.G. Characterization of a nonclathrin endocytic pathway: Membrane cargo and lipid requirements. Mol. Biol. Cell 2004, 15, 3542–3552. [Google Scholar] [CrossRef] [PubMed]
- Renard, H.F.; Simunovic, M.; Lemiere, J.; Boucrot, E.; Garcia-Castillo, M.D.; Arumugam, S.; Chambon, V.; Lamaze, C.; Wunder, C.; Kenworthy, A.K.; et al. Endophilin-A2 functions in membrane scission in clathrin-independent endocytosis. Nature 2015, 517, 493–496. [Google Scholar] [CrossRef]
- Boucrot, E.; Ferreira, A.P.; Almeida-Souza, L.; Debard, S.; Vallis, Y.; Howard, G.; Bertot, L.; Sauvonnet, N.; McMahon, H.T. Endophilin marks and controls a clathrin-independent endocytic pathway. Nature 2015, 517, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Casamento, A.; Boucrot, E. Molecular mechanism of Fast Endophilin-Mediated Endocytosis. Biochem. J. 2020, 477, 2327–2345. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Boucrot, E. Fast and ultrafast endocytosis. Curr. Opin. Cell Biol. 2017, 47, 64–71. [Google Scholar] [CrossRef]
- Simunovic, M.; Manneville, J.B.; Renard, H.F.; Evergren, E.; Raghunathan, K.; Bhatia, D.; Kenworthy, A.K.; Voth, G.A.; Prost, J.; McMahon, H.T.; et al. Friction mediates scission of tubular membranes scaffolded by BAR proteins. Cell 2017, 170, 172.e11–184.e11. [Google Scholar] [CrossRef]
- Day, C.A.; Baetz, N.W.; Copeland, C.A.; Kraft, L.J.; Han, B.; Tiwari, A.; Drake, K.R.; De Luca, H.; Chinnapen, D.J.; Davidson, M.W.; et al. Microtubule motors power plasma membrane tubulation in clathrin-independent endocytosis. Traffic 2015, 16, 572–590. [Google Scholar] [CrossRef]
- Ferreira, A.P.A.; Casamento, A.; Carrillo Roas, S.; Halff, E.F.; Panambalana, J.; Subramaniam, S.; Schutzenhofer, K.; Chan Wah Hak, L.; McGourty, K.; Thalassinos, K.; et al. Cdk5 and GSK3beta inhibit fast endophilin-mediated endocytosis. Nat. Commun. 2021, 12, 2424. [Google Scholar] [CrossRef] [PubMed]
- Mercier, V.; Laporte, M.H.; Destaing, O.; Blot, B.; Blouin, C.M.; Pernet-Gallay, K.; Chatellard, C.; Saoudi, Y.; Albiges-Rizo, C.; Lamaze, C.; et al. ALG-2 interacting protein-X (Alix) is essential for clathrin-independent endocytosis and signaling. Sci. Rep. 2016, 6, 26986. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.A.; Jobling, M.G.; Saslowsky, D.E.; Kern, E.; Drake, K.R.; Kenworthy, A.K.; Holmes, R.K.; Lencer, W.I. Attenuated endocytosis and toxicity of a mutant cholera toxin with decreased ability to cluster GM1. Infect. Immun. 2008, 76, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Jobling, M.G.; Yang, Z.; Kam, W.R.; Lencer, W.I.; Holmes, R.K. A single native ganglioside GM1-binding site is sufficient for cholera toxin to bind to cells and complete the intoxication pathway. mBio 2012, 3, e00401-12. [Google Scholar] [CrossRef]
- Johannes, L.; Wunder, C.; Shafaq-Zadah, M. Glycolipids and lectins in endocytic uptake pocesses. J. Mol. Biol. 2016, 428, 4792–4818. [Google Scholar] [CrossRef]
- Ebrahimkutty, M.P.; Galic, M. Receptor-free signaling at curved cellular membranes. Bioessays 2019, 41, e1900068. [Google Scholar] [CrossRef]
- Galic, M.; Begemann, I.; Viplav, A.; Matis, M. Force-control at cellular membranes. Bioarchitecture 2014, 4, 164–168. [Google Scholar]
- Klokk, T.I.; Kavaliauskiene, S.; Sandvig, K. Cross-linking of glycosphingolipids at the plasma membrane: Consequences for intracellular signaling and traffic. Cell Mol. Life Sci. 2016, 73, 1301–1316. [Google Scholar] [CrossRef]
- Utskarpen, A.; Massol, R.; van Deurs, B.; Lauvrak, S.U.; Kirchhausen, T.; Sandvig, K. Shiga toxin increases formation of clathrin-coated pits through Syk kinase. PLoS ONE 2010, 5, e10944. [Google Scholar] [CrossRef]
- Hehnly, H.; Sheff, D.; Stamnes, M. Shiga toxin facilitates its retrograde transport by modifying microtubule dynamics. Mol. Biol. Cell 2006, 17, 4379–4389. [Google Scholar] [CrossRef] [PubMed]
