Elucidating the Venom Diversity in Sri Lankan Spectacled Cobra (Naja naja) through De Novo Venom Gland Transcriptomics, Venom Proteomics and Toxicity Neutralization
Abstract
:1. Introduction
2. Results and Discussion
2.1. De Novo RNA Sequencing and Transcriptome Assembly
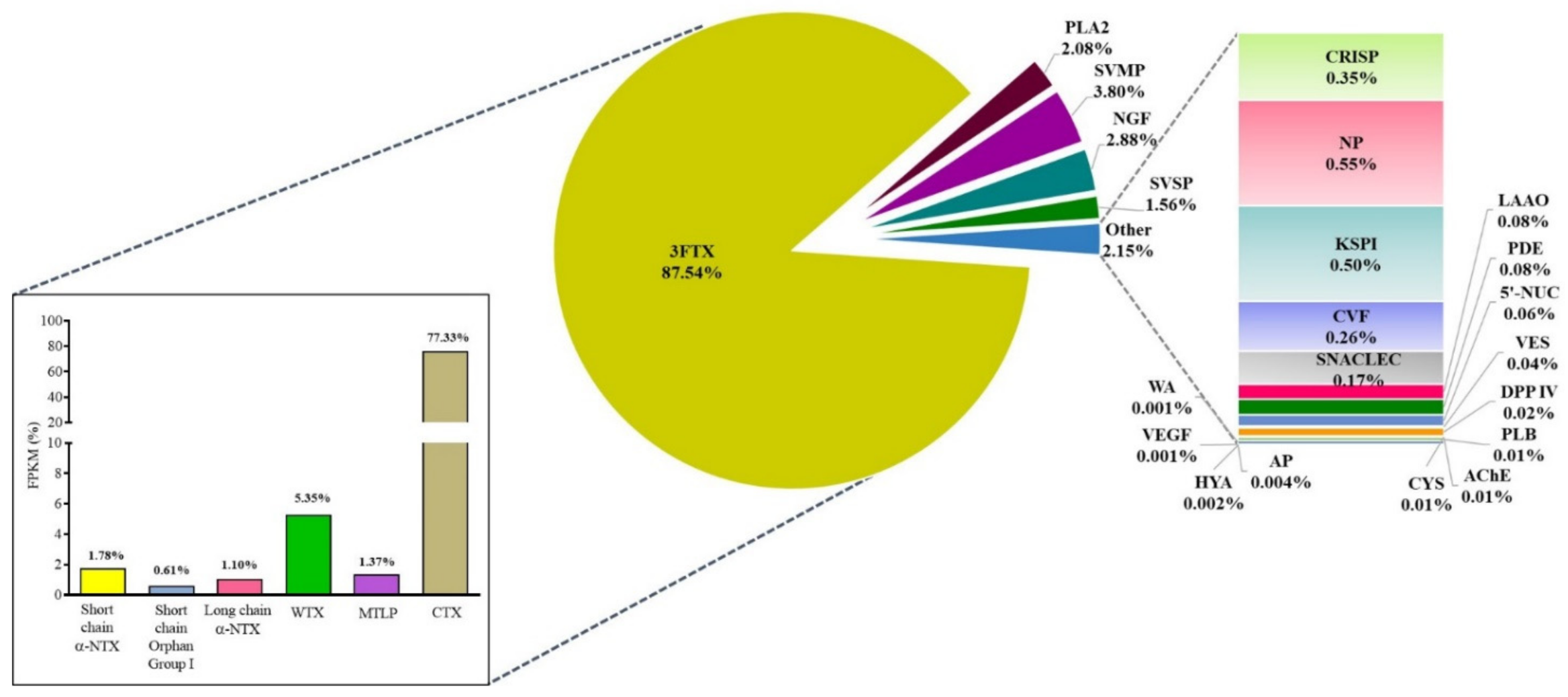
2.2. Venom Gland Transcriptome of NN-SL
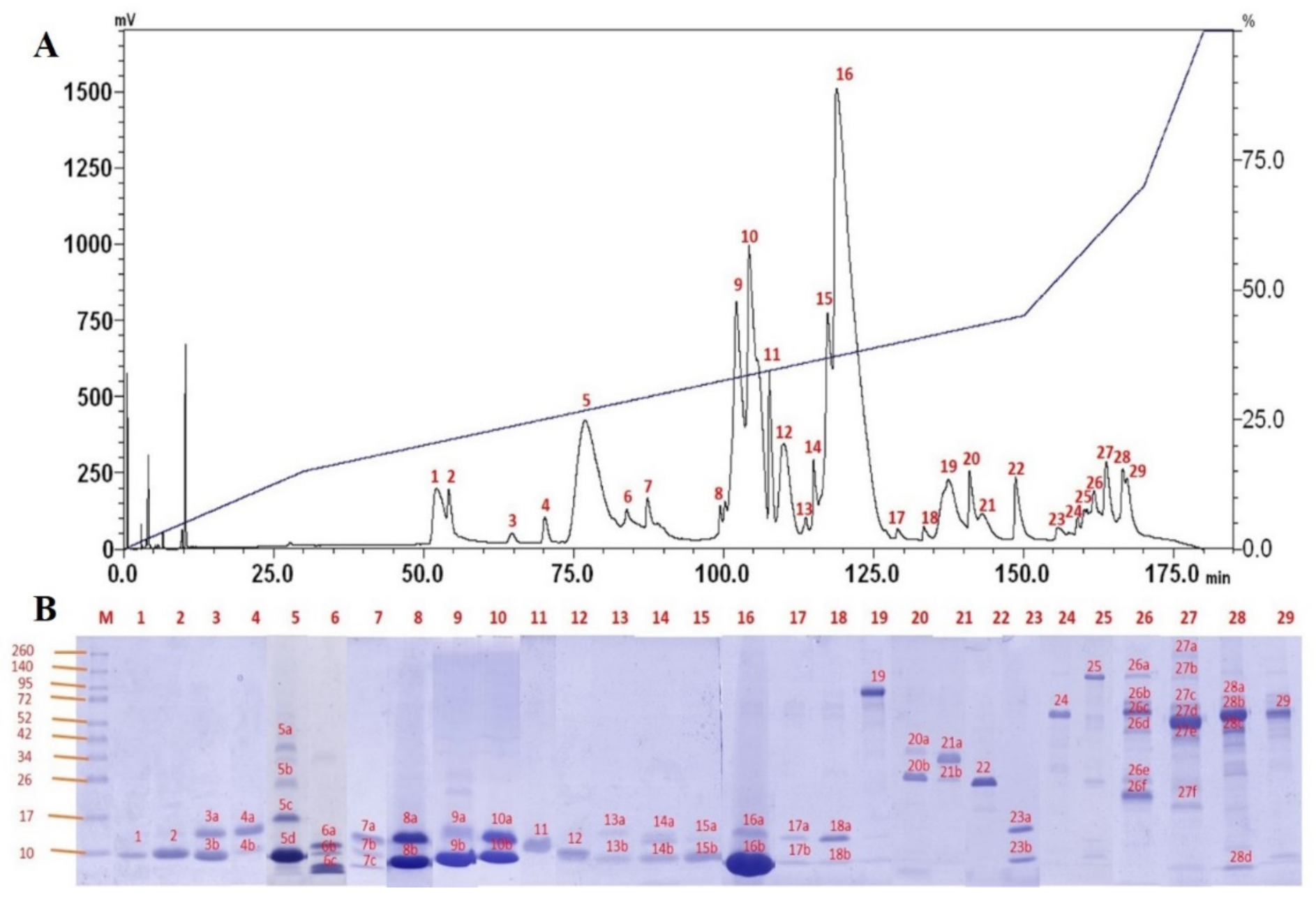
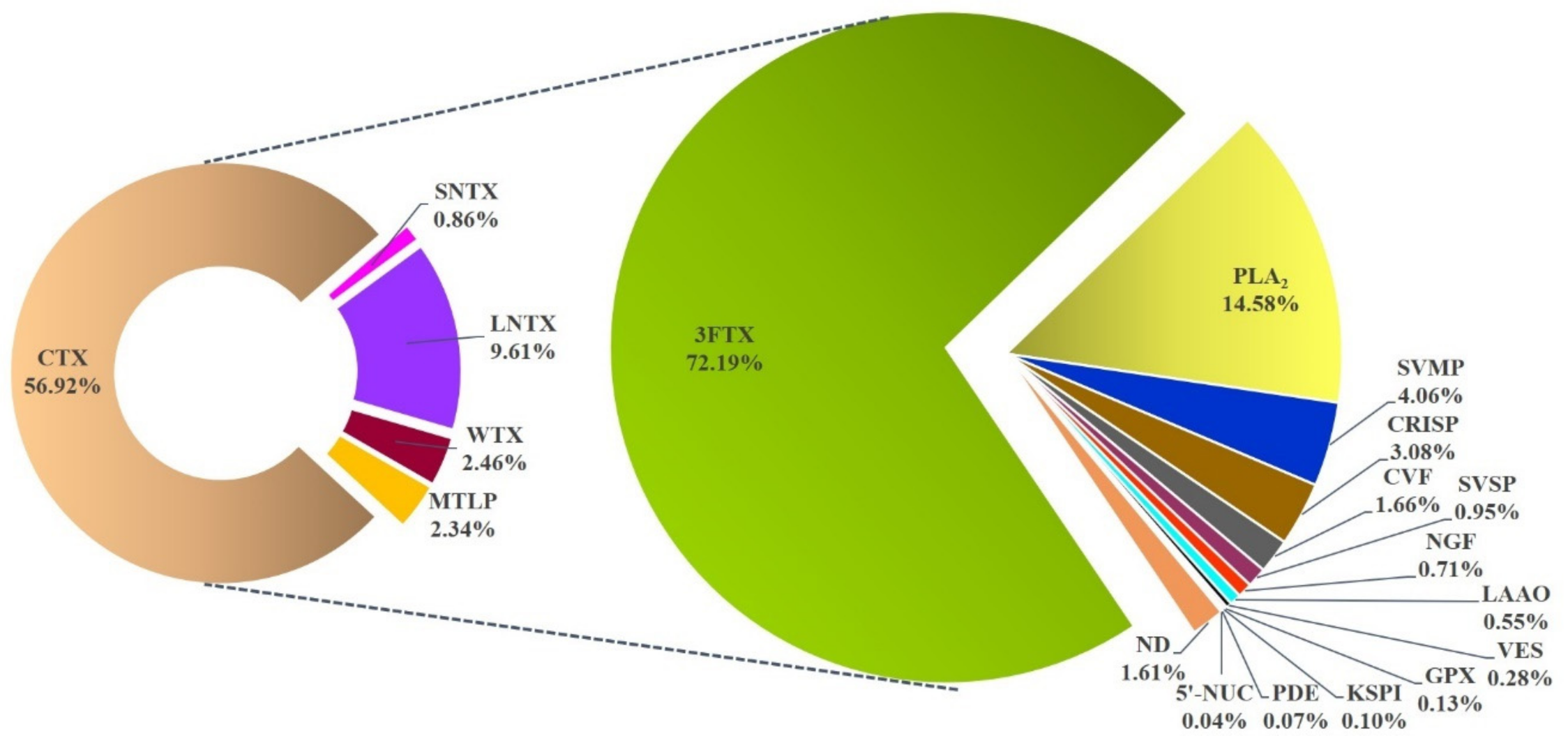
2.3. Venom Proteome of NN-SL
2.4. Three-Finger Toxins (3FTX)
2.5. Phospholipase A2 (PLA2)
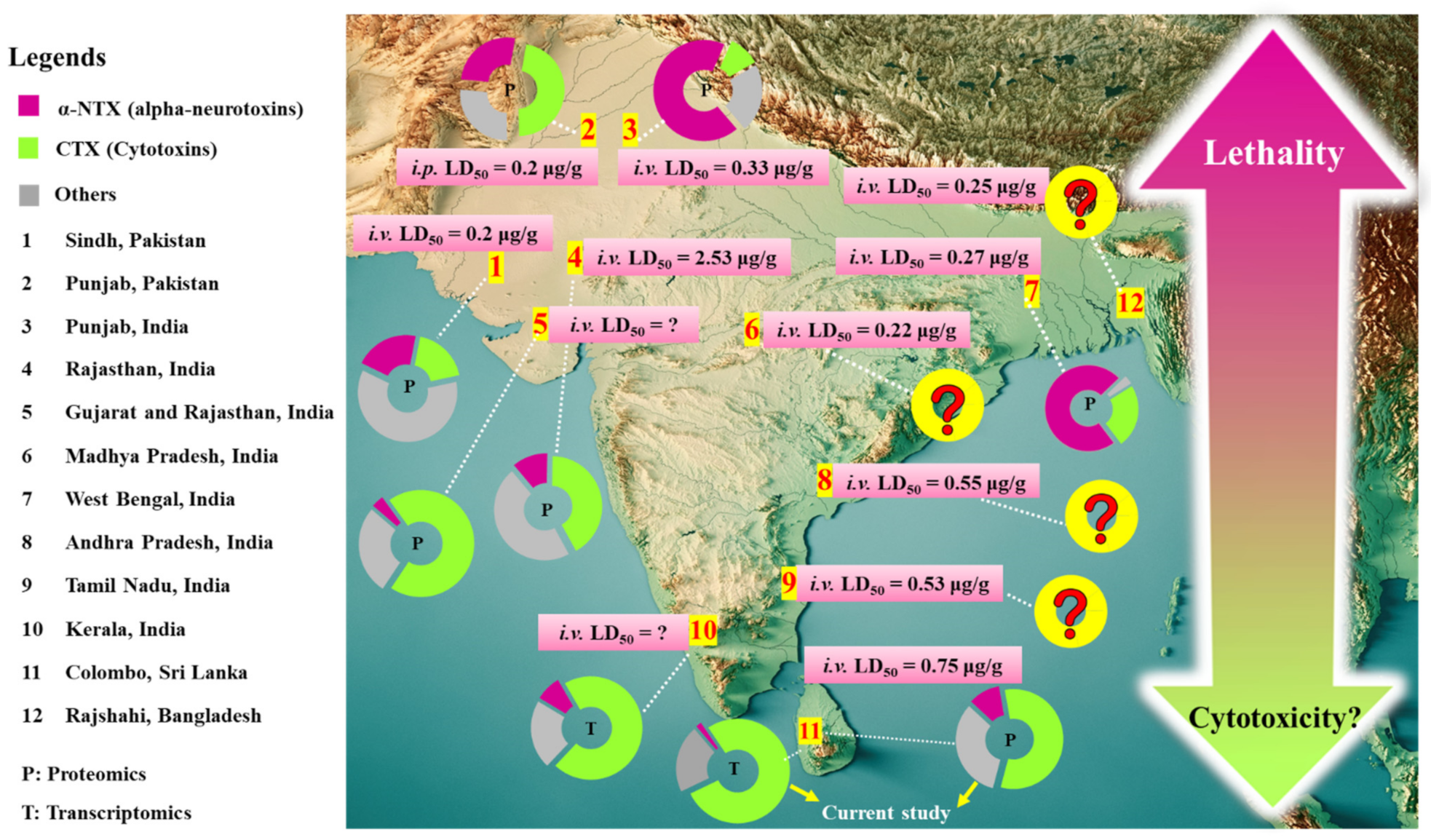
2.6. Comparison of Naja naja Venomics and Implication on Antivenom Treatment for Cobra Bite Envenomation
3. Conclusions
4. Materials and Methods
4.1. Snake Venom, Venom Gland Preparation and Antivenom
4.2. Chemicals and Materials
4.3. RNA Extraction and Purification
4.4. Raw Sequenced Reads and De Novo Transcriptome Assembly
4.5. Transcripts Clustering and Functional Annotation
4.6. Quantification of Transcript Abundance
4.7. Categorization of Transcripts
4.8. Multiple Sequence Alignment
4.9. Correlation Analysis
4.10. Heatmap of Differentially Expressed Toxins Genes and Proteins
4.11. Reverse-Phase High-Performance Liquid Chromatography (RP-HPLC)
4.12. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
4.13. In-Gel Tryptic Digestion and Tandem Mass Spectrometry
4.14. Estimation of Protein Relative Abundance
4.15. Determination of Venom Lethality and Neutralization by Antivenom
4.16. Supporting Data
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Snakebite Envenoming. Available online: https://www.who.int/news-room/fact-sheets/detail/snakebite-envenoming (accessed on 12 June 2021).
- Kasturiratne, A.; Wickremasinghe, A.R.; de Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; de Silva, H.J. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008, 5, e218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Primers 2017, 3, 17063. [Google Scholar] [CrossRef]
- Ralph, R.; Sharma, S.K.; Faiz, M.A.; Ribeiro, I.; Rijal, S.; Chappuis, F.; Kuch, U. The timing is right to end snakebite deaths in South Asia. BMJ Clin. Res. Ed. 2019, 364, k5317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longbottom, J.; Shearer, F.M.; Devine, M.; Alcoba, G.; Chappuis, F.; Weiss, D.J.; Ray, S.E.; Ray, N.; Warrell, D.A.; Ruiz de Castaneda, R.; et al. Vulnerability to snakebite envenoming: A global mapping of hotspots. Lancet 2018, 392, 673–684. [Google Scholar] [CrossRef] [Green Version]
- Senji Laxme, R.R.; Attarde, S.; Khochare, S.; Suranse, V.; Martin, G.; Casewell, N.R.; Whitaker, R.; Sunagar, K. Biogeographical venom variation in the Indian spectacled cobra (Naja naja) underscores the pressing need for pan-India efficacious snakebite therapy. PLoS Negl. Trop. Dis. 2021, 15, e0009150. [Google Scholar] [CrossRef] [PubMed]
- Sintiprungrat, K.; Watcharatanyatip, K.; Senevirathne, W.D.; Chaisuriya, P.; Chokchaichamnankit, D.; Srisomsap, C.; Ratanabanangkoon, K. A comparative study of venomics of Naja naja from India and Sri Lanka, clinical manifestations and antivenomics of an Indian polyspecific antivenom. J. Proteom. 2016, 132, 131–143. [Google Scholar] [CrossRef]
- Wong, K.Y.; Tan, C.H.; Tan, K.Y.; Quraishi, N.H.; Tan, N.H. Elucidating the biogeographical variation of the venom of Naja naja (spectacled cobra) from Pakistan through a venom-decomplexing proteomic study. J. Proteom. 2018, 175, 156–173. [Google Scholar] [CrossRef]
- Faisal, T.; Tan, K.Y.; Sim, S.M.; Quraishi, N.; Tan, N.H.; Tan, C.H. Proteomics, functional characterization and antivenom neutralization of the venom of Pakistani Russell’s viper (Daboia russelii) from the wild. J. Proteom. 2018, 183, 1–13. [Google Scholar] [CrossRef]
- Bhatia, S.; Vasudevan, K. Comparative proteomics of geographically distinct saw-scaled viper (Echis carinatus) venoms from India. Toxicon X 2020, 7, 100048. [Google Scholar] [CrossRef]
- Kalita, B.; Patra, A.; Das, A.; Mukherjee, A.K. Proteomic analysis and immuno-profiling of eastern India Russell’s viper (Daboia russelii) Venom: Correlation between RVV composition and clinical manifestations post RV bite. J. Proteom. Res. 2018, 17, 2819–2833. [Google Scholar] [CrossRef] [PubMed]
- Maduwage, K.; Silva, A.; O’Leary, M.A.; Hodgson, W.C.; Isbister, G.K. Efficacy of Indian polyvalent snake antivenoms against Sri Lankan snake venoms: Lethality studies or clinically focussed in vitro studies. Sci. Rep. 2016, 6, 26778. [Google Scholar] [CrossRef] [Green Version]
- Kularatne, S.A.; Budagoda, B.D.; Gawarammana, I.B.; Kularatne, W.K. Epidemiology, clinical profile and management issues of cobra (Naja naja) bites in Sri Lanka: First authenticated case series. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Ediriweera, D.S.; Kasturiratne, A.; Pathmeswaran, A.; Gunawardena, N.K.; Wijayawickrama, B.A.; Jayamanne, S.F.; Isbister, G.K.; Dawson, A.; Giorgi, E.; Diggle, P.J.; et al. Mapping the risk of snakebite in Sri Lanka—A national survey with geospatial analysis. PLoS Negl. Trop. Dis. 2016, 10, e0004813. [Google Scholar] [CrossRef] [PubMed]
- Rathnayaka, R.; Kularathna, S.A.M.; Sugathapala, A.G.H.; Pathiraja, P.; Ranathunga, P. A cobra bite—Uncommon bitten site. Sri Lankan J. Anaesthesiol. 2015, 23, 89. [Google Scholar] [CrossRef] [Green Version]
- Seneviratne, U.; Dissanayake, S. Neurological manifestations of snake bite in Sri Lanka. J. Postgrad. Med. 2002, 48, 275–278; discussion 278–279. [Google Scholar]
- Madhushani, U.; Thakshila, P.; Hodgson, W.C.; Isbister, G.K.; Silva, A. Effect of Indian Polyvalent Antivenom in the Prevention and Reversal of Local Myotoxicity Induced by Common Cobra (Naja naja) Venom from Sri Lanka In Vitro. Toxins 2021, 13, 308. [Google Scholar] [CrossRef] [PubMed]
- Pla, D.; Sanz, L.; Quesada-Bernat, S.; Villalta, M.; Baal, J.; Chowdhury, M.A.W.; León, G.; Gutiérrez, J.M.; Kuch, U.; Calvete, J.J. Phylovenomics of Daboia russelii across the Indian subcontinent. Bioactivities and comparative in vivo neutralization and in vitro third-generation antivenomics of antivenoms against venoms from India, Bangladesh and Sri Lanka. J. Proteom. 2019, 207, 103443. [Google Scholar] [CrossRef] [PubMed]
- Kularatne, S.A.M.; Silva, A.; Weerakoon, K.; Maduwage, K.; Walathara, C.; Paranagama, R.; Mendis, S. Revisiting Russell’s viper (Daboia russelii) bite in Sri Lanka: Is abdominal pain an early feature of systemic envenoming? PLoS ONE 2014, 9, e90198. [Google Scholar] [CrossRef] [Green Version]
- Theakston, R.D.; Phillips, R.E.; Warrell, D.A.; Galagedera, Y.; Abeysekera, D.T.; Dissanayaka, P.; de Silva, A.; Aloysius, D.J. Envenoming by the common krait (Bungarus caeruleus) and Sri Lankan cobra (Naja naja naja): Efficacy and complications of therapy with Haffkine antivenom. Trans. R. Soc. Trop. Med. Hyg. 1990, 84, 301–308. [Google Scholar] [CrossRef]
- Keyler, D.E.; Gawarammana, I.; Gutierrez, J.M.; Sellahewa, K.H.; McWhorter, K.; Malleappah, R. Antivenom for snakebite envenoming in Sri Lanka: The need for geographically specific antivenom and improved efficacy. Toxicon 2013, 69, 90–97. [Google Scholar] [CrossRef]
- Villalta, M.; Sánchez, A.; Herrera, M.; Vargas, M.; Segura, Á.; Cerdas, M.; Estrada, R.; Gawarammana, I.; Keyler, D.E.; McWhorter, K.; et al. Development of a new polyspecific antivenom for snakebite envenoming in Sri Lanka: Analysis of its preclinical efficacy as compared to a currently available antivenom. Toxicon 2016, 122, 152–159. [Google Scholar] [CrossRef]
- Tan, K.Y.; Tan, C.H.; Fung, S.Y.; Tan, N.H. Venomics, lethality and neutralization of Naja kaouthia (monocled cobra) venoms from three different geographical regions of Southeast Asia. J. Proteom. 2015, 120, 105–125. [Google Scholar] [CrossRef]
- Huang, H.W.; Liu, B.S.; Chien, K.Y.; Chiang, L.C.; Huang, S.Y.; Sung, W.C.; Wu, W.G. Cobra venom proteome and glycome determined from individual snakes of Naja atra reveal medically important dynamic range and systematic geographic variation. J. Proteom. 2015, 128, 92–104. [Google Scholar] [CrossRef]
- Deka, A.; Reza, M.A.; Faisal Hoque, K.M.; Deka, K.; Saha, S.; Doley, R. Comparative analysis of Naja kaouthia venom from North-East India and Bangladesh and its cross reactivity with Indian polyvalent antivenoms. Toxicon 2019, 164, 31–43. [Google Scholar] [CrossRef]
- Dutta, S.; Chanda, A.; Kalita, B.; Islam, T.; Patra, A.; Mukherjee, A.K. Proteomic analysis to unravel the complex venom proteome of eastern India Naja naja: Correlation of venom composition with its biochemical and pharmacological properties. J. Proteom. 2017, 156, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.Y.; Tan, C.H.; Chanhome, L.; Tan, N.H. Comparative venom gland transcriptomics of Naja kaouthia (monocled cobra) from Malaysia and Thailand: Elucidating geographical venom variation and insights into sequence novelty. PeerJ 2017, 5, e3142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.-C.; You, C.-H.; Wang, P.-J.; Yu, J.-S.; Huang, G.-J.; Liu, C.-H.; Hsieh, W.-C.; Lin, C.-C. Analysis of the efficacy of Taiwanese freeze-dried neurotoxic antivenom against Naja kaouthia, Naja siamensis and Ophiophagus hannah through proteomics and animal model approaches. PLoS Negl. Trop. Dis. 2017, 11, e0006138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petras, D.; Sanz, L.; Segura, Á.; Herrera, M.; Villalta, M.; Solano, D.; Vargas, M.; León, G.; Warrell, D.A.; Theakston, R.D.G.; et al. Snake venomics of African spitting cobras: Toxin composition and assessment of congeneric cross-reactivity of the pan-african EchiTAb-Plus-ICP antivenom by antivenomics and neutralization approaches. J. Proteom. Res. 2011, 10, 1266–1280. [Google Scholar] [CrossRef]