- Johannes, L.; Popoff, V. Tracing the retrograde route in protein trafficking. Cell 2008, 135, 1175–1187. [Google Scholar] [CrossRef]
- Cho, J.A.; Chinnapen, D.J.; Aamar, E.; Te Welscher, Y.M.; Lencer, W.I.; Massol, R. Insights on the trafficking and retro-translocation of glycosphingolipid-binding bacterial toxins. Front. Cell. Infect. Microbiol. 2012, 2, 51. [Google Scholar] [CrossRef] [PubMed]
- Zuverink, M.; Barbieri, J.T. Protein toxins that utilize gangliosides as host receptors. Prog. Mol. Biol. Transl. Sci. 2018, 156, 325–354. [Google Scholar]
- Sandvig, K.; Garred, O.; Prydz, K.; Kozlov, J.V.; Hansen, S.H.; van Deurs, B. Retrograde transport of endocytosed Shiga toxin to the endoplasmic reticulum. Nature 1992, 358, 510–512. [Google Scholar] [CrossRef] [PubMed]
- Johannes, L.; Tenza, D.; Antony, C.; Goud, B. Retrograde transport of KDEL-bearing B-fragment of Shiga toxin. J. Biol. Chem. 1997, 272, 19554–19561. [Google Scholar] [CrossRef]
- Simpson, M.S.; Lencer, W.I.; Luong, P. A quantitative single-cell flow cytometry assay for retrograde membrane trafficking using engineered cholera toxin. Bio-Protocol 2020, 10, e3707. [Google Scholar] [CrossRef]
- Saslowsky, D.E.; te Welscher, Y.M.; Chinnapen, D.J.; Wagner, J.S.; Wan, J.; Kern, E.; Lencer, W.I. Ganglioside GM1-mediated transcytosis of cholera toxin bypasses the retrograde pathway and depends on the structure of the ceramide domain. J. Biol. Chem. 2013, 288, 25804–25809. [Google Scholar] [CrossRef]
- Maxfield, F.R.; McGraw, T.E. Endocytic recycling. Nat. Rev. Mol. Cell Biol. 2004, 5, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Sandvig, K.; Ryd, M.; Garred, O.; Schweda, E.; Holm, P.K.; van Deurs, B. Retrograde transport from the Golgi complex to the ER of both Shiga toxin and the nontoxic Shiga B-fragment is regulated by butyric acid and cAMP. J. Cell Biol. 1994, 126, 53–64. [Google Scholar] [CrossRef]
- Garcia-Castillo, M.D.; Chinnapen, D.J.; Te Welscher, Y.M.; Gonzalez, R.J.; Softic, S.; Pacheco, M.; Mrsny, R.J.; Kahn, C.R.; von Andrian, U.H.; Lau, J.; et al. Mucosal absorption of therapeutic peptides by harnessing the endogenous sorting of glycosphingolipids. eLife 2018, 7, e34469. [Google Scholar] [CrossRef]
- Lencer, W.I.; Moe, S.; Rufo, P.A.; Madara, J.L. Transcytosis of cholera toxin subunits across model human intestinal epithelia. Proc. Natl. Acad. Sci. USA 1995, 92, 10094–10098. [Google Scholar] [CrossRef]
- Nelms, B.; Dalomba, N.F.; Lencer, W. A targeted RNAi screen identifies factors affecting diverse stages of receptor-mediated transcytosis. J. Cell Biol. 2017, 216, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Castillo, M.D.; Lencer, W.I.; Chinnapen, D.J. Transcytosis assay for transport of glycosphingolipids across MDCK-II cells. Bio-Protocol 2018, 8, e3049. [Google Scholar] [CrossRef] [PubMed]
- Cuatrecasas, P. Interaction of Vibrio cholerae enterotoxin with cell membranes. Biochemistry 1973, 12, 3547–3558. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, J.; Lonnroth, I.; Svennerholm, L. Tissue receptor for cholera exotoxin: Postulated structure from studies with GM1 ganglioside and related glycolipids. Infect. Immun. 1973, 8, 208–214. [Google Scholar] [CrossRef]
- Schengrund, C.L.; Ringler, N.J. Binding of Vibrio cholera toxin and the heat-labile enterotoxin of Escherichia coli to GM1, derivatives of GM1, and nonlipid oligosaccharide polyvalent ligands. J. Biol. Chem. 1989, 264, 13233–13237. [Google Scholar] [CrossRef]
- Jobling, M.G.; Holmes, R.K. Mutational analysis of ganglioside GM(1)-binding ability, pentamer formation, and epitopes of cholera toxin B (CTB) subunits and CTB/heat-labile enterotoxin B subunit chimeras. Infect. Immun. 2002, 70, 1260–1271. [Google Scholar] [CrossRef]