- Chanda, A.; Patra, A.; Kalita, B.; Mukherjee, A.K. Proteomics analysis to compare the venom composition between Naja naja and Naja kaouthia from the same geographical location of eastern India: Correlation with pathophysiology of envenomation and immunological cross-reactivity towards commercial polyantivenom. Expert Rev. Proteom. 2018, 15, 949–961. [Google Scholar] [CrossRef]
- Suryamohan, K.; Krishnankutty, S.P.; Guillory, J.; Jevit, M.; Schröder, M.S.; Wu, M.; Kuriakose, B.; Mathew, O.K.; Perumal, R.C.; Koludarov, I.; et al. The Indian cobra reference genome and transcriptome enables comprehensive identification of venom toxins. Nat. Genet. 2020, 52, 106–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.; Tan, K.Y.; Tan, N. A protein decomplexation strategy in snake venom proteomics. Funct. Proteom. 2019, 1871, 83–92. [Google Scholar]
- Tan, C.H.; Tan, K.Y. De novo venom-gland transcriptomics of spine-bellied sea snake (Hydrophis curtus) from Penang, Malaysia–Next-generation sequencing, functional annotation and toxinological correlation. Toxins 2021, 13, 127. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Tan, K.Y.; Fung, S.Y.; Tan, N.H. Venom-gland transcriptome and venom proteome of the Malaysian king cobra (Ophiophagus hannah). BMC Genom. 2015, 16, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Chong, H.P.; Tan, K.Y.; Tan, N.H.; Tan, C.H. Exploring the diversity and novelty of toxin genes in Naja sumatrana, the equatorial spitting cobra from Malaysia through de novo venom-gland transcriptomics. Toxins 2019, 11, 104. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Li, Y.; Lee, W.; Xu, X.; Zhang, Y.; Zhao, R.; Zhang, Y.; Wang, W. Venom gland transcriptomes of two elapid snakes (Bungarus multicinctus and Naja atra) and evolution of toxin genes. BMC Genom. 2011, 12, 1. [Google Scholar] [CrossRef] [Green Version]
- Margres, M.J.; Aronow, K.; Loyacano, J.; Rokyta, D.R. The venom-gland transcriptome of the eastern coral snake (Micrurus fulvius) reveals high venom complexity in the intragenomic evolution of venoms. BMC Genom. 2013, 14, 531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, N.H.; Wong, K.Y.; Tan, C.H. Venomics of Naja sputatrix, the Javan spitting cobra: A short neurotoxin-driven venom needing improved antivenom neutralization. J. Proteom. 2017, 157, 18–32. [Google Scholar] [CrossRef]
- Tan, C.H.; Wong, K.Y.; Chong, H.P.; Tan, N.H.; Tan, K.Y. Proteomic insights into short neurotoxin-driven, highly neurotoxic venom of Philippine cobra (Naja philippinensis) and toxicity correlation of cobra envenomation in Asia. J. Proteom. 2019, 206, 103418. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, M.; McCleary, R.J.R.; Kesherwani, M.; Kini, R.M.; Velmurugan, D. Comparison of proteomic profiles of the venoms of two of the ‘Big Four’ snakes of India, the Indian cobra (Naja naja) and the common krait (Bungarus caeruleus), and analyses of their toxins. Toxicon 2017, 135, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Yap, M.K.; Fung, S.Y.; Tan, K.Y.; Tan, N.H. Proteomic characterization of venom of the medically important Southeast Asian Naja sumatrana (Equatorial spitting cobra). Acta Trop. 2014, 133, 15–25. [Google Scholar] [CrossRef]
- Laustsen, A.H.; Gutierrez, J.M.; Lohse, B.; Rasmussen, A.R.; Fernandez, J.; Milbo, C.; Lomonte, B. Snake venomics of monocled cobra (Naja kaouthia) and investigation of human IgG response against venom toxins. Toxicon 2015, 99, 23–35. [Google Scholar] [CrossRef] [Green Version]
- Shan, L.L.; Gao, J.F.; Zhang, Y.X.; Shen, S.S.; He, Y.; Wang, J.; Ma, X.M.; Ji, X. Proteomic characterization and comparison of venoms from two elapid snakes (Bungarus multicinctus and Naja atra) from China. J. Proteom. 2016, 138, 83–94. [Google Scholar] [CrossRef]
- Asad, M.H.H.B.; McCleary, R.J.R.; Salafutdinov, I.; Alam, F.; Shah, H.S.; Bibi, S.; Ali, A.; Khalid, S.; Hasan, S.M.F.; Sabatier, J.-M.; et al. Proteomics study of Southern Punjab Pakistani cobra (Naja naja: Formerly Naja naja karachiensis) venom. Toxicol. Environ. Chem. 2019, 101, 91–116. [Google Scholar] [CrossRef]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef]
- Utkin, Y.N. Last decade update for three-finger toxins: Newly emerging structures and biological activities. World J. Biol Chem 2019, 10, 17–27. [Google Scholar] [CrossRef]
- Kini, R.M.; Doley, R. Structure, function and evolution of three-finger toxins: Mini proteins with multiple targets. Toxicon 2010, 56, 855–867. [Google Scholar] [CrossRef]
- Chien, K.Y.; Chiang, C.M.; Hseu, Y.C.; Vyas, A.A.; Rule, G.S.; Wu, W. Two distinct types of cardiotoxin as revealed by the structure and activity relationship of their interaction with zwitterionic phospholipid dispersions. J. Biol. Chem. 1994, 269, 14473–14483. [Google Scholar] [CrossRef]
- Suzuki-Matsubara, M.; Athauda, S.B.; Suzuki, Y.; Matsubara, K.; Moriyama, A. Comparison of the primary structures, cytotoxicities, and affinities to phospholipids of five kinds of cytotoxins from the venom of Indian cobra, Naja naja. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2016, 179, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.-L.; Chiu, C.-R.; Huang, W.-N.; Wu, W.-G. The role of sulfatide lipid domains in the membrane pore-forming activity of cobra cardiotoxin. Biochim. Biophys. Acta (BBA) Biomembr. 2012, 1818, 1378–1385. [Google Scholar] [CrossRef] [Green Version]
- Forouhar, F.; Huang, W.N.; Liu, J.H.; Chien, K.Y.; Wu, W.G.; Hsiao, C.D. Structural basis of membrane-induced cardiotoxin A3 oligomerization. J. Biol. Chem. 2003, 278, 21980–21988. [Google Scholar] [CrossRef] [Green Version]
- Chong, H.P.; Tan, K.Y.; Tan, C.H. Cytotoxicity of snake venoms and cytotoxins from two southeast Asian cobras (Naja sumatrana, Naja kaouthia): Exploration of anticancer potential, selectivity, and cell death mechanism. Front. Mol. Biosci. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Konshina, A.G.; Boldyrev, I.A.; Omelkov, A.V.; Utkin, Y.N.; Efremov, R.G. Anionic lipids: Determinants of binding cytotoxins from snake venom on the surface of cell membranes. Acta Nat. 2010, 2, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Wong, K.Y.; Tan, C.H.; Tan, N.H. Venom and purified toxins of the spectacled cobra (Naja naja) from Pakistan: Insights into toxicity and antivenom neutralization. Am. J. Trop. Med. Hyg. 2016, 94, 1392–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, K.Y.; Tan, C.H.; Fung, S.Y.; Tan, N.H. Neutralization of the principal toxins from the venoms of Thai Naja kaouthia and Malaysian Hydrophis schistosus: Insights into toxin-specific neutralization by two different antivenoms. Toxins 2016, 8, 86. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-C.; Chou, Y.-S.; Chen, C.-Y.; Liu, K.-L.; Huang, G.-J.; Yu, J.-S.; Wu, C.-J.; Liaw, G.-W.; Hsieh, C.-H.; Chen, C.-K. Pathogenesis of local necrosis induced by Naja atra venom: Assessment of the neutralization ability of Taiwanese freeze-dried neurotoxic antivenom in animal models. PLoS Negl. Trop. Dis. 2020, 14, e0008054. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.C.; Huang, Y.K.; Chen, Y.W.; Chen, M.H.; Tu, A.T.; Chen, Y.C. Venom ophthalmia and ocular complications caused by snake venom. Toxins 2020, 12, 567. [Google Scholar] [CrossRef] [PubMed]
- Konshina, A.G.; Boldyrev, I.A.; Utkin, Y.N.; Omel’kov, A.V.; Efremov, R.G. Snake Cytotoxins bind to membranes via interactions with phosphatidylserine head groups of lipids. PLoS ONE 2011, 6, e19064. [Google Scholar] [CrossRef] [Green Version]
- Tjong, S.C.; Wu, P.L.; Wang, C.M.; Huang, W.N.; Ho, N.L.; Wu, W.G. Role of glycosphingolipid conformational change in membrane pore forming activity of cobra cardiotoxin. Biochemistry 2007, 46, 12111–12123. [Google Scholar] [CrossRef]
- Tönsing, L.; Potgieter, D.J.; Louw, A.I.; Visser, L. The binding of snake venom cardiotoxins to heart cell membranes. Biochim. Biophys. Acta 1983, 732, 282–288. [Google Scholar] [CrossRef]
- Lee, C.Y.; Chang, C.C.; Chiu, T.H.; Chiu, P.J.S.; Tseng, T.C.; Lee, S.Y. Pharmacological properties of cardiotoxin isolated from Formosan cobra venom. Naunyn-Schmiedebergs Arch. Für Pharmakol. Exp. Pathol. 1968, 259, 360–374. [Google Scholar] [CrossRef]
- Panagides, N.; Jackson, T.; Ikonomopoulou, M.; Arbuckle, K.; Pretzler, R.; Yang, D.; Ali, S.; Koludarov, I.; Dobson, J.; Sanker, B.; et al. How the cobra got its flesh-eating venom: Cytotoxicity as a defensive innovation and its co-evolution with hooding, aposematic marking, and spitting. Toxins 2017, 9, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauridsen, L.P.; Laustsen, A.H.; Lomonte, B.; Gutierrez, J.M. Exploring the venom of the forest cobra snake: Toxicovenomics and antivenom profiling of Naja melanoleuca. J. Proteom. 2017, 150, 98–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourne, Y.; Talley, T.T.; Hansen, S.B.; Taylor, P.; Marchot, P. Crystal structure of a Cbtx-AChBP complex reveals essential interactions between snake alpha-neurotoxins and nicotinic receptors. EMBO J. 2005, 24, 1512–1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.C.; Lee, C.Y. Electrophysiological study of neuromuscular blocking action of cobra neurotoxin. Br. J. Pharmacol. Chemother. 1966, 28, 172–181. [Google Scholar] [CrossRef] [Green Version]
- Nirthanan, S.; Gwee, M.C.E. Three-finger alpha-neurotoxins and the nicotinic acetylcholine receptor, forty years on. J. Pharmacol. Sci. 2004, 94, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, P.C.; Henikoff, S. SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003, 31, 3812–3814. [Google Scholar] [CrossRef] [Green Version]
- Sim, N.-L.; Kumar, P.; Hu, J.; Henikoff, S.; Schneider, G.; Ng, P.C. SIFT web server: Predicting effects of amino acid substitutions on proteins. Nucleic Acids Res. 2012, 40, W452–W457. [Google Scholar] [CrossRef]
- Nirthanan, S.; Awal, W.; Niranjan, N.R. Snake α-neurotoxins and the nicotinic acetylcholine receptor. In Snake Venoms; Gopalakrishnakone, P., Inagaki, H., Mukherjee, A.K., Rahmy, T.R., Vogel, C.-W., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 1–39. [Google Scholar]
- Silva, A.; Cristofori-Armstrong, B.; Rash, L.D.; Hodgson, W.C.; Isbister, G.K. Defining the role of post-synaptic alpha-neurotoxins in paralysis due to snake envenoming in humans. Cell Mol. Life Sci 2018, 75, 4465–4478. [Google Scholar] [CrossRef]
- Chicheportiche, R.; Vincent, J.P.; Kopeyan, C.; Schweitz, H.; Lazdunski, M. Structure-function relationship in the binding of snake neurotoxins to the torpedo membrane receptor. Biochemistry 1975, 14, 2081–2091. [Google Scholar] [CrossRef] [PubMed]
- Leong, P.K.; Tan, N.H.; Fung, S.Y.; Sim, S.M. Cross neutralisation of Southeast Asian cobra and krait venoms by Indian polyvalent antivenoms. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 731–737. [Google Scholar] [CrossRef]
- Leong, P.K.; Sim, S.M.; Fung, S.Y.; Sumana, K.; Sitprija, V.; Tan, N.H. Cross neutralization of afro-Asian cobra and Asian krait venoms by a thai polyvalent snake antivenom (neuro polyvalent snake antivenom). PLoS Negl. Trop. Dis. 2012, 6, e1672. [Google Scholar] [CrossRef]
- Leong, P.K.; Tan, C.H.; Sim, S.M.; Fung, S.Y.; Sumana, K.; Sitprija, V.; Tan, N.H. Cross neutralization of common southeast Asian viperid venoms by a Thai polyvalent snake antivenom (Hemato polyvalent snake antivenom). Acta Trop. 2014, 132, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Leong, P.K.; Fung, S.Y.; Sim, S.M.; Ponnudurai, G.; Ariaratnam, C.; Khomvilai, S.; Sitprija, V.; Tan, N.H. Cross neutralization of Hypnale hypnale (hump-nosed pit viper) venom by polyvalent and monovalent Malayan pit viper antivenoms in vitro and in a rodent model. Acta Trop. 2011, 117, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Liew, J.L.; Tan, N.H.; Tan, C.H. Proteomics and preclinical antivenom neutralization of the mangrove pit viper (Trimeresurus purpureomaculatus, Malaysia) and white-lipped pit viper (Trimeresurus albolabris, Thailand) venoms. Acta Trop. 2020, 209, 105528. [Google Scholar] [CrossRef]
- Utkin, Y.N.; Kukhtina, V.V.; Kryukova, E.V.; Chiodini, F.; Bertrand, D.; Methfessel, C.; Tsetlin, V.I. Weak toxin from Naja kaouthia is a nontoxic antagonist of alpha 7 and muscle-type nicotinic acetylcholine receptors. J. Biol. Chem. 2001, 276, 15810–15815. [Google Scholar] [CrossRef] [Green Version]
- Kukhtina, V.V.; Weise, C.; Muranova, T.A.; Starkov, V.G.; Franke, P.; Hucho, F.; Wnendt, S.; Gillen, C.; Tsetlin, V.I.; Utkin, Y.N. Muscarinic toxin-like proteins from cobra venom. Eur. J. Biochem. 2000, 267, 6784–6789. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.S.; Chou, Y.C.; Lin, S.R.; Wu, B.N.; Lin, J.; Hong, E.; Sun, Y.J.; Hsiao, C.D. A novel neurotoxin, cobrotoxin b, from Naja naja atra (Taiwan cobra) venom: Purification, characterization, and gene organization. J. Biochem. 1997, 122, 1252–1259. [Google Scholar] [CrossRef]
- Pereira, M.F.; Novello, J.C.; Cintra, A.C.O.; Giglio, J.R.; Landucci, E.T.; Oliveira, B.; Marangoni, S. The amino acid sequence of Bothropstoxin-II, an Asp-49 myotoxin from Bothrops jararacussu (Jararacucu) venom with low phospholipase A2 activity. J. Protein Chem. 1998, 17, 381–386. [Google Scholar] [CrossRef] [PubMed]
- van den Bergh, C.J.; Slotboom, A.J.; Verheij, H.M.; de Haas, G.H. The role of aspartic acid-49 in the active site of phospholipase A2. A site-specific mutagenesis study of porcine pancreatic phospholipase A2 and the rationale of the enzymatic activity of [lysine49]phospholipase A2 from Agkistrodon piscivorus piscivorus’ venom. Eur. J. Biochem. 1988, 176, 353–357. [Google Scholar] [CrossRef]
- Vonk, F.J.; Casewell, N.R.; Henkel, C.V.; Heimberg, A.M.; Jansen, H.J.; McCleary, R.J.; Kerkkamp, H.M.; Vos, R.A.; Guerreiro, I.; Calvete, J.J. The king cobra genome reveals dynamic gene evolution and adaptation in the snake venom system. Proc. Natl. Acad. Sci. USA 2013, 110, 20651–20656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.H.; Wong, K.Y.; Tan, N.H.; Ng, T.S.; Tan, K.Y. Distinctive Distribution of Secretory Phospholipases A2 in the Venoms of Afro-Asian Cobras (Subgenus: Naja, Afronaja, Boulengerina and Uraeus). Toxins 2019, 11, 116. [Google Scholar] [CrossRef] [Green Version]
- Tan, K.Y.; Wong, K.Y.; Tan, N.H.; Tan, C.H. Quantitative proteomics of Naja annulifera (sub-Saharan snouted cobra) venom and neutralization activities of two antivenoms in Africa. Int. J. Biol. Macromol. 2020, 158, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Sudarshan, S.; Dhananjaya, B.L. The antimicrobial activity of an acidic phospholipase A2 (NN-XIa-PLA2) from the venom of Naja naja naja (indian cobra). Appl. Biochem. Biotechnol. 2015, 176, 2027–2038. [Google Scholar] [CrossRef]
- Mukherjee, A.K. Non-covalent interaction of phospholipase A2 (PLA2) and kaouthiotoxin (KTX) from venom of Naja kaouthia exhibits marked synergism to potentiate their cytotoxicity on target cells. J. Venom Res. 2010, 1, 37–42. [Google Scholar] [PubMed]
- Gasanov, S.E.; Alsarraj, M.A.; Gasanov, N.E.; Rael, E.D. Cobra venom cytotoxin free of phospholipase A2 and its effect on model membranes and T leukemia cells. J. Membr. Biol. 1997, 155, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Bustillo, S.; Garcia-Denegri, M.E.; Gay, C.; Van de Velde, A.C.; Acosta, O.; Angulo, Y.; Lomonte, B.; Gutierrez, J.M.; Leiva, L. Phospholipase A2 enhances the endothelial cell detachment effect of a snake venom metalloproteinase in the absence of catalysis. Chem.-Biol. Interact. 2015, 240, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Kazandjian, T.D.; Petras, D.; Robinson, S.D.; van Thiel, J.; Greene, H.W.; Arbuckle, K.; Barlow, A.; Carter, D.A.; Wouters, R.M.; Whiteley, G.; et al. Convergent evolution of pain-inducing defensive venom components in spitting cobras. Science 2021, 371, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Hia, Y.L.; Tan, K.Y.; Tan, C.H. Comparative venom proteomics of banded krait (Bungarus fasciatus) from five geographical locales: Correlation of venom lethality, immunoreactivity and antivenom neutralization. Acta Trop. 2020, 207, 105460. [Google Scholar] [CrossRef]