- Pang, H.; Le, P.U.; Nabi, I.R. Ganglioside GM1 levels are a determinant of the extent of caveolae/raft-dependent endocytosis of cholera toxin to the Golgi apparatus. J. Cell Sci. 2004, 117, 1421–1430. [Google Scholar] [CrossRef]
- Holmgren, J.; Lonnroth, I.; Mansson, J.; Svennerholm, L. Interaction of cholera toxin and membrane GM1 ganglioside of small intestine. Proc. Natl. Acad. Sci. USA 1975, 72, 2520–2524. [Google Scholar] [CrossRef]
- Moss, J.; Fishman, P.H.; Manganiello, V.C.; Vaughan, M.; Brady, R.O. Functional incorporation of ganglioside into intact cells: Induction of choleragen responsiveness. Proc. Natl. Acad. Sci. USA 1976, 73, 1034–1037. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.A.; Jobling, M.G.; Wimer-Mackin, S.; Ferguson-Maltzman, M.; Madara, J.L.; Holmes, R.K.; Lencer, W.I. Ganglioside structure dictates signal transduction by cholera toxin and association with caveolae-like membrane domains in polarized epithelia. J. Cell Biol. 1998, 141, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Fishman, P.H.; Pacuszka, T.; Hom, B.; Moss, J. Modification of ganglioside GM1. Effect of lipid moiety on choleragen action. J. Biol. Chem. 1980, 255, 7657–7664. [Google Scholar] [CrossRef]
- Pacuszka, T.; Bradley, R.M.; Fishman, P.H. Neoglycolipid analogues of ganglioside GM1 as functional receptors of cholera toxin. Biochemistry 1991, 30, 2563–2570. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, J.; Fredman, P.; Lindblad, M.; Svennerholm, A.M.; Svennerholm, L. Rabbit intestinal glycoprotein receptor for Escherichia coli heat-labile enterotoxin lacking affinity for cholera toxin. Infect. Immun. 1982, 38, 424–433. [Google Scholar] [CrossRef]
- Holmgren, J.; Lindblad, M.; Fredman, P.; Svennerholm, L.; Myrvold, H. Comparison of receptors for cholera and Escherichia coli enterotoxins in human intestine. Gastroenterology 1985, 89, 27–35. [Google Scholar] [CrossRef]
- Fukuta, S.; Magnani, J.L.; Twiddy, E.M.; Holmes, R.K.; Ginsburg, V. Comparison of the carbohydrate-binding specificities of cholera toxin and Escherichia coli heat-labile enterotoxins LTh-I, LT-IIa, and LT-IIb. Infect. Immun. 1988, 56, 1748–1753. [Google Scholar] [CrossRef]
- Pacuszka, T.; Fishman, P.H. Generation of cell surface neoganglioproteins. GM1-neoganglioproteins are non-functional receptors for cholera toxin. J. Biol. Chem. 1990, 265, 7673–7678. [Google Scholar] [CrossRef]
- Orlandi, P.A.; Critchley, D.R.; Fishman, P.H. The heat-labile enterotoxin of Escherichia coli binds to polylactosaminoglycan-containing receptors in CaCo-2 human intestinal epithelial cells. Biochemistry 1994, 33, 12886–12895. [Google Scholar] [CrossRef]
- Kuziemko, G.M.; Stroh, M.; Stevens, R.C. Cholera toxin binding affinity and specificity for gangliosides determined by surface plasmon resonance. Biochemistry 1996, 35, 6375–6384. [Google Scholar] [CrossRef] [PubMed]
- Masserini, M.; Freire, E.; Palestini, P.; Calappi, E.; Tettamanti, G. Fuc-GM1 ganglioside mimics the receptor function of GM1 for cholera toxin. Biochemistry 1992, 31, 2422–2426. [Google Scholar] [CrossRef] [PubMed]
- Sethi, A.; Wands, A.M.; Mettlen, M.; Krishnamurthy, S.; Wu, H.; Kohler, J.J. Cell type and receptor identity regulate cholera toxin subunit B (CTB) internalization. Interface Focus 2019, 9, 20180076. [Google Scholar] [CrossRef]
- Aigal, S.; Claudinon, J.; Romer, W. Plasma membrane reorganization: A glycolipid gateway for microbes. Biochim. Biophys. Acta 2015, 1853, 858–871. [Google Scholar] [CrossRef] [PubMed]
- Johannes, L.; Billet, A. Glycosylation and raft endocytosis in cancer. Cancer Metastasis Rev. 2020, 39, 375–396. [Google Scholar] [CrossRef] [PubMed]
- Johannes, L. The cellular and chemical biology of endocytic trafficking and intracellular delivery-the GL-Lect hypothesis. Molecules 2021, 26, 3299. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).