- Tan, K.Y.; Tan, N.H.; Tan, C.H. Venom proteomics and antivenom neutralization for the Chinese eastern Russell’s viper, Daboia siamensis from Guangxi and Taiwan. Sci. Rep. 2018, 8, 8545. [Google Scholar] [CrossRef]
- Senji Laxme, R.R.; Khochare, S.; Attarde, S.; Suranse, V.; Iyer, A.; Casewell, N.R.; Whitaker, R.; Martin, G.; Sunagar, K. Biogeographic venom variation in Russell’s viper (Daboia russelii) and the preclinical inefficacy of antivenom therapy in snakebite hotspots. PLoS Negl. Trop. Dis. 2021, 15, e0009247. [Google Scholar] [CrossRef]
- Chanda, A.; Kalita, B.; Patra, A.; Senevirathne, W.; Mukherjee, A.K. Proteomic analysis and antivenomics study of Western India Naja naja venom: Correlation between venom composition and clinical manifestations of cobra bite in this region. Expert Rev. Proteom. 2019, 16, 171–184. [Google Scholar] [CrossRef]
- Senji Laxme, R.R.; Khochare, S.; de Souza, H.F.; Ahuja, B.; Suranse, V.; Martin, G.; Whitaker, R.; Sunagar, K. Beyond the ‘big four’: Venom profiling of the medically important yet neglected Indian snakes reveals disturbing antivenom deficiencies. PLoS Negl. Trop. Dis. 2019, 13, e0007899. [Google Scholar] [CrossRef]
- Oh, A.M.F.; Tan, C.H.; Tan, K.Y.; Quraishi, N.H.; Tan, N.H. Venom proteome of Bungarus sindanus (Sind krait) from Pakistan and in vivo cross-neutralization of toxicity using an Indian polyvalent antivenom. J. Proteom. 2018, 193, 243–254. [Google Scholar] [CrossRef]
- Sanz, L.; Quesada-Bernat, S.; Ramos, T.; Casais-e-Silva, L.L.; Corrêa-Netto, C.; Silva-Haad, J.J.; Sasa, M.; Lomonte, B.; Calvete, J.J. New insights into the phylogeographic distribution of the 3FTx/PLA2 venom dichotomy across genus Micrurus in South America. J. Proteom. 2019, 200, 90–101. [Google Scholar] [CrossRef]
- Tan, C.H.; Tan, K.Y.; Ng, T.S.; Sim, S.M.; Tan, N.H. Venom proteome of spine-bellied sea snake (Hydrophis curtus) from Penang, Malaysia: Toxicity correlation, immunoprofiling and cross-neutralization by sea snake antivenom. Toxins 2019, 11, 3. [Google Scholar] [CrossRef] [Green Version]
- Sanz, L.; Pla, D.; Pérez, A.; Rodríguez, Y.; Zavaleta, A.; Salas, M.; Lomonte, B.; Calvete, J.J. Venomic analysis of the poorly studied desert coral snake, Micrurus tschudii tschudii, supports the 3FTx/PLA2 dichotomy across Micrurus venoms. Toxins 2016, 8, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández, J.; Rey-Súarez, P.; Pla, D.; Sanz, L.; Sasa, M.; Núñez, V.; Gutiérrez, J.; Calvete, J.; Lomonte, B. Proteomic studies on Micrurus (Coral Snakes) venom reveal a dichotomy of phenotypes. Toxicon 2018, 150, 319–320. [Google Scholar] [CrossRef]
- Oh, A.M.F.; Tan, K.Y.; Tan, N.H.; Tan, C.H. Proteomics and neutralization of Bungarus multicinctus (many-banded krait) venom: Intra-specific comparisons between specimens from China and Taiwan. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 247, 109063. [Google Scholar] [CrossRef] [PubMed]
- Lomonte, B.; Díaz, C.; Chaves, F.; Fernández, J.; Ruiz, M.; Salas, M.; Zavaleta, A.; Calvete, J.J.; Sasa, M. Comparative characterization of Viperidae snake venoms from Perú reveals two compositional patterns of phospholipase A2 expression. Toxicon X 2020, 7, 100044. [Google Scholar] [CrossRef] [PubMed]
- Zancolli, G.; Calvete, J.J.; Cardwell, M.D.; Greene, H.W.; Hayes, W.K.; Hegarty, M.J.; Herrmann, H.-W.; Holycross, A.T.; Lannutti, D.I.; Mulley, J.F.; et al. When one phenotype is not enough: Divergent evolutionary trajectories govern venom variation in a widespread rattlesnake species. Proc. R. Soc. B Biol. Sci. 2009, 286, 20182735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Punde, D.P. Management of snake-bite in rural Maharashtra: A 10-year experience. Natl. Med. J. India 2005, 18, 71–75. [Google Scholar]
- Bawaskar, H.S.; Bawaskar, P.H.; Punde, D.P.; Inamdar, M.K.; Dongare, R.B.; Bhoite, R.R. Profile of snakebite envenoming in rural Maharashtra, India. J. Assoc. Phys. India 2008, 56, 88–95. [Google Scholar]
- Xu, N.; Zhao, H.Y.; Yin, Y.; Shen, S.S.; Shan, L.L.; Chen, C.X.; Zhang, Y.X.; Gao, J.F.; Ji, X. Combined venomics, antivenomics and venom gland transcriptome analysis of the monocoled cobra (Naja kaouthia) from China. J. Proteom. 2017, 159, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Pandey, S. A study of snake bite cases in rural area of south eastern Rajasthan. Int. J. Biol. Med. Res. 2012, 3, 2348–2351. [Google Scholar]
- Al-Mamun, M.; Hakim, M.; Zaman, M.; Hoque, K.; Ferdousi, Z.; Reza, M. Histopathological alterations induced by Naja naja crude venom on renal, pulmonary and intestinal tissues of mice model. Br. Biotechnol. J. 2015, 6, 119–125. [Google Scholar] [CrossRef]
- Wong, O.F.; Lam, T.S.; Fung, H.T.; Choy, C.H. Five-year experience with Chinese cobra (Naja atra)-related injuries in two acute hospitals in Hong Kong. Hong Kong Med. J. 2010, 16, 36–43. [Google Scholar]
- Mao, Y.C.; Liu, P.Y.; Chiang, L.C.; Lai, C.S.; Lai, K.L.; Ho, C.H.; Wang, T.H.; Yang, C.C. Naja atra snakebite in Taiwan. Clin. Toxicol. 2017, 56, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wongtongkam, N.; Wilde, H.; Sitthi-Amorn, C.; Ratanabanangkoon, K. A study of thai cobra (Naja kaouthia) bites in Thailand. Mil. Med. 2005, 170, 336–341. [Google Scholar] [CrossRef] [Green Version]
- Ratanabanangkoon, K.; Tan, K.Y.; Pruksaphon, K.; Klinpayom, C.; Gutiérrez, J.M.; Quraishi, N.H.; Tan, C.H. A pan-specific antiserum produced by a novel immunization strategy shows a high spectrum of neutralization against neurotoxic snake venoms. Sci. Rep. 2020, 10, 11261. [Google Scholar] [CrossRef]
- Ratanabanangkoon, K.; Tan, K.Y.; Eursakun, S.; Tan, C.H.; Simsiriwong, P.; Pamornsakda, T.; Wiriyarat, W.; Klinpayom, C.; Tan, N.H. A Simple and novel strategy for the production of a pan-specific antiserum against elapid snakes of Asia. PLoS Negl. Trop. Dis. 2016, 10, e0004565. [Google Scholar] [CrossRef] [Green Version]
- Ratanabanangkoon, K. A quest for a universal plasma-derived antivenom against all elapid neurotoxic snake venoms. Front. Immunol. 2021, 12, 1363. [Google Scholar] [CrossRef] [PubMed]
- Wery, M.; Descrimes, M.; Thermes, C.; Gautheret, D.; Morillon, A. Zinc-mediated RNA fragmentation allows robust transcript reassembly upon whole transcriptome RNA-Seq. Methods 2013, 63, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Conesa, A.; Madrigal, P.; Tarazona, S.; Gomez-Cabrero, D.; Cervera, A.; McPherson, A.; Szcześniak, M.W.; Gaffney, D.J.; Elo, L.L.; Zhang, X.; et al. A survey of best practices for RNA-seq data analysis. Genome Biol. 2016, 17, 13. [Google Scholar] [CrossRef] [Green Version]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Lieber, M.; et al. De novo transcript sequence reconstruction from RNA-seq using the trinity platform for reference generation and analysis. Nat. Protoc. 2013, 8, 1494–1512. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [Green Version]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Muller, A.; Waterhouse, A.; Procter, J.; Barton, G.J.; Plewniak, F.; Poch, O. MACSIMS: Multiple alignment of complete sequences information management system. BMC Bioinform. 2006, 7, 318. [Google Scholar] [CrossRef] [Green Version]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.A.; Clamp, M.; Barton, G.J. Jalview Version 2—A multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [Green Version]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.Y.; Tan, K.Y.; Tan, N.H.; Tan, C.H. A neurotoxic snake venom without phospholipase A2: Proteomics and cross-neutralization of the venom from Senegalese cobra, Naja senegalensis (Subgenus: Uraeus). Toxins 2021, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Finney, D.J. Probit Analysis, 2nd ed.; Cambridge University Press: New York, NY, USA, 1952. [Google Scholar]
- Morais, V.; Ifran, S.; Berasain, P.; Massaldi, H. Antivenoms: Potency or median effective dose, which to use? J. Venom. Anim. Toxins Incl. Trop. Dis. 2010, 16, 191–193. [Google Scholar] [CrossRef]
- Tan, C.H.; Tan, K.Y. Functional application of snake venom proteomics in in vivo antivenom assessment. In Functional Proteomics: Methods and Protocols; Wang, X., Kuruc, M., Eds.; Springer: New York, NY, USA, 2019; pp. 153–158. [Google Scholar]
- Ma, J.; Chen, T.; Wu, S.; Yang, C.; Bai, M.; Shu, K.; Li, K.; Zhang, G.; Jin, Z.; He, F.; et al. iProX: An integrated proteome resource. Nucleic Acids Res. 2019, 47, 1211–1217. [Google Scholar] [CrossRef] [Green Version]



| Parameter | Sequencing Output |
|---|---|
| Total raw reads | 54,477,234 |
| Total clean reads | 51,595,008 |
| Total clean nucleotides (nt) | 4,643,550,720 |
| Q20 (%) | 98.50 |
| N (%) | 0.01 |
| GC (%) | 44.62 |
| Contigs assembly statistics | 106,637 |
| Total length (nt) | 28,724,569 |
| Mean length (nt) | 269 |
| N50 length (bp) | 361 |
| Unigenes assembly statistics | 53,595 |
| Total length (nt) | 25,919,321 |
| Mean length (nt) | 484 |
| N50 length (bp) | 676 |
| Gene ID | Protein Family/ Subfamily | Annotated Accession (NCBI/Uniprot) | Species | Coverage A (%) | Transcript B Abundance (%) |
|---|---|---|---|---|---|
| Three-finger toxins (3FTX) | 87.54% | ||||
| Short-chain 3FTX: Short α-NTX (SNTX) | 1.78% | ||||
| NN-3FTX01 | Short neurotoxin 2 | P62376 | Hydrophis cyanocinctus | 13–79 | 0.11% |
| NN-3FTX02 * | Cobrotoxin | P60770 | Naja atra | 1–82 (98.80) | 0.69% |
| NN-3FTX03 * | Cobrotoxin-b | P80958 | Naja atra | 1–82 (100) | 0.97% |
| Short-chain 3FTX: Orphan Group I | 0.61% | ||||
| NN-3FTX04 | Neurotoxin-like protein NTL2 | Q9W717 | Naja atra | 1–75 | 0.61% |
| Short-chain 3FTX: Muscarinic toxin-like protein (MTLP) | 1.37% | ||||
| NN-3FTX05 * | Neurotoxin homolog NL1 | Q9DEQ3 | Naja atra | 6–86 (94.19) | 0.96% |
| NN-3FTX06 | Muscarinic toxin-like protein 2 | P82463 | Naja kaouthia | 1–46 | 0.41% |
| NN-3FTX07 | Haditoxin | A8N286 | Ophiophagus hannah | 36–84 | 0.001% |
| Short-chain 3FTX: Cytotoxin/cardiotoxin (CTX) | 77.33% | ||||
| NN-3FTX08 * | Cytotoxin 5 | Q98961 | Naja atra | 1–81 (100) | 77.33% |
| Long-chain 3FTX: Long α-NTX (LNTX) | 1.10% | ||||
| NN-3FTX09 * | Long neurotoxin 7 | O42257 | Naja sputatrix | 12–88 (97.78) | 1.00% |
| NN-3FTX10 | Long neurotoxin 1 | Q8UW29 | Hydrophis hardwickii | 12–91 | 0.01% |
| NN-3FTX11 | Long neurotoxin-like OH-31 | Q53B55 | Ophiophagus hannah | 20–59 | 0.08% |
| NN-3FTX12 | Long neurotoxin-like OH-31 | Q53B55 | Ophiophagus hannah | 5–59 | 0.003% |
| Non-conventional 3FTX: Weak neurotoxin (WTX) | 5.35% | ||||
| NN-3FTX13 | Probable weak neurotoxin NNAM1 | Q9YGI2 | Naja atra | 15–86 | 5.35% |
| Phospholipase A2 (PLA2) | 2.08% | ||||
| NN-PLA01 | Basic phospholipase A2 | P00610 | Hydrophis schistosus | 23–119 | 0.01% |
| NN-PLA02 | Basic phospholipase A2 | P00610 | Hydrophis schistosus | 23–119 | 0.002% |
| NN-PLA03 | Acidic phospholipase A2 57 | Q8UW31 | Hydrophis hardwickii | 51–135 | 0.01% |
| NN-PLA04 * | Acidic phospholipase A2 2 | P00597 | Naja kaouthia | 1–146 (100) | 2.05% |
| NN-PLA05 | Basic phospholipase A2 73 | Q8UW30 | Hydrophis hardwickii | 1–56 | 0.01% |
| Snake venom metalloproteinase (SVMP) | 3.80% | ||||
| NN-SVMP01 | Scutatease-1 | B5KFV7 | Notechis scutatus | 337–388 | 0.04% |
| NN-SVMP02 | Zinc metalloproteinase mocarhagin | Q10749 | Naja mossambica | 502–568 | 0.03% |
| NN-SVMP03 | Zinc metalloproteinase mocarhagin | Q10749 | Naja mossambica | 191–241 | 0.03% |
| NN-SVMP04 | Zinc metalloproteinase mocarhagin | Q10749 | Naja mossambica | 143–195 | 0.03% |
| NN-SVMP05 | Zinc metalloproteinase-disintegrin cobrin | Q9PVK7 | Naja kaouthia | 159–499 | 0.27% |
| NN–SVMP06 | Zinc metalloproteinase-disintegrin cobrin | Q9PVK7 | Naja kaouthia | 159–600 | 0.16% |
| NN-SVMP07 | Zinc metalloproteinase-disintegrin cobrin | Q9PVK7 | Naja kaouthia | 159–600 | 0.13% |
| NN-SVMP08 | Zinc metalloproteinase-disintegrin atrase-B | D6PXE8 | Naja atra | 322–369 | 0.01% |
| NN-SVMP09 | Zinc metalloproteinase-disintegrin atrase-A | D5LMJ3 | Naja atra | 456–607 | 0.10% |
| NN-SVMP10 | Zinc metalloproteinase-disintegrin atrase-A | D5LMJ3 | Naja atra | 456–607 | 0.10% |
| NN-SVMP11 | Zinc metalloproteinase-disintegrin atrase-A | D5LMJ3 | Naja atra | 456–607 | 0.15% |
| NN-SVMP12 | Zinc metalloproteinase-disintegrin atrase-A | D5LMJ3 | Naja atra | 403–607 | 0.13% |
| NN-SVMP13 | Zinc metalloproteinase-disintegrin atrase-A | D5LMJ3 | Naja atra | 403–607 | 0.17% |
| NN-SVMP14 | Zinc metalloproteinase-disintegrin atrase-A | D5LMJ3 | Naja atra | 456–607 | 0.28% |
| NN-SVMP15 | Zinc metalloproteinase-disintegrin atrase-A | D5LMJ3 | Naja atra | 1–229 | 1.25% |
| NN-SVMP16 | Hemorrhagic metalloproteinase-disintegrin kaouthiagin | P82942 | Naja kaouthia | 6–64 | 0.03% |
| NN-SVMP17 | Zinc metalloproteinase-disintegrin-like atragin | D3TTC2 | Naja atra | 527–613 | 0.80% |
| NN-SVMP18 | Hemorrhagic metalloproteinase-disintegrin kaouthiagin | P82942 | Naja kaouthia | 130–201 | 0.07% |
| Cysteine-rich secretory protein (CRISP) | 0.35% | ||||
| NN-CRISP01 * | Natrin-1 | Q7T1K6 | Naja atra | 1–239 (100) | 0.35% |
| Snake venom serine protease (SVSP) | 1.56% | ||||
| NN-SVSP01 * | Serine protease harobin | Q5MCS0 | Hydrophis hardwickii | 1–265 (96.60) | 1.56% |
| NN-SVSP02 | Serine protease HTRA1 | V8NGT0 | Ophiophagus hannah | 79–304 | 0.001% |
| NN-SVSP03 * | Serine protease 23 | V8N8N4 | Ophiophagus hannah | 8–372 (98.12) | 0.001% |
| Cobra venom factor (CVF) | 0.26% | ||||
| NN-CVF01 | Cobra venom factor | Q91132 | Naja kaouthia | 1586–1642 | 0.13% |
| NN-CVF02 * | Cobra venom factor | Q91132 | Naja kaouthia | 1–1592 (96.96) | 0.10% |
| NN-CVF03 | Cobra venom factor | Q91132 | Naja kaouthia | 1197–1592 | 0.02% |
| NN-CVF04 | Cobra venom factor | Q91132 | Naja kaouthia | 1–640 | 0.01% |
| Kunitz-type serine protease inhibitor (KSPI) | 0.50% | ||||
| NN-KSPI01 * | Putative Kunitz-type serine protease inhibitor | B2BS84 | Austrelaps labialis | 1–252 (98.81) | 0.02% |
| NN-KSPI02 * | Kunitz-type protease inhibitor | U3FZD6 | Micrurus fulvius | 1–511 (99.61) | 0.004% |
| NN-KSPI03 | Protease inhibitor 3 | C1IC51 | Walterinnesia aegyptia | 1–67 | 0.17% |
| NN-KSPI04 | Kunitz-type serine protease inhibitor NACI | Q5ZPJ7 | Naja atra | 43–81 | 0.30% |
| Phosphodiesterase (PDE) | 0.08% | ||||
| NN-PDE01 * | Snake venom phosphodiesterase | A0A2D0TC04 | Naja atra | 21–850 (97.65) | 0.08% |
| 5’-Nucleotidase (5’-NUC) | 0.06% | ||||
| NN-NUC01 * | Ecto-5’-nucleotidase | A0A194AS98 | Micrurus tener | 1–569 (99.13) | 0.05% |
| NN-NUC02 * | Ecto-5’-nucleotidase | A0A194AS98 | Micrurus tener | 1–536 (93.38) | 0.008% |
| NN-NUC03 * | Ecto-5’-nucleotidase | A0A194AS98 | Micrurus tener | 1–536 (93.38) | 0.004% |
| NN-NUC04 | 5’ nucleotidase | A6MFL8 | Demansia vestigiata | 134–559 | 0.001% |
| Acetylcholinesterase (AChE) | 0.01% | ||||
| NN-ACHE01 * | Acetylcholinesterase | Q92035 | Bungarus fasciatus | 1–566 (95.55) | 0.004% |
| NN-ACHE02 | Acetylcholinesterase | Q92035 | Bungarus fasciatus | 1–566 (95.55) | 0.002% |
| Hyaluronidase (HYA) | 0.002% | ||||
| NN-HYA01 | Hyaluronidase | V8PHI0 | Ophiophagus hannah | 26–71 | 0.001% |
| NN-HYA02 | Hyaluronidase | A0A2D4JS30 | Micrurus lemniscatus lemniscatus | 89–208 | 0.0005% |
| NN-HYA03 | Hyaluronidase | A0A2D4JS30 | Micrurus lemniscatus lemniscatus | 27–91 | 0.0003% |
| NN-HYA04 * | Hyaluronidase | A0A194APD1 | Micrurus tener | 1–440 (98.43) | 0.0003% |
| Nerve growth factor (NGF) | 2.88% | ||||
| NN-NGF01 * | Venom nerve growth factor 2 | Q5YF89 | Naja sputatrix | 1–241 (100) | 2.88% |
| Vespryn (VES) | 0.04% | ||||
| NN-VES01 * | Ohanin | P83234 | Ophiophagus hannah | 1–181 (100) | 0.03% |
| NN-VES02 * | Ohanin | P83234 | Ophiophagus hannah | 1–181 (100) | 0.02% |
| Vascular endothelial growth factor (VEGF) | 0.001% | ||||
| NN-VEGF01 * | Vascular endothelial growth factor 1 | U3F558 | Micrurus fulvius | 1–142 (95.95) | 0.001% |
| NN-VEGF02 * | Vascular endothelial growth factor 1 | U3F558 | Micrurus fulvius | 1–142 (95.95) | 0.0003% |
| Dipeptidyl peptidase IV (DPP IV) | 0.02% | ||||
| NN-DPP01 | Dipeptidyl peptidase 4 | V8P9G9 | Ophiophagus hannah | 0.02% | 0.02% |
| Phospholipase B (PLB) | 0.01% | ||||
| NN-PLB01 * | Phospholipase-B 81 | F8J2D3 | Drysdalia coronoides | 1–553 (100) | 0.01% |
| NN-PLB02 | Phospholipase B-like | V8NLQ9 | Ophiophagus hannah | 146–196 | 0.001% |
| Snake C-type lectin (SNACLEC) | 0.17% | ||||
| NN-SNAC01 * | C-type lectin BfL-1 | Q90WI8 | Bungarus fasciatus | 1–158 (100) | 0.06% |
| NN-SNAC02 * | C-type lectin BfL-1 | Q90WI8 | Bungarus fasciatus | 1–148 (98.67) | 0.11% |
| NN-SNAC03 | Venom C-type lectin mannose binding isoform 1 variant 3 | D2YVK0 | Tropidechis carinatus | 57–158 | 0.001% |
| Cystatin (CYS) | 0.01% | ||||
| NN-CYS01 | Cystatin | E3P6P4 | Naja kaouthia | 0.01% | 0.01% |
| Aminopeptidase (AP) | 0.004% | ||||
| NN-AP01 * | Aminopeptidase | U3FZS8 | Micrurus fulvis | 1–992 (98.29) | 0.003% |
| NN-AP02 * | Aminopeptidase | U3FZS8 | Micrurus fulvius | 1–992 (98.29) | 0.001% |
| Waprin (WA) | 0.001% | ||||
| NN-WAP01 | Waprin-Phi3 | A7X4M7 | Philodryas olfersii | 33–80 | 0.001% |
| Natriuretic peptide (NP) | 0.55% | ||||
| NN-NP01 | Natriuretic peptide Na-NP | D9IX97 | Naja atra | 34–77 | 0.41% |
| NN-NP02 | Natriuretic peptide Na-NP | D9IX97 | Naja atra | 115–165 | 0.08% |
| NN-NP03 | Natriuretic peptide Na-NP | D9IX97 | Naja atra | 25–80 | 0.06% |
| L-amino acid oxidase (LAAO) | 0.08% | ||||
| NN-LAAO01 | L-amino-acid oxidase | Q4JHE3 | Oxyuranus scutellatus scutellatus | 1–517 | 0.04% |
| NN-LAAO02 | L-amino-acid oxidase | Q4JHE3 | Oxyuranus scutellatus scutellatus | 1–517 | 0.02% |
| NN-LAAO03 | L-amino-acid oxidase | Q4JHE3 | Oxyuranus scutellatus scutellatus | 1–517 | 0.02% |
| Protein Name | Accession Number * | Species * | Relative Abundance |
|---|---|---|---|
| 3FTX | 72.19% | ||
| SNTX | 0.86% | ||
| Short neurotoxin 1 | P01427 | Naja oxiana | 0.86% |
| LNTX | 9.61% | ||
| Alpha-elapitoxin-Nk2a | P01391 | Naja kaouthia | 2.22% |
| Long neurotoxin 1 | P25668 | Naja naja | 0.54% |
| Long neurotoxin 1 | P25671 | Naja kaouthia | 6.60% |
| Long neurotoxin 4 | P25672 | Naja naja | 0.25% |
| MTLP | 2.34% | ||
| Muscarinic toxin-like protein 3 | P82464 | Naja kaouthia | 2.16% |
| Muscarinic toxin-like protein 2 | P82463 | Naja kaouthia | 0.18% |
| WTX | 2.46% | ||
| Weak neurotoxin 7 | P29181 | Naja naja | 0.15% |
| Weak neurotoxin 6 | P29180 | Naja naja | 2.31% |
| CTX | 56.92% | ||
| Cytotoxin 3 | P01446 | Naja kaouthia | 0.06% |
| Cytotoxin 1d/1e | Q98958 | Naja atra | 7.83% |
| Cytotoxin-like basic protein | P62377 | Naja naja | 4.34% |
| Cytotoxin 4 | P60303 | Naja kaouthia | 0.38% |
| Cytotoxin 2c | O93472 | Naja sputatrix | 37.34% |
| Cytotoxin 1 | P01447 | Naja naja | 6.97% |
| PLA2 | 14.58% | ||
| Neutral phospholipase A2 muscarinic inhibitor | Q92084 | Naja sputatrix | 1.88% |
| Acidic phospholipase A2 2 | P15445 | Naja naja | 11.63% |
| Basic phospholipase A2 homolog 1 | P10117 | Laticauda colubrina | 0.54% |
| Acidic phospholipase A2 1 | P00596 | Naja kaouthia | 0.32% |
| Acidic phospholipase A2 2 | P00597 | Naja kaouthia | 0.21% |
| KSPI | 0.10% | ||
| Protease inhibitor 2 | Unigene36921_ESM | Hydrophis schistosus | 0.10% |
| NGF | 0.71% | ||
| Venom nerve growth factor | P01140 | Naja naja | 0.58% |
| Venom nerve growth factor | P61898 | Naja atra | 0.12% |
| VES | 0.28% | ||
| Thaicobrin | P82885 | Naja kaouthia | 0.28% |
| SVMP | 4.06% | ||
| Zinc metalloproteinase-disintegrin-like atrase-A | D5LMJ3 | Naja atra | 3.91% |
| Hemorrhagic metalloproteinase kaouthiagin | P82942 | Naja kaouthia | 0.15% |
| CRISP | 3.08% | ||
| Cysteine-rich venom protein natrin-1 | Q7T1K6 | Naja atra | 3.08% |
| SVSP | 0.95% | ||
| Snake venom serine protease NaSP | A8QL53 | Naja atra | 0.95% |
| CVF | 1.66% | ||
| Cobra venom factor precursor | Q91132 | Naja kaouthia | 1.66% |
| PDE | 0.07% | ||
| Phosphodiesterase | A0A194ARD7 | Micrurus tener | 0.04% |
| Phosphodiesterase 1 | Unigene5869_Naja-sumatrana | Naja sumatrana | 0.03% |
| LAAO | 0.55% | ||
| L-amino acid oxidase | A8QL58 | Naja atra | 0.44% |
| L-amino-acid oxidase precursor | Q4JHE3 | Oxyuranus scutellatus scutellatus | 0.11% |
| 5’-NUC | 0.04% | ||
| Snake venom 5’-nucleotidase | CL3600.Contig1_NS2A | Naja sumatrana | 0.04% |
| GPX | 0.13% | ||
| Glutathione peroxidase | V8P395 | Ophiophagus hannah | 0.13% |
| ND | 1.61% |
| Snake Locality | i.v. LD50 (mg/kg) | Antivenom Used | Challenge Dose | ED50 (µL) a | ER50 (mg/mL) b | Neutralization c Potency (mg/mL) | Reference |
|---|---|---|---|---|---|---|---|
| Sri Lanka | 0.75 (0.48–1.18) | VINS polyvalent antivenom | 2.5 LD50 | 35.00 | 1.23 (1.11–1.37) | 0.74 | Current study |
| Sri Lanka | 1.13 (0.80–1.49) | VINS polyvalent antivenom | 3.0 LD50 | 96.86 | 0.70 (0.60–0.90) | 0.47 | [22] |
| Instituto Clodomiro Picado polyvalent antivenom | 3.0 LD50 | 195 | 0.40 (0.30–0.50) | 0.23 | |||
| Sri Lanka | 0.67 (0.48–0.99) | VINS polyvalent antivenom | 5.0 LD50 | # | - | [12] | |
| Bharat polyvalent antivenom | 5.0 LD50 | # | - | ||||
| Maharashtra, West India | 0.73 (0.50–0.88) | Premium Serums antivenom | 5.0 LD50 | 81.27 | 0.90 (0.74–1.08) | 0.72 | [94] |
| Punjab, North India | 0.33 (0.28–0.38) | Premium Serums antivenom | 5.0 LD50 | 67.37 | 0.49 | 0.39 | [6] |
| Rajasthan, West India | 2.53 | Premium Serums antivenom | 5.0 LD50 | NE | NE | NE | |
| West Bengal, East India | 0.27 (0.22–0.32) | Premium Serums antivenom | 5.0 LD50 | 60.45 | 0.45 | 0.36 | |
| Andhra Pradesh, Southeast India | 0.55 (0.45–0.65) | Premium Serums antivenom | 5.0 LD50 | 54.24 | 1.01 | 0.81 | |
| Madhya Pradesh, Central India | 0.22 (0.18–0.25) | Premium Serums antivenom | 5.0 LD50 | 60.45 | 0.36 | 0.29 | |
| Pakistan | 0.22 (0.12–0.40) | VINS polyvalent antivenom | 5.0 LD50 | 32.77 | 0.77 (0.69–0.85) | 0.61 | [8,54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, K.Y.; Tan, K.Y.; Tan, N.H.; Gnanathasan, C.A.; Tan, C.H. Elucidating the Venom Diversity in Sri Lankan Spectacled Cobra (Naja naja) through De Novo Venom Gland Transcriptomics, Venom Proteomics and Toxicity Neutralization. Toxins 2021, 13, 558. https://doi.org/10.3390/toxins13080558
Wong KY, Tan KY, Tan NH, Gnanathasan CA, Tan CH. Elucidating the Venom Diversity in Sri Lankan Spectacled Cobra (Naja naja) through De Novo Venom Gland Transcriptomics, Venom Proteomics and Toxicity Neutralization. Toxins. 2021; 13(8):558. https://doi.org/10.3390/toxins13080558
Chicago/Turabian StyleWong, Kin Ying, Kae Yi Tan, Nget Hong Tan, Christeine Ariaranee Gnanathasan, and Choo Hock Tan. 2021. "Elucidating the Venom Diversity in Sri Lankan Spectacled Cobra (Naja naja) through De Novo Venom Gland Transcriptomics, Venom Proteomics and Toxicity Neutralization" Toxins 13, no. 8: 558. https://doi.org/10.3390/toxins13080558
APA StyleWong, K. Y., Tan, K. Y., Tan, N. H., Gnanathasan, C. A., & Tan, C. H. (2021). Elucidating the Venom Diversity in Sri Lankan Spectacled Cobra (Naja naja) through De Novo Venom Gland Transcriptomics, Venom Proteomics and Toxicity Neutralization. Toxins, 13(8), 558. https://doi.org/10.3390/toxins13080558